1 Department of Ophthalmology, Eye Institute of Chinese PLA, Xijing Hospital, Fourth Military Medical University, 710032 Xi′an, Shaanxi, China
2 Department of Chinese Materia Medica, College of Life Sciences, Northwestern University, 710069 Xi′an, Shaanxi, China
3 Department of Ophthalmology, The First Affiliated Hospital of Northwest University (Xi′an First Hospital), 710002 Xi′an, Shaanxi, China
†These authors contributed equally.
Academic Editor: Shikun He
Abstract
In recent years, in-depth research on anti-tumor therapy has brought the emergence of new active chemotherapeutic agents and combination regimens. However, as one of them, taxane drugs are widely used in clinical practice, but it should be noted that many side reactions caused by their application bring some difficulties to routine management. Among the side reactions related to taxane anti-tumor therapy, ocular adverse reactions are occasionally reported and are not life-threatening but may seriously affect patients’ life quality. Thus, the continuation, reduction and cessation of taxane chemotherapy still need to be further evaluated by ophthalmologists and oncologists once the side effects show up. To prevent ocular side reactions, close attention should be paid to complications during medication. To facilitate the oncology department and ophthalmologists to comprehensively understand the ophthalmic adverse reactions of taxane drugs and their possible mechanisms and improve drug use efficiency, we collected relevant literature and reviewed and provided some suggestions for the monitoring and managing of ophthalmic toxicity.
Keywords
- paclitaxel
- taxane
- eye diseases
- drug-related side effects and adverse reactions
As the first natural phytochemical drug approved by Food and Drug Administration (FDA), paclitaxel (Taxol) is an anti-tumor drug with high efficacy and low toxicity. Paclitaxel is used to treat breast cancer, ovarian, lung cancer, sarcoma, lymphoma and other diseases [1, 2]. Breast cancer is the most common cancer among women in most countries. The number of women diagnosed with breast cancer will increase to almost 3.2 million per year by 2030 [3]. Taxane is a first- and second-line anticancer drug in treating breast cancer, ovarian cancer and non-small cell lung cancer. However, served as an anti-tumor drug, the number of reports on Paclitaxel’s adverse side effects is increasing rapidly, mainly including bone marrow suppression, allergic reaction, digestive system reaction, numbness of the hand and foot, liver injury, and etc. Moreover, in recent years, there have been increasing reports of adverse ocular reactions caused by taxane drugs, such as vision loss [4], dry eye [5, 6], lacrimal duct obstruction [7], conjunctivitis [8], glaucoma [9], optic neuropathy [10, 11, 12], epiphora [13], macular edema [14, 15], blindness caused by combination with cisplatin [16], and so on. Therefore, clinicians must be aware of potential vision-threatening complications [17]. Prompt consultation with an ophthalmologist can lead to early detection, proper diagnosis, and appropriate therapeutic measures. Dose reduction or discontinuation of incriminated drugs may help reduce the severity and the duration of side effects. Generally, the ocular side effects caused by taxane chemotherapy can be relieved after stopping the administration of chemotherapy [18]. All those side effects mentioned above may not threaten the life span of the patients while may cause various troubles in their life after anti-cancer treatment. Herein, we review the relevant literatures and reports to conclude a comprehensive understanding of the ocular adverse side effects caused by taxane drugs, and also discuss underlining toxic mechanisms in order to provide a few instructions to its clinical administration.
Paclitaxel is the first microtubule-stabilizing drug of the taxane family isolated from the yew root. In 1967, Mansukh Wani and Monroe Wall isolated and identified the active ingredient from the bark of T. brevifolia. They named it taxol, based on its species of origin and the presence of hydroxyl groups [19]. Paclitaxel entered phase I clinical trials in 1894 and phase II clinical trials in 1895. In the United States, the Phase III clinical trial was completed in-ears from 1983 and was approved by the FDA in 1992 [1]. Paclitaxel has been obtained by chemical semi-synthesis, total chemical synthesis, tissue and cell culture, microbial fermentation, and biosynthesis. In 1995, docetaxel, the second family member, was waived for medical use. Due to the poor water-solubility of paclitaxel before, solvent-increasing polyoxyethylene castor oil and anhydrous ethanol will be added when it is used [20]. As the solvent cause severe allergic reactions, there were various limitations to clinical use.
