1 Henan Key Laboratory of Brain Targeted Bio-Nanomedicine, School of Life Sciences & School of Pharmacy, Henan University, 475004 Kaifeng, Henan, China
2 Henan-Macquarie University Joint Centre for Biomedical Innovation, School of Life Sciences, Henan University, 475004 Kaifeng, Henan, China
†These authors contributed equally.
Abstract
Adoptive chimeric antigen receptor (CAR) T cells designed to recognize specific
tumor antigens have shown promising results in cancer therapy. While CAR T cell
therapy has demonstrated notable clinical effectiveness for hematologic disease,
efforts to develop therapies for solid tumors, including glioblastoma (GBM), have
been hampered by heterogeneity, an immunosuppressive tumor microenvironment, and
difficulty in trafficking. Several specific tumor antigens, such as
IL13R
Keywords
- CAR T
- glioblastoma
- immunotherapy
- cancer therapy
- cell therapy
Glioblastoma (GBM) is a primary malignant tumor of the central nervous system (CNS) that is the most common and aggressive with World Health Organization (WHO) grade IV tumors [1, 2]. Standard therapies for GBM include microsurgical resection followed by adjuvant chemotherapy and radiotherapy. However, despite such aggressive therapy and intense research efforts, the overall survival (OS) rate has remained at 15–18 months [1, 2]. Therefore, new therapeutic approaches in GBM are urgently warranted [3].
Immunotherapy that uses natural defense mechanisms was first attempted as adoptive cell transfer using autologous lymphocytes in patients with hematological cancer [4] and now could revolutionize the treatment of even solid cancers. However, these approaches lack specificity for cancer cells, and to address this limitation, the exploration of genetically engineered T cell receptors (TCR) to identify specific target cancer-associated antigens ultimately generated chimeric antigen receptor (CAR) T cell therapies [5]. CAR T cell therapy has shown the potential to prolong patient survival and induce remission, even in cases where standard treatment has failed [6]. However, since the first CAR was reported in 1993, it has taken decades for CAR T cells targeting CD19 to be approved by the Food and Drug Administration (FDA) as a therapy for patients with relapsed or refractory B cell acute lymphoblastic leukemia, and investigators are continually seeking to extend the therapeutic effects of CAR T cells [7, 8]. Although recent clinical and ongoing studies have yielded some promising results, the progress of CAR T cell therapy for GBM remains challenging, with only suboptimal efficacy achieved to date [9].
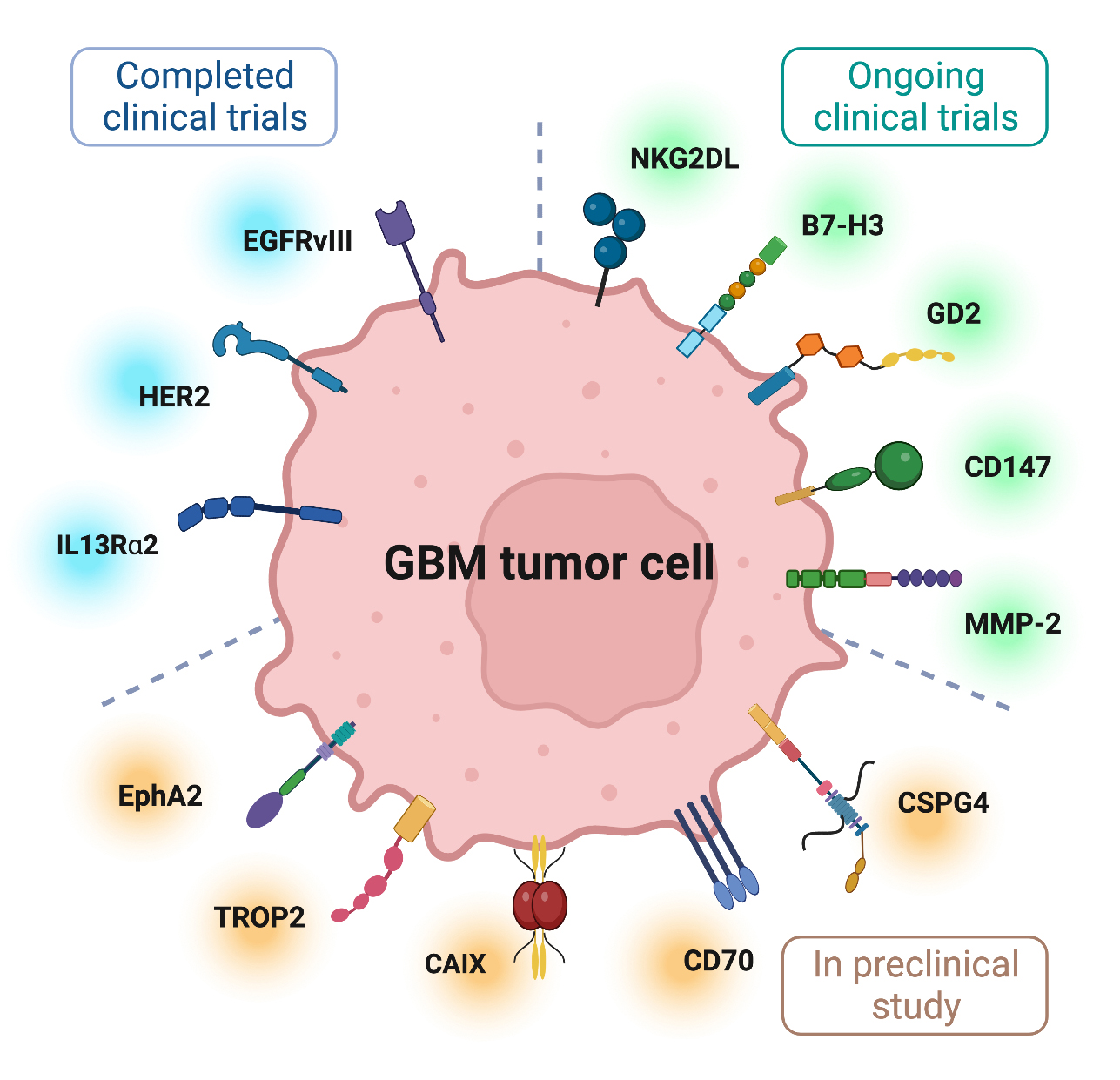
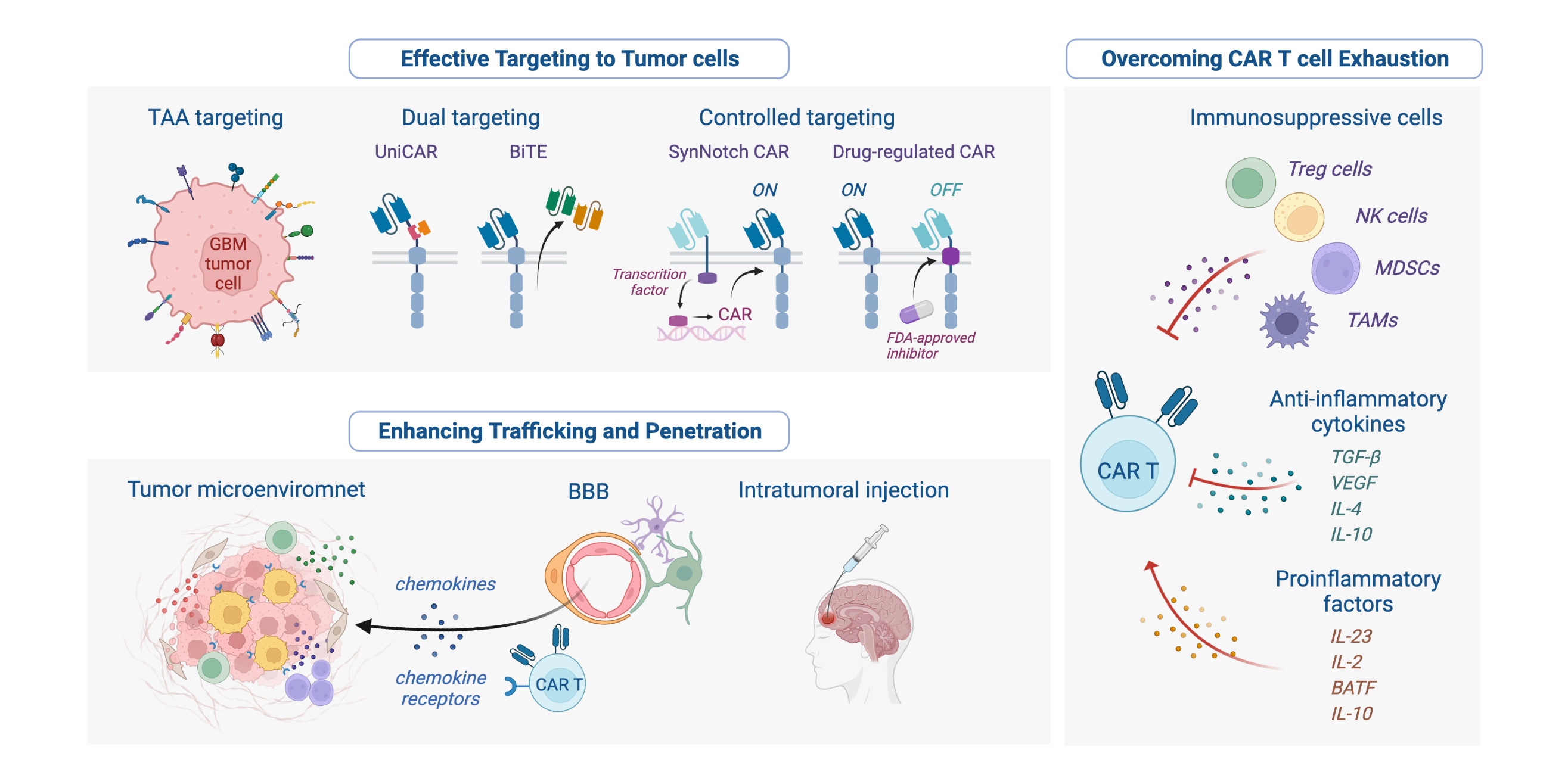
