Background: Muscle atrophy
resulting wholly or partially from disuse represents a serious medical
complication that decreases quality of life and increases morbidity and
mortality. The accumulation of misfolded/unfolded proteins disrupts endoplasmic
reticulum (ER) homeostasis and thus causes ER stress. Growing evidence indicates
that ER stress plays an essential role in skeletal muscle remodeling under
various physiological or pathophysiological conditions. However, whether ER
stress is involved in disuse-induced muscle atrophy remains unclear.
Methods: To induce muscle atrophy, 8-week-old C57BL/6JNifdc male mice
were subjected to 3, 7, or 14 days of hindlimb unloading (HU), and rhesus
macaques (Macaca mulatta) were subjected to 10 head-down
tilted bed rest (HDBR) for 6 weeks. Tauroursodeoxycholic acid (TUDCA) (500
mg/kg/d) was orally administered to mice during HU to inhibit ER stress.
Quantitative PCR, Western blotting, and immunohistochemistry were conducted to
evaluate gene, protein, and structural changes, respectively. Results:
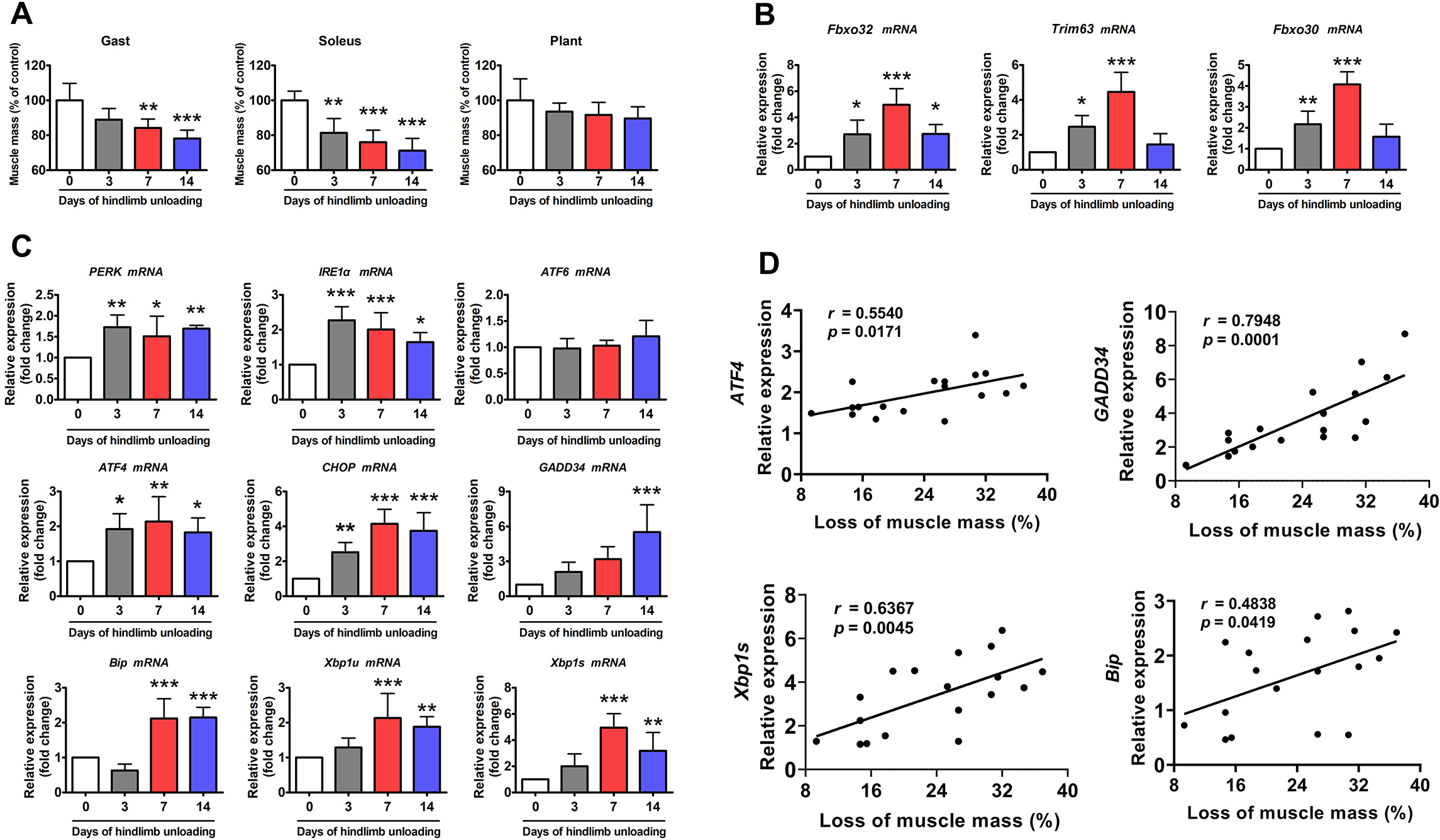
ER stress marker genes were rapidly induced by HU in a similar trend to that
observed with atrophy-related genes such as Atrogin-1, muscle RING
finger 1 (MuRF1), and muscle ubiquitin ligase of SCF complex in
atrophy-1 (MUSA1). Inhibition of ER stress with TUDCA, a pan-ER stress
inhibitor, attenuated HU-induced muscle atrophy and the upregulation of ubiquitin
ligases via the AKT/forkhead box O3a pathway. In addition, the
oxidative-to-glycolytic myofiber type transition caused by HU was also inhibited
by TUDCA treatment. ER stress activation was also confirmed in HDBR-induced
rhesus soleus muscle atrophy. Conclusions: The strong positive
correlation between ER stress activation and both HU- and HDBR-induced muscle
atrophy indicates that ER stress activation is ubiquitously involved in
disuse-induced muscle atrophy, regardless of species. Thus, inhibiting ER stress
may be an effective therapeutic strategy to prevent muscle atrophy during
disuse.