Currently, the paclitaxel family includes traditional paclitaxel, paclitaxel liposome, paclitaxel nanoparticle albumin-bound (NAB) (Abraxane), and docetaxel (Taxotere). Unlike other microtubule-stabilizing anticancer drugs which prevent the assembly of tubulin into microtubules, it is a microtubule stabilizer (anticontractile agent) and a mitotic inhibitor (antiproliferative agent) [19]. Paclitaxel promotes the assembly of tubulin to microtubules and prevents the dissociation of microtubules and blocks cell cycle progression, prevents mitosis, and inhibits the growth of cancer cells [1]. Thus, paclitaxel has become a widely accepted chemotherapeutic drug in treating ovarian cancer, breast cancer, non-small cell lung cancer, and other malignant solid tumors [2].
According to the Drug Instructions, the injectable paclitaxel (albumin-bound) specification states that eye/visual adverse events occurred in 13% population (48/366) of clinical studies in the United States and Europe, while severe cases occurred in 1% population. The major diseases were keratitis and blurred vision, which were usually reversible. In a phase Ⅰ clinical study of Chinese patients, 1 out of 104 patients developed transient blurred vision and diplopia. In a randomized controlled clinical study of Chinese patients with metastatic breast cancer, 4 out of 100 patients developed mild blurred vision, which was transient and self-healing. Noguchi has conducted a retrospective study of risk factors concerning ocular disorders caused by paclitaxel and NAB-paclitaxel [21]. This retrospective study targeted patients who were newly treated with paclitaxel or NAB-paclitaxel at Kyoto Okamoto Memorial Hospital between April 1 2012, and March 31 2017 of 128 subjects, 13 (10.2%) had ocular disorders with symptoms ranging from grades 1 and 2 (Eye disorders and peripheral were graded by using the Common Terminology Criteria for Adverse Events version are grade 1, which are asymptomatic, asymptomatic only detected during examination, or asymptomatic but not affecting function without treatment. Grade 2 is characterized by symptoms that affect function but do not interfere with daily activities, requiring correction or simple drug treatment). The symptoms mainly of these 13 patients included conjunctivitis or subconjunctival hemorrhage (3.1%), visual acuity reduction (2.3%), blurred vision and eye pain (1.6% each), eye mucus, blepharitis, stye, watering eyes, photopsia, and muscae volitantes (0.8% each). Meanwhile, Noguchi conducted a retrospective study on the risk factors of eye diseases caused by docetaxel administration every 3 weeks [22]. This case-control study targeted newly administered docetaxel patients at the Kyoto Okamoto Memorial Hospital between July 1 2015 and June 30 2018. Of the 89 subjects, 7 (7.9%) had eye disorders. Another retrospective analysis of clinical characteristics, treatment regimens, and concurrent systemic adverse reactions was performed in patients treated with taxanes at a single center from January 1 2010 to February 29 2020. In this study, 22 (1.1%) of 1918 patients experienced ophthalmic side effects [23]. Cancer chemotherapy has the potential to produce acute and chronic damage in any organ system. However, some organs are more sensitive than others. In this context, the eye is usually considered a sanctuary site, but has a potentially high degree of sensitivity to toxic substances. Ocular toxicity induced by cancer chemotherapy is not uncommon. Still, the broad spectrum of reactions to injury displayed by the eye reflects the unique anatomical, physiological, and biochemical features of this essential organ.
The most common symptoms of ocular adverse effects are discomfort of the eyelids, conjunctiva, and lacrimal gland organs, including dry eye [5, 6], epiphora [13], nasolacrimal duct obstruction [29], lacrimal duct obstruction [29, 30, 31], erosive conjunctivitis and punctate stenosis [8], corneal epithelial lesions [27, 32], chalazion [26], corneal disorder [27] and limbal stem cell deficiency [7]. According to previous reports, the nasolacrimal duct obstruction may be related to the interstitial fibrosis of lacrimal duct mucosa [29]. It was presumable that conjunctiva, keratopathy, and dry eye might be associated with the cytotoxicity of the drug. The drug inhibits cell proliferation in the cornea and eye surface, leading to stem cell dysfunction, and then triggers inflammation of the conjunctiva and epithelial defects [32]. Docetaxel-induced meibomian duct inflammation and blockage are the likely causes of chalazion [26]. However, the mechanisms of the ocular surface and accessory organ injury still need to be investigated.
| Ocular disorders | Main symptoms | Incidence rate |
| Dry eyes/ocular surface discomfort | Foreign body sensation Grit or sand in eyes | 51.7% [6] |
| Limbal stem cell deficiency | Eye pain; Blurred vision in both eyes [7] | NA |
| Conjunctivitis | Eye redness and lacrimation [8] | NA |
| Glaucoma | Vision loss [9, 24] | NA |
| Optic Neuropathy | Transient obscurations of vision lasting seconds [10] | NA |
| Vision reduce, narrowed field of vision, Color vision is impaired [11] | ||
| Loss of visual acuity with blind spots [25] | ||
| Epiphora | Not described | 12.5% [13] |
| 7.9% [22] | ||
| Macular edema | Visually impaired/Sudden, catastrophic vision loss | 0.5% [14] |
| Chalazion | Eye irritation and dryness [26] | NA |
| Corneal disorder | Blurred vision [27] | NA |
| Cataract | Not described [28] | NA |
| NA, not applicable. | ||
Intraocular tissue (lens and vitreous) damage caused by taxane is rare. Kuwata et al. [28] evaluated the ocular toxicity of different doses of paclitaxel to Sprague-Dawley (SD) rats of different ages. The results showed that the amount of 4 or 8 mg/kg of paclitaxel injected into 0-day-old rats resulted in apoptotic epithelial cells of the lens, and the lens fibers were degenerative on the 7th day. These phenomena suggested the possible occurrence of cataracts. However, no ocular lesions were observed being injured at the dose of 2 mg/kg of paclitaxel injection. Also, no ocular lesions were observed injured in rats injected intraperitoneally with 4 mg/kg paclitaxel at 14 days of age and 8 mg/kg paclitaxel at 12 to 18 weeks of age. These results suggested that the ocular toxicity of paclitaxel may be dose-related and age-related, and attention should be paid to the ocular toxicity of paclitaxel in the early developmental stage. But the mechanisms are still underwater.
The first symptom of macular edema caused by taxane is impaired visual acuity. Kaya et al. [14] analyzed 202 patients who received taxane-based therapy to treat various cancer. Taxane-related cystoid macular edema (CME) was detected only in one patient on paclitaxel. Generally, the proportion of taxane-related maculopathy was 0.5% (1/202) of all patients in the taxane group. In a case report and literature review shared by Alvarez⁃Fernandez et al. [33], a 73-year-old patient with metastatic breast cancer developed macular edema after being treated with paclitaxel, which resolved after drug withdrawal. In addition, 57 cases of paclitaxel-related macular edema reported in 52 literatures were analyzed. The median time of occurrence of macular edema after taking the drug among these patients was 4.25 months. Among these patients, 92.86% were diagnosed with macular edema and bilateral vision loss in the initial examination, and most of their symptoms reversed after paclitaxel withdrawal. So far, we have retrieved 18 cases of taxane-induced macular edema that spontaneously resolved after stopping chemotherapy [33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48].
However, this particular macular edema, unlike another common macular edema such as diabetic macular edema (DME), macular edema secondary to retinal vein occlusion or uveitis, presents unique clinical characteristics. In taxane-induced macular edema, optical coherence tomography (OCT) showed intraretinal cystoid spaces and dome-shaped configuration of the fovea in both eyes. However, there was no evidence of any fluorescein leakage shown on fluorescein angiography (FA) in both eyes. In addition, most taxane-induced macular edema will resolve spontaneously after drug withdrawal. The pathogenesis of this type of macular edema is still unclear. So far, its specific mechanisms are not well studied, except for several speculations in several case reports [4, 15, 34, 36, 38, 41, 49, 50, 51, 52, 53, 54, 55, 56]. Among them, the primary underlining mechanism is mainly focused on the toxicity of Müller cells and etinal pigment epithelial (RPE) cells in the retina. Paclitaxel may lead to cellular dysfunction and accumulation of intracellular fluid [4], resulting in the disturbing of the nerve sensory osmotic gradient maintained by Müller cells in the retina. Also, paclitaxel would cause minimal impairment to the blood-retina barrier (BRB), where RPE cells play an essential role in maintaining and participating in phagocytosis and visual circulation metabolism [15].
Meanwhile, through experiments on animals, Kuwata et al. [28] found that ocular toxicity of different doses of paclitaxel to newborn SD rats of different ages also showed that retinal dysplasia occurred after intraperitoneal injection of 4 or 8 mg/kg paclitaxel in 0-day-old SD rats. This suggested that the ocular side effects of paclitaxel were related to the duration and concentration of the drug.