Solid tumors such as GBM display a high antigen heterogeneity compared to liquid tumors. This allows tumor cells to evade recognition by CAR T cells, typically encode specificity toward a single antigenic target, and thus cannot identify all cancer cells in a tumor. In addition, expanding T cell specificity to multiple antigens can increase the risk of on-target, off-tumor toxicity. Moreover, physical barriers, such as collagen-rich stroma surrounding tumor cells, can prevent T cell infiltration in solid tumors. Furthermore, the CAR T cells administered to patients with GBM encounter significant challenges in a highly immunosuppressive TME with molecular, cellular, and metabolic profiles that eventually create exhaustion and dysfunction of T cells. In GBM therapy, CAR T cells also need to penetrate the brain tissue protected by the blood-brain barrier (BBB). Early-phase clinical studies of CAR T cell therapy for patients with GBM have demonstrated safety but did not achieve sufficient antitumor activity. Despite this, some CAR T cells have shown tumor control, and complete responses have been reported [9]. In this review, we summarize the targets for completed and ongoing clinical trials in GBM therapy (Fig. 1) (Tables 1,2, Ref. [10, 11, 12, 13, 14]) and discuss the challenges and prospects of CAR T application (Fig. 2) to guide subsequent research for the treatment of GBM.
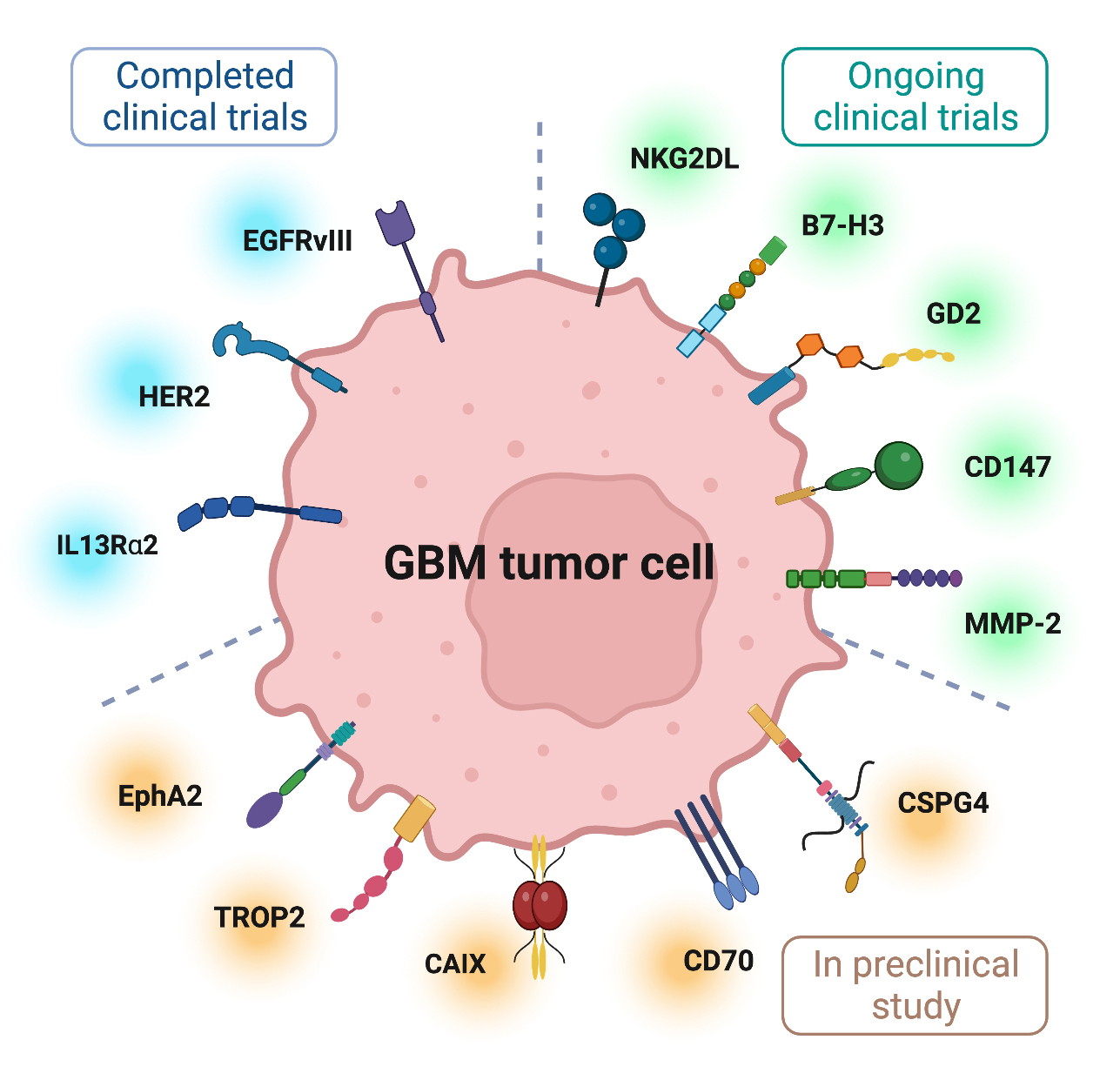 Fig. 1.
