-
- Academic Editor
-
-
-
†These authors contributed equally.
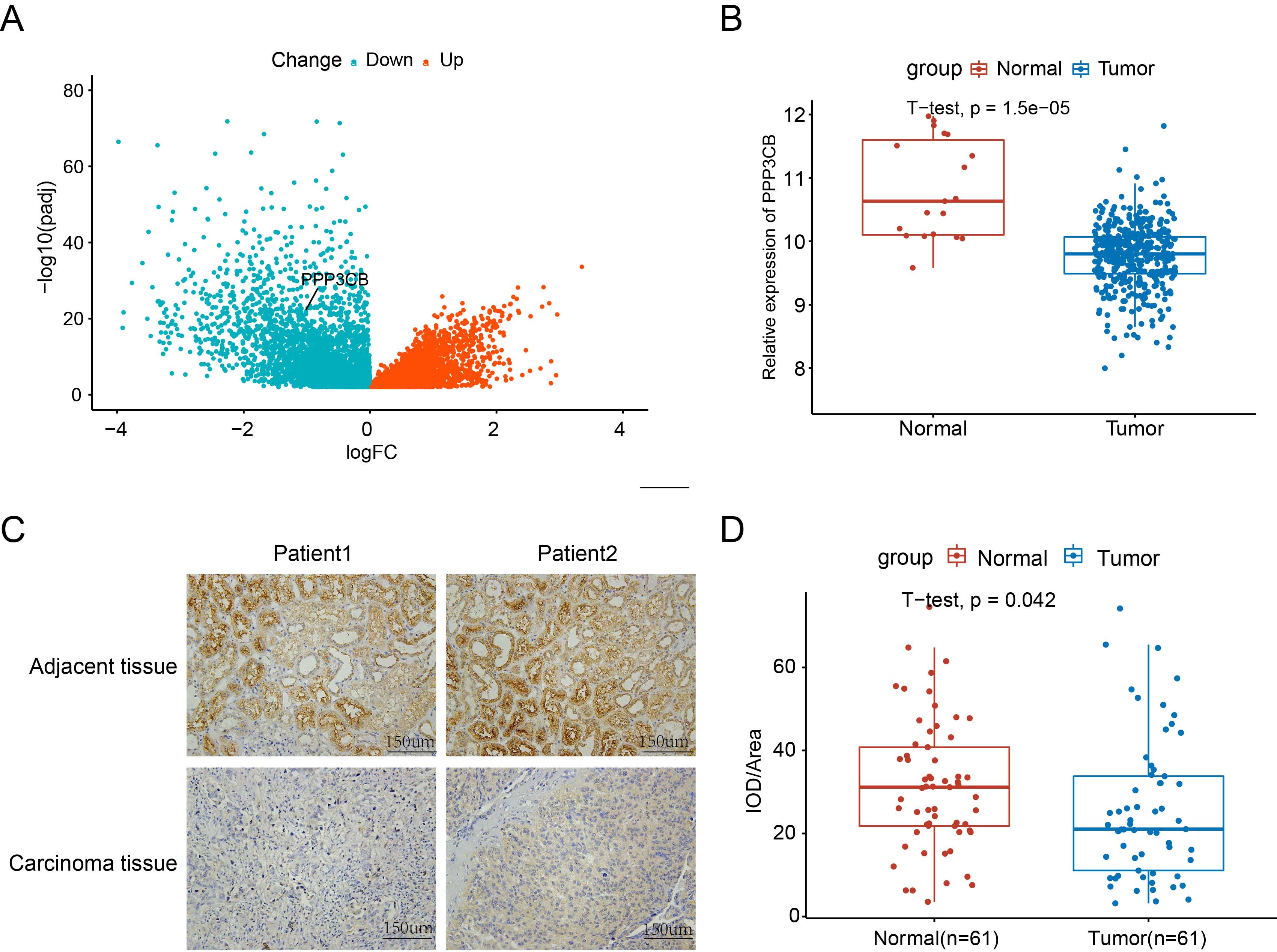
Background: Cancer treatment has recently shifted towards metabolic approaches aimed at enhancing therapeutic efficacy. Somewhat surprisingly, a known regulator of energy metabolism in normal tissues, PPP3CB, is down-regulated in bladder cancer. This suggests that PPP3CB could exert an inhibitory effect on bladder cancer through its role in energy metabolism. Methods: To explore the above hypothesis, we employed non-targeted metabolism screening in bladder cancer cells with knockdown of PPP3CB. Glucose uptake and lactate production were carefully measured using specialized assay kits for glucose/lactic acid content. Western blot analysis was also used to evaluate the expression levels of pyruvate dehydrogenase kinase 1 (PDHK1) and p-PDHA1 in cells with PPP3CB knockdown. To substantiate the findings, co-immunoprecipitation (co-IP) experiments were performed to validate the interaction between PPP3CB and PDHK1. Various in vitro assays were also performed, including clone formation assay and Cell Counting Kit-8 (CCK8) viability assays. The in vivo anti-tumor potential of PPP3CB in bladder cancer was also studied using a nude mouse tumorigenesis model. Results: Significant down-regulation of PPP3CB was observed in bladder tumors, and potent anti-tumor effects of PPP3CB were observed in vitro. Investigation of the underlying mechanism by which PPP3CB hampers glycolysis in bladder cancer cells revealed that it interacted with PDHK1 to inhibit its protein stabilization. PDHK1 thus appears to be a crucial mediator through which PPP3CB exerts its inhibitory effects on bladder cancer cells. Conclusions: In summary, PPP3CB exerts strong inhibitory influences on bladder cancer cell proliferation and glycolysis via its destabilization of PDHK1. These results highlight the potential of PPP3CB as a novel regulator of the Warburg effect. Interestingly, the downregulation of PPP3CB in bladder cancer cells increases the Warburg effect, thereby generating more lactic acid and reshaping the tumor microenvironment so as to promote tumor cell proliferation.