Hofstra et al. [25] mentioned a case where an ovarian cancer patient
experienced a sudden vision loss with scotomas after receiving six chemotherapy
cycles of intraperitoneal paclitaxel infusion. Physical examination by an
ophthalmologist revealed bilateral left-sided hemianopsia because of a prolonged
vascular spasm resulting in a neurological defect of the optical cortex. When
paclitaxel was infused, the patient experienced scotomata, small luminous dotsor
“flies” in the visual fields of both eyes, lasting a few minutes to several
hours. The speculation is that paclitaxel may damage the optic nerve [57].
Sediman et al. [10] estimated that among 25 breast cancer patients who
received paclitaxel at 250–275 mg/m
It should be noted that optic neuropathy may also be induced by taxane-induced
glaucoma, although further study was needed. A young female patient with
metastatic breast cancer who underwent chemotherapy of docetaxel in combination
with both low-dose and high-pulse corticosteroid treatment. She developed
symptomatic vision loss during the course of treatment and had high intraocular
pressure, optic nerve cupping, and bilateral visual-field loss and was then
diagnosed as open-angle glaucoma [9]. As docetaxel was discontinued with local
treatment by a
Paclitaxel inhibited cell viability and proliferation in a dose-dependent
manner. Paclitaxel may inhibit the proliferation, differentiation, and apoptosis
of human adipose stem cells (hASC) regulated by the tumor necrosis
factor-
Microtubules are involved in many vital functions like signal transduction and intracellular transport. Paclitaxel can bind to the interior of microtubules, thus making them more stable, interfering with the regular cycling of microtubule depolymerization and repolymerization. In dividing cells such as cancer cells, this disrupts normal spindle dynamics, interferes with mitosis, and ultimately leads to cell death [66]. Meanwhile, paclitaxel can interfere with normal cellular functions, including cell division and microtubule-dependent intra-cellular and axonal transport. Axons are enriched in microtubules where increased microtubule stability may cause neurotoxicity. Microtubule-dependent transport is critical for axonal function and health, both in the central and peripheral nervous systems; as such, disruption of axonal transport is implicated in various central nervous system disorders and peripheral neuropathies [67, 68]. Thus, neurotoxicity could be induced primarily by affecting local effects in the distal axon.
It has been reported that paclitaxel treatment increased the
production of reactive oxygen species (ROS) in BCBL-1 cells, resulting in
cytotoxicity. As is known to all, caspase-2 was activated in paclitaxel-treated
BCBL-1 cells, and caspase-2 can induce mitochondrial dysfunction directly or
indirectly [61]. In addition, paclitaxel can also induce mitochondrial swelling
and transport dysfunction in peripheral nerves [69, 70]. It impairs not only the
axonal transport of mitochondria but also their morphology and function. The
paclitaxel-induced alterations in the permeability transition pore opening in
mitochondria then cause changes in calcium flow. Calcium flow changes induce
deficiencies in the mitochondrial respiratory chain, which leads to ATP deficits.
Furthermore, the response to oxidative stress is impaired by paclitaxel
treatment, further increasing the ATP deficits [71]. Mitochondrial damage and ROS
are closely related since mitochondrial damage causes the production of ROS,
i.e., H
Paclitaxel causes degeneration of both central and peripheral axon branches of dorsal root ganglia in mice, but this is dose-dependent. At lower doses (lower than the maximum tolerated dose), dark inclusions, as well as clear vacuolations in the cytoplasm of neurons and satellite cells, can be observed. At relatively high doses (at the maximum tolerated dose), a proportion of the cytoplasms of dorsal root ganglion (DRG) neurons appeared much darker than that of normal neurons, and a few degenerating neuronal cells were reported [66, 70]. The enhanced stabilization of the microtubule is likely to be the effect of paclitaxel that initiates the downstream neurotoxic, while the degeneration of DRG neurons caused by taxol is mainly due to paclitaxel changes in tubulin modifications [69].
Paclitaxel induces the classical development of stem cell-like cells. It
promotes the regeneration of neurons, which may lead to rapid and sustainable
functional improvement in Spinal cord injury (SCI) rats. When it comes to the
differentiation of neural stem cells, researchers found that paclitaxel reduces
scar formation after SCI and enhances intrinsic axonal regeneration.