Fig. 1.Several targetable tumor-associated antigens for GBM CAR T cell therapy. The figure was created with BioRender.com.
 Fig. 2.
Fig. 2.Summary of challenges and solutions for CAR T cell-based therapeutic in GBM. The figure was created with BioRender.com.
| Molecular target | Clinical trial identifier and title | Study phase | CAR T cell dosage | Sponsor/site | Response (Median OS) | Ref. |
| IL13R |
NCT00730613 | I | - intravenous infusion | City of Hope Medical Center | 11 months | [10] |
| Cellular adoptive immunotherapy using genetically modified T-lymphocytes in treating patients with recurrent or refractory high-grade malignant glioma | - up to 10 |
National Cancer Institute (NCI) | ||||
| NCT01082926 | I | - intravenous infusion | City of Hope Medical Center | 19.7 month | [11] | |
| Phase I study of cellular immunotherapy for recurrent/refractory malignant glioma using intratumoral infusions of GRm13z40-2, an allogeneic CD8+ cytolitic T-cell line genetically modified to express the il 13-Zetakine and hytk and to be resistant to glucocorticoids, in combination with interleukin-2 | - 1 × 10 |
National Cancer Institute (NCI) | ||||
| EGFRvIII | NCT02209376 | I | - intravenous infusion | University of Pennsylvania | 8 months | [12] |
| Autologous T cells redirected to EGFRVIII-with a chimeric antigen receptor in patients with EGFRVIII + GBM | - 1.75 × 10 |
University of California, San Francisco | ||||
| NCT01454596 | I/II | - 6.3 × 10 |
National Cancer Institute (NCI) | 6.9 months | [13] | |
| CAR T Cell receptor immunotherapy targeting EGFRvIII for patients with malignant gliomas expressing EGFRvIII | ||||||
| HER2 | NCT01109095 | I | - 1 × 10 |
Baylor College of Medicine | 11.1 months (95% CI, 4.1–27.2 months) from the first T cell infusion | [14] |
| CMV-specific cytotoxic T lymphocytes expressing CAR targeting HER2 in patients with GBM | The Methodist Hospital Research Institute | 24.5 months (95% CI, 17.2–34.6 months) from diagnosis | ||||
| Center for Cell and Gene Therapy, Baylor College of Medicine |
| Molecular target | Clinical trial identifier and title | Study phase | CAR T cell dosage | Sponsor/site |
| NKG2DL | NCT04270461 | I | - intravenous administration or hepatic portal artery injection | Jiujiang University Affiliated Hospital KAEDI |
| NKG2D-based CAR T cells immunotherapy for patients with r/r NKG2DL+ solid tumors | - NKG2D-CAR T cells: 1–10 × 10 |
|||
| B7-H3 | NCT04077866 | I/II | - three intratumoral or intracerebroventricular injections of CAR T cells at two doses in between temozolomide cycles | Second Affiliated Hospital Zhejiang |
| B7-H3 CAR T for recurrent or refractory glioblastoma | Ningbo Yinzhou People’s Hospital | |||
| Huizhou Municipal Central Hospital | ||||
| BoYuan RunSheng Pharma Co., Ltd. | ||||
| NCT04385173 | I | - three intratumoral or Intracerebroventricular injections of B7-H3 CAR T with 1–2 weeks of intervals between cycles of temozolomide treatment | Second Affiliated Hospital, School of Medicine, Zhejiang University | |
| Pilot study of B7-H3 CAR T in treating patients with recurrent and refractory GBM | BoYuan RunSheng Pharma Co., Ltd. | |||
| GD2 | NCT04196413 | I | - rolling-6 dose escalation design test GD2-CAR T cells in subjects with H3K27M-mutant DIPG | Crystal Mackall, MD |
| GD2 CAR T cells in diffuse intrinsic pontine gliomas (DIPG) & spinal diffuse midline glioma (DMG) | California Institute for Regenerative Medicine (CIRM) | |||
| Cure Search National Institutes of Health (NIH) | ||||
| CD147 | NCT04045847 | Early I | - intracavity injection | Xijing Hospital |
| CD147-CAR T cells in patients with recurrent malignant glioma | - every 7 days for 3 weeks | |||
| MMP2 | NCT04214392 | I | - each treatment cycle begins with one or two CAR T cell infusions (one at each catheter site) and lasts for 1 week | City of Hope Medical Center, National Cancer Institute (NCI) |
| CAR T cells with a chlorotoxin tumor-targeting domain for the treatment of MMP2+ recurrent or progressive GBM |
GBM tumors exhibit high expression of interleukin 13 receptor alpha 2
(IL13R
IL13R
Epidermal growth factor receptor (EGFR) is a receptor tyrosine kinase (RTK) commonly amplified and/or mutated in about half of GBM patients. Among EGFR mutations, the most common is the activated EGFR variant III mutant (EGFRvIII), produced by in-frame deletion of exons 2–7 of the coding sequence and found in approximately 45% of GBM patients [25]. EGFRvIII has been an attractive target because of the assumed role in tumorigenesis and because the selective expression on tumor tissue has been well-characterized [26]. In addition, EGFRvIII expression is specific to GBM and is not found in healthy tissues, so EGFRvIII has been assessed for good targets. Although inhibitors targeting EGFRvIII have been used in GBM patients, they have not shown significant clinical effectiveness because of signaling bypass, whereby inhibition of the EGFRvIII pathway activates other RTKs [27]. Consequently, EGFRvIII could be a viable and appealing target for CAR T cell therapy in GBM.
