- Academic Editor
-
-
-
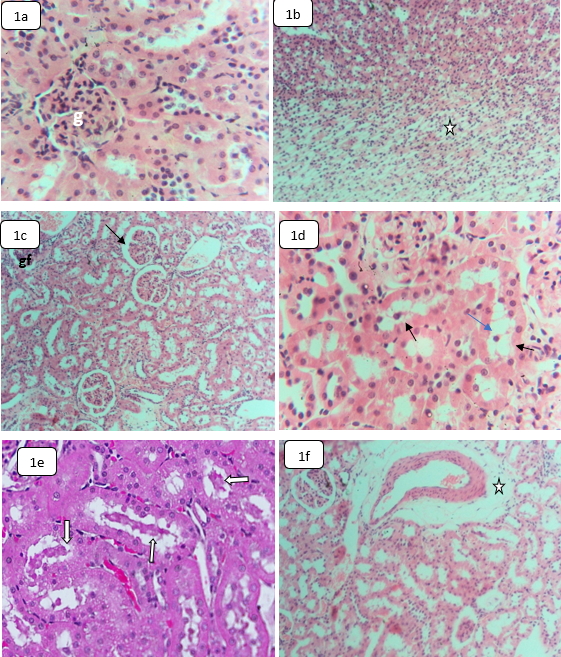
Background: Hydroxychloroquine (HCQ) toxicity can adversely affect vital organs, cause pathologic ocular damage, and can have direct cardiovascular effects. This study aims to identify the biochemical, hematological, and histological alterations of the vital organs associated with the effects of HCQ. Methods: Male albino rats were exposed to the equivalent of HCQ therapeutic doses given to human patients being affected by malaria, lupus erythematosus, and COVID-19. The animal blood samples were subjected to hematological analysis, biochemical analysis, liver function tests, kidney function tests, and cardiac biomarkers. Liver, kidney, heart, spleen, and testis biopsies were subjected to histological examination. Results: HCQ significantly lowered the values of erythrocytes, hemoglobin, hematocrit, platelets, leucocytes, and lymphocytes but significantly increased the values of aspartate aminotransferase (AST), alanine aminotransferase (ALT), amylase, alkaline phosphatase, lactate dehydrogenase, cholesterol, and chlorine ions. The renal tissues of HCQ-treated animals demonstrated glomerular fragmentation, partial atrophy degeneration, renal tubules hydropic degeneration, hyaline cast formation, and interstitial edema formation. Additionally, the heart exhibited myofiber necrosis, myolysis, wavy appearance, disorganization, and disarray. The testicular tissues also demonstrated spermatocyte degeneration, spermatogenic cell sloughing, testicular interstitial edema, and occasional spermatogenic arrest. Additionally, the spleen showed a decrease in the number and size of the white pulp follicles, a decrease in the number of apoptotic activity, and a decline in the number of T-rich cells. However, the red pulp demonstrated a diffuse decline in B rich-lymphocytes and macrophages. The liver was also the least affected but showed Kupffer cell hyperplasia and occasional hepatocyte dysplasia. Conclusions: The results indicate that chronic exposure to HCQ could alter the structures and functions of the vital organs.