Paclitaxel-triggered neuronal differentiation occurs through the
Wnt/
Paclitaxel chemotherapy may cause chest pain, and bradycardia, resulting in
myocardial ischemia. Symptoms improved when chemotherapy was stopped [78]. In
addition, it may reduce ischemic ventricular arrhythmias (Vas) by stabilizing
microtubules to protect connexin 43 (Cx43), and the dynamic movement of Cx43 is
regulated by microtubules and their related proteins. Dysfunction of Cx43 in
ischemic myocardium is associated with Vas [79]. Also, cerebral ischemia is
another side effect that should be paid enough attention to [80]. On the other
hand, paclitaxel enhances the myocardial protective effect of myocardial ischemia
preconditioning through stabilizing microtubules of cardiomyocytes and promoting
HIF-1
Intercellular communication occurs through specific membrane pores called gap junctions. Taxol significantly affects gap-junction-mediated intercellular communication (GJIC) by impairing the transfer of Cx43 to the plasma membrane by stabilizing microtubules. Reversibility of taxol-induced reduction of GJIC as well as microtubular and Cx43 arrangements were demonstrated after taxol depletion from the nutrient medium. However, taxol does not immediately reduce GJIC, depleting connexin reserves and thus affecting GJIC [82]. Müller cells and RPE are critical to sustaining the BRB. As we mentioned before, taxane may disturb the functions of these cells. Thus the cellular junctions may be influenced as well.
Paclitaxel has an anti-fibrosis effect. In the thioacetamide (TAA)-induced rat
liver fibrosis model, paclitaxel prevented TAA-induced liver fibrosis in rats,
possibly by inhibiting the expression of transforming growth factor-
For patients under taxane administration, it is recommended to have a baseline examination of ophthalmology before treatment, including visual acuity, intraocular pressure, fundus, color vision, visual field examination, and etc. and to closely observe whether there are new ocular symptoms during the treatment process. Patients with ocular symptoms in the course of medication should be referred to have ocular consultancy and have a complete ocular checkup in time. For the ocular surface diseases caused by paclitaxel drugs, such as dry eye, conjunctivitis, keratitis, and etc. Usually, it is recommended to use artificial tears, non-steroidal anti-inflammatory drugs, antibiotic eye ointment and other symptomatic treatments. Lacrimal duct obstruction or narrow, tear overflow can be used for lacrimal duct irrigation or lacrimal duct exploration [32]. The adverse side effects on the ocular surface caused by paclitaxel are reversible and can be recovered after withdrawal and symptomatic treatment, within several days to 5 months [32].
In conclusion, the adverse ocular side effects of taxane drugs mainly appear on the ocular surface, ocular appendage and intraocular tissues, with various clinical manifestations such as dry eye, conjunctivitis, keratitis, macular edema, retinal injury, optic nerve injury, and etc (see in Fig. 1). The underlining mechanisms remain to be further investigated. The macular edema induced by taxane drugs may be related to the dysfunction of RPE cells and Müller cells. Other adverse reactions may be associated with the cytotoxicity of paclitaxel. If baseline eye exams were available, it would be helpful for clinicians to identify and diagnose whether eye disorders are caused by taxane chemotherapy. The concern on baseline exams has been raised in several cases [23, 85]. We also highlight those ocular symptoms should be closely observed during taxane chemotherapy. Chemotherapy regimens should be adjusted according to comprehensive evaluation, and symptomatic treatments should also be given to avoid irreversible damage.
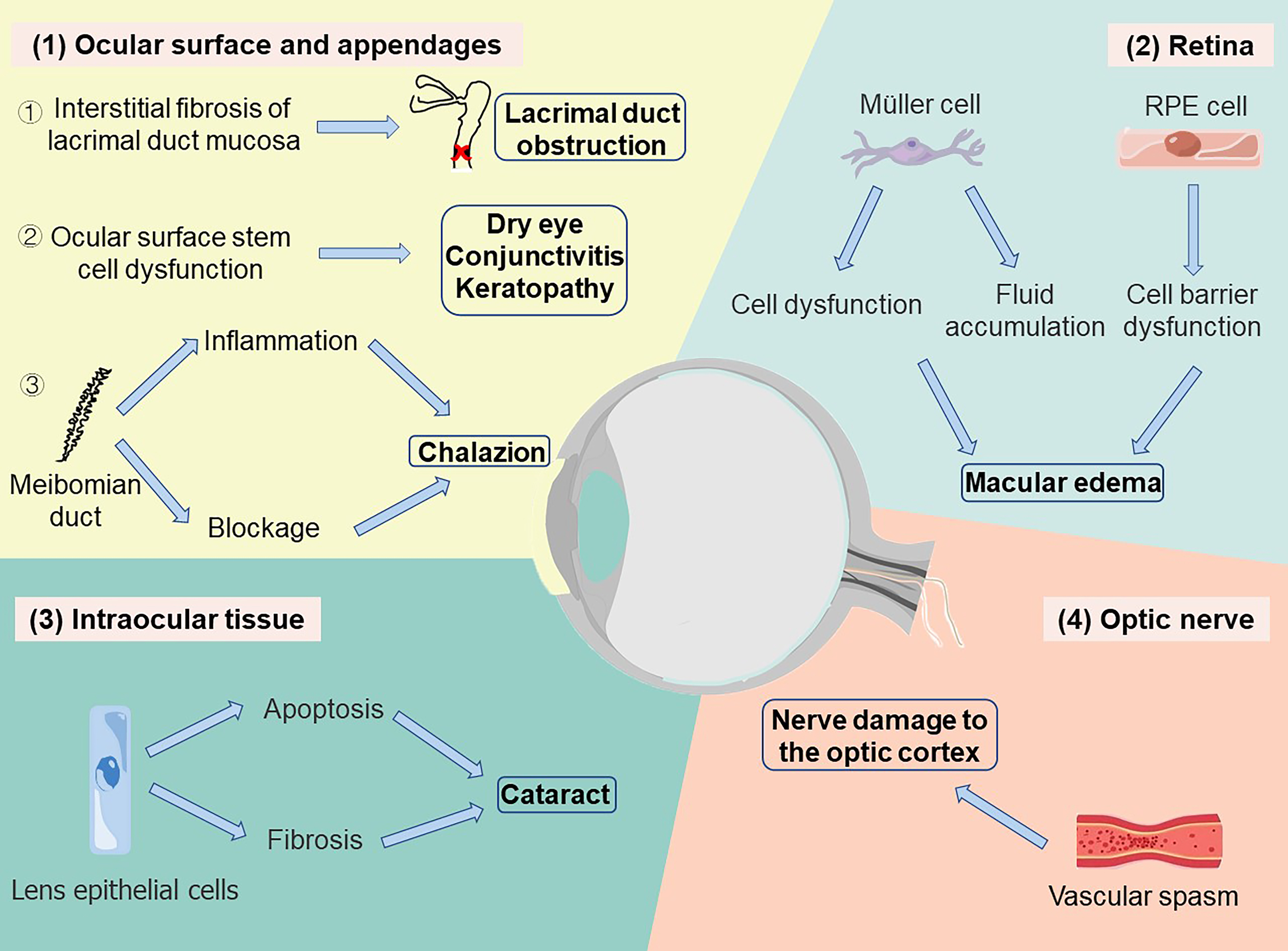 Fig. 1.
Fig. 1.Taxane-induced ocular diseases and their mechanisms. (1) Diseases in ocular surface: ① Taxane promotes interstitial fibrosis of lacrimal duct mucosa and leads to lacrimal duct obstruction. ② Taxane inhibits cell proliferation in the cornea and eye surface, causing the dysfunction of stem cells, which may result in dry eye, conjunctivitis, and keratopathy. ③ Taxane leads to chalazion by inducing the inflammation and blockage of the meibomian duct. (2) Diseases in retina: Taxane results in the dysfunction of Müller cells and the accumulation of intracellular fluid as well as damage of RPE barrier function which might cause further macular edema. (3) Diseases in intraocular tissue: Taxane induces the apoptosis of lens epithelial cells and the formation of lens fibers, leading to cataracts. (4) Diseases in optic nerve: Taxane injection may cause transient vascular spasm, resulting in neurological defects of the optical cortex.
YTY and ZYZ wrote the manuscript. LSW, and YS participate to assist this work. GRD and ZJC reviewed the manuscript. GRD provided thoughtful comments and suggestions during this review preparation. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Not applicable.
Thanks to all the peer reviewers for their opinions and suggestions.
This article is supported by the National Natural Science Foundation of China
(81970814, 81670863), Professional Promotion Program of Xijing Hospital
(XJZT19ML19), Key Research and Development Program of Shaanxi Province
(2021SF-335), Science and Technology Project of Xi
The authors declare no conflict of interest.