The first clinical trial with EGFRvIII CAR T cells was reported in 2017 that used a single dose of EGFRvIII CAR T cells that was intravenously administered to 10 patients with recurrent GBM (NCT02209376) and which exhibited safety and a restricted antitumor response with downregulated expression of the targeted antigen [12]. In another clinical trial, third-generation EGFRvIII CAR T cells were administered after lymphatic regression chemotherapy and intravenous IL-2 injections, but they did not show clinical success and were well-tolerated without any apparent toxicity (NCT01454596) [13]. Additionally, EGFRvIII CAR T cell preparation using humanized scFv has been proven safe but failed to show clinical benefit [28].
Further analysis of patients receiving a single whole-body dose of the
second-generation EGFRvIII targeting CAR T cell formulation was conducted on
tumor tissues to assess the antitumor immune response and CAR T cell
effectiveness. These CAR T cells were shown to be present in some patient
tissues, and increased frequency of autologous non-CAR T cells and
immunosuppressive Tregs were also found in treated tumor tissues [12].
Immunohistochemical staining showed the increased expression of other soluble
immunosuppressive molecules such as programmed cell death ligand 1 (PD-L1),
indoleamine 2, 3-dioxygenase 1 (IDO1), and transforming growth factor-
Human epidermal growth factor receptor 2 (HER2), also known as ERBB2, is a member of the EGFR family that is overexpressed in approximately 15% of patients with GBM contributing to poor survival rate [33]. Aberrant HER2 signaling deregulates apoptosis and cell proliferation and increases the degree of aplasia in the GBM [34]. Although HER2 is overexpressed in numerous tumors, such as GBM, breast, and ovarian, it can be expressed in some healthy tissues, leading to safety issues.
The first reported use of HER2 CAR T cell therapy in metastatic colon cancer
resulted in respiratory distress within 15 min of administration and death five
days later [35]. In addition, analysis of serum samples after cell infusion
showed elevated levels of cytokines, including interferon-
The NK group 2-member D (NKG2D) receptor, a C-type lectin-like homodimeric
receptor, is expressed on various immune cells, including NK cells, CD8+ T cells,
In 2015, CAR T cells targeting NKG2D were first proposed but exhibited significant clinical toxicity in animals [41]. Since then, NKG2D CAR T cells have demonstrated practical anticancer effects in hepatocellular carcinoma, pancreatic, colorectal, and gastrointestinal tube adenocarcinomas [42, 43, 44, 45]. In addition, combined with radiotherapy, CAR T cells that express full-length murine NKG2D have been shown to considerably extend OS in orthotopic xenograft models that use murine GBM cells [46]. Moreover, human NKG2D CAR T cells eradicated human GBM and GBM-stem-like cells in vitro and a subcutaneous tumor model [39]. However, a phase 1 clinical trial for solid tumors, including GBM, aimed at evaluating the safety and clinical effects of NKG2D CAR T cells was withdrawn due to administrative reasons (NCT04270461).
B7-Homolog 3 protein (B7-H3, also known as CD276) is a member of the B7 family
of immunoregulatory proteins that bind to receptors on lymphocytes that regulate
the immune response [47]. B7-H3 is a related cell surface protein ligand that is
overexpressed in tumor tissues, with high expression in
The constructed B7-H3 CAR T cells have been examined for cytotoxic effects on GBM cells since 2019 [51, 55, 56]. In the enhanced B7-H3 CAR T cells, the internal stimulatory structural domains of CD28 or IL-7 receptors were fused to extracellular PD-1 to create PD-1 decoy receptors, which produced a more enduring anticancer activity [55]. In 2022, the results from a human trial of B7-H3 CAR T for multiple basal cell carcinoma demonstrated that intratumoral injection of these cells partially controlled tumor growth in patients with BCC with minor adverse events. However, the capability and safety of B7-H3 CAR T therapy require further investigation in a larger patient cohort [57]. In addition, an ongoing randomized, phase I/II study (NCT04077866) is currently evaluating the safety and performance of B7-H3 CAR T cells in patients with refractory or recurrent GBM between cycles of temozolomide [58]. Another ongoing phase I study (NCT04385173) is evaluating the safety and efficacy of B7-H3 CAR T in patients with GBM who have relapsed on temozolomide or have not responded to standard therapy.
Gangliosides (GD) are frequently expressed in normal tissue. However, disialoganglioside (GD2) is a specific type of GD that is mainly expressed in fetal tissue [59] and is also highly expressed in several cancer tissues, including melanoma, retinoblastoma, neuroblastoma, and GBM [60, 61]. In addition, GD2 has been identified as a cancer stem cell marker in breast cancer [62] and with the in vitro neurosphere formation capacity of GBM patient-derived cells [63].
In preclinical studies, CAR T cells targeting GD2 have demonstrated a solid ability to kill neuroblastoma cell lines in vitro as well as in subcutaneous xenografts [64] and patient-derived diffuse midline glioma orthotopic xenografts [65]. Moreover, genetically engineered CAR T cells with a truncated form of anti-GD2 (GD2 tCAR) exhibited enhanced specific targeting [60], and the bifunctional mesenchymal stem cells (MSCs), expressing GD2 tCAR and the proapoptotic agent tumor necrosis factor-related apoptosis-inducing ligand, showed potent antitumor activity against GD2-positive GBM cells. Furthermore, the preclinical efficacy of GD2-directed CAR T cells was recently demonstrated against H3K27M mutant GBM cells, providing a theoretical basis for the first human phase I clinical trial (NCT04196413) [66]. CAR T cells manufactured using a clinical retroviral vector and a GD2-specific CAR encoding an IL-15 transgene further improved tumor control and achieved a striking 50% bioluminescence imaging response rate to intracranial tumors [67].
Immunohistochemical analysis was used to evaluate the expression pattern of CD147 in GBM samples from 206 patients and healthy brain tissue samples from 36 individuals [68, 69]. CD147 expression was found to be higher in GBM samples than that in healthy tissues. Increased CD147 expression was related to a worse OS rate in patients with GBM. These findings show that CD147 is abundantly expressed in GBM [69]. CAR T cells and NK cells were transduced with a CD147-specific CAR, which detected the CD147 surface marker and targeted hepatocellular carcinoma (HCC) via a logic-gated (log) GPC3-synNotch mechanism. CD147-CAR did not induce significant targeted/tumor removal toxicity in a human CD147 transgenic mice model but did selectively kill double antigen (GPC3+CD147+)-positive HCC cells while failing to kill the single antigen (GPC3-CD147+)-positive HCC cells [70]. An early phase I clinical trial tested the safety, tolerability, and efficacy of CD147-specific CAR T cell therapy in patients with recurrent GBM (NCT04045847).
The primary chlorotoxin (CLTX) can interact with isoforms of matrix metalloproteinase-2 (MMP-2) that were identified on the surface of GBM cells and are significantly elevated in GBM and associated malignancies [71]. CLTX peptide has the ability to bind to GBM specifically, and when combined with CAR T cells, it results in a potent anti-GBM activity that can target tumors that do not express other GBM-associated antigens. Importantly, CLTX CAR T cells were observed to specifically target cells expressing MMP-2 without showing any off-target effector activity against healthy cells or after transfer to mice [72]. A phase I study was initiated to treat MMP-2-positive relapsed or progressing GBM with T cells carrying CLTX CARs (NCT04214392).
Identifying tumor-specific antigens is a crucial factor in the development of CAR T cell therapy however, it is also challenging due to the heterogeneity of tumors, lack of tumor-specific antigens, and expression of shared target candidates in important healthy tissue, which increases the risk of off-target tumor toxicity.
Tumor antigens are classified into tumor-associated antigens (TAA) and tumor-specific antigens (TSA). Both tumor cells and healthy tissue cells have a high expression level of TAAs, albeit at a low level. Only tumor cells express TSAs, which makes them a perfect target for antigens [73]. As the discovery and screening of TSAs are challenging, the most used recognition target of CAR is TAA, so the diverse expression of TAA in different kinds of tumor cells may affect the recognition of cancer cells by CAR T cells and reduce the effect of CAR T therapy. Numerous potential targets have therefore been identified and yielded encouraging results in the preclinical setting (Table 3, Ref. [74, 75, 76, 77, 78, 79, 80, 81, 82]). Antigens such as carbonic anhydrase IX (CAIX) [74, 75], CSPG4 [76, 77], or TRPO [78, 79] that are primarily investigated in non-CNS tumors are upregulated in GBM and have therefore been considered as potential targets. Expression of antigens as stem-like cell surface markers, including CD133 [83], CD70 [80, 84], and EphA2 [81, 82], is enhanced by alkylating chemotherapy or radiotherapy typically provided as first-line therapy in GBM, thus making these antigens promising in treating recurrent disease. Other targets that have been primarily selected because of their relatively high expression in GBM tissue include heparan sulfate proteoglycan glypican-2 (GPC2) [85, 86] and TWEAK receptor Fn14 [87].
| Molecular target | Characteristics | Research advances | Ref. |
| CAIX | - a surrogate marker for hypoxia | - in GBM mice model, direct intratumoral injection of anti-CAIX CAR T cells is effective with limiting side effects | [74, 75] |
| - particularly upregulated in GBM | |||
| - associated with a poor patient outcome and survival rate | |||
| CSPG4 | - related to cell proliferation and migration in vitro and in vivo | - to prevent the emergence of antigen-negative clones, it is worth exploring strategies that combine CSPG4-CAR T cells with CAR T cells targeting IL13 |
[76, 77] |
| - as a marker for GBM stem cells (GSCs) | |||
| TROP2 | - play a role in tumor growth, invasion, and metastasis of various solid tumors | - CD27-based TROP2 CAR T cells showed higher antitumor activity, upregulating IL-7R |
[78, 79] |
| - highly expressed in GBM, but low expressed in normal brain | |||
| CD70 | - type II transmembrane protein binding to CD27 | - CD70 CAR T cells effectively induced anti-tumor responses against CD70+ gliomas both in vitro and in vivo | [80] |
| - expressed on activated T cells and mature DCs | |||
| - overexpressed in primary and recurrent GBM, but not on tumor infiltrating T cells | - no toxicity in xenograft and syngeneic models | ||
| - associated with poor survival in GBM | |||
| EphA2 | - Eph family of RTKs | - the third-generation of EphA2 CAR T cells obviously improved survival rate in tumor bearing mice in vivo, showing better antitumor activity | [81, 82] |
| - associated with tumorigenesis, angiogenesis, invasion and metastasis | |||
| - highly expressed in GBM but only at low levels in normal brain tissue |
Several approaches for overcoming the heterogeneity in tumor antigens highlight
the complexity of developing effective CAR T cell therapies for GBM, thus
increasing tumor cell subpopulations while targeting a single antigen. One is a
simultaneous administration of each CAR T cell targeting a different GBM antigen.
The other is dual-targeting, using CAR T cells expressing two scFvs to simplify
the number of injections. However, clinical treatment with bispecific CARs or T
cells expressing two CARs, such as in CD19/CD22- and CD19/CD20-targeting
approaches, showed limited results in leukemia. Another method is engineering
CARs, whose antigen-binding structural domain consists of a ligand binding
various different antigens (called a universal CAR); the representative example
is the T1E peptide that attaches to the EGFR family of receptors for solid tumors
[88]. Furthermore, bispecific T cell engager (BiTE) expressed in T cells provides
an opportunity to target multiple antigens and redirect bystander T cells to
tumor cells. In addition to targeting various antigens, efforts to activate
bystander T cells to identify tumor cells are being pursued, including the
transgenic expression of cytokines (e.g., IL-18 or IL-36
As a switch-controlled CAR T, the synthetic notch (synNotch) CARs can induce the
expression of a second CAR upon activation by an antigen-specific synNotch [91].
SynNotch-GD2-B7H3 [92] and SynNotch-HER2 [93] were designed to express the kill
switch consistently. Recently, dual administration of synNotch CARs, (1)
EGFRvIII-SynNotch CAR and EphA2 or IL13R
CAR T cell therapy is a promising modality for cancer, but its effectiveness is often limited by poor infiltration of CAR T cells into tumors and the presence of a highly immunosuppressive tumor microenvironment (TME). The GBM TME consists of stromal cells, immune cells along with enzymes, growth factors, inflammatory cytokines, and pathogenic stimuli within the extracellular matrix (ECM), as well as highly proliferative malignant astrocytoma cells and likely cancer stem cells [97], all of which form discrete niches inside the tumor [98]. All these individual components of the TME can act as a physical barrier that limits the effectiveness of CAR T therapy. As a result, numerous studies have focused on developing strategies to improve the ability of infused CAR T cells to penetrate and traffic into the TME to enhance their antitumor activity. Overall, these approaches aim to overcome the challenges posed by the TME and improve the therapeutic potential of CAR T cell therapy.
In tumor evasion of immune responses, chemokine ligands, and receptors play different roles. However, low expression of chemokine receptors can reduce immune cell infiltration, contributing to tumor evasion of immune responses [99]. In recent preclinical studies, a strategy to improve the tumor-homing and antitumor efficacy of CAR T cells has been explored by overexpressing CCR2 (ligand; CCL2, CCL7, CCL8, CCL13, and CCL16) [100], CCR4 (ligand; CCL4, CCL3, CCL5, CCL17, and CCL22) [101], and CCR6 (ligand CCL20) [102, 103]. Thus, these approaches can attract CAR T cells to tumors with high expression of specific chemokines, leading to adequate tumor clearance. In addition, in parallel to the development of technologies to enable real-time imaging of CAR T cells in vivo allows for non-invasive monitoring of CAR T cell trafficking to tumor cells and facilitates the examination of novel interventions to enhance CAR T cell effects [104]. Moreover, combined treatment of oncolytic adenovirus and chemokine CXCL11 increased CAR T cell infiltration and reprogramed the immunosuppressive TME, thereby improving therapeutic efficacy [105]. These advances hold promise for improving the efficacy of CAR T cell therapy against solid tumors by enhancing their ability to penetrate the tumor microenvironment and target cancer cells.
In the case of GBM tumors, another physical barrier that hampers CAR T cell trafficking is the BBB. The BBB is a highly selective semipermeable boundary that separates circulating blood from the extracellular fluid in the brain and CNS. The BBB consists of endothelial cells of the capillary wall, astrocytes that wrap around the capillaries, and pericytes embedded in the basement membrane of the capillaries [105]. The BBB acts as a protective barrier to prevent the entry of potentially harmful substances, including immune cells, as well as CAR T cells. Therefore, strategies to enhance the ability of CAR T cells to cross the BBB and reach GBM cells are needed to improve the efficacy of CAR T cell therapy against GBM. CAR T cells can be transferred across the BBB in association with constitutively or inducible expressed cytokines, chemokines, or other molecules and can be actively utilized for CAR design. In addition, using in vitro BBB models, the iPSC-derived brain endothelial-like cell trans-well coculture model can be a valuable tool for deciphering the mechanism of CAR T-induced BBB destruction, concomitant toxicity, and effector functions of target cells after BBB [106].
Furthermore, another aspect of enhancing CAR T cell trafficking is the route of
CAR T infusion. Although most studies of CAR T cell therapy have used intravenous
injection, a more site-specific delivery method may be beneficial in the therapy
of solid tumors, including those of breast, pleura, and liver [107] as well as
brain [21, 66], by bypassing circumventing multiple barriers to CAR T cell
trafficking as well as restricting on-target, off-tumor toxicity. In HER2 CAR T
cell treatment for breast cancer brain metastasis, local intracranial
administration demonstrated in vivo antitumor potency using three administration
routes: intravenous, local intratumoral, and regional intraventricular [108].
Similarly, another study on the delivery route and therapeutic strength
demonstrated that local intracranial local delivery of IL13R
CAR T cell exhaustion is a phenomenon that arises from prolonged exposure to antigen stimulation and an immunosuppressive TME, including various suppressive immune cells such as Treg cells, NK cells, myeloid-derived suppressor cells (MDSCs), and tumor-associated macrophages (TAMs). Maintaining CAR T cell effector function, persistence, and overcoming exhaustion remains a major challenge in achieving clinical potency [110]. Exhausted T cells have a low proliferation rate in response to antigenic stimuli (e.g., the response to antigenic stimuli), progressive loss of effector functions (e.g., reduced cytokine production and weakened killing effects), expression of multiple inhibitory receptors (e.g., PD-1, Tim3, and LAG3). Furthermore, altered metabolism from oxidative phosphorylation to glycolysis is also a feature of T cell exhaustion [111]. The comparison between monocyte-derived T cells of the dysfunctional and functional remission groups revealed a significant difference in the proportion of CD8+ T cells expressing PD-1 and LAG3 (lymphocyte activation gene 3, CD223). The findings imply that the initial cell phenotype before infusion may strongly correlate with treatment failure and short-term [112]. It has been demonstrated that introducing a dominant-negative PD-1 receptor can enhance the functional persistence of CAR T and prevent exhaustion [113]. CAR T cells targeting human CAIX and engineered to secrete human anti-PD-L1 antibodies exhibited significantly enhanced activity, elevated cytokine production, and greater uptake by immune cells, leading to a substantial reduction in tumor growth of clear cell renal cell carcinoma [114].
Treg cells negatively affect suppressor cytotoxic T cells through multiple mechanisms, including the secretion of immunosuppressive cytokines, competitively consuming IL-2, and the inhibition of antigen-presenting cells and obstructing T cell activation by cytotoxic T lymphocyte antigen 4 (CTLA4) [115]. To overcome the immunosuppressive effects of Treg cells, one group generated a BiTE that induced the expression of CAR targeting EGFRvIII and allowed the conversion of Treg cells into cytotoxic T cells [116]. In the GBM model, combining GD2-targeted CAR T cells and NKG2D CAR NK cells, NKG2D CAR NK cells selectively allowed MDSCs and restored impaired CAR T cell activity [117]. In addition, targeting MDSC induced the release of proinflammatory cytokines and chemokines, resulting in tumor regression and increased survival, compared to CAR T cell therapy alone [117]. Moreover, the inhibition of colony-stimulating factor 1 receptor (CSF1R) to deplete TAM and targeted ablation of PD-1 in myeloid progenitor cells revealed more potent antitumor activity and tumor growth suppression [118]. TAMs, the most abundant immune-infiltrating cells in the TME, prevent T cell-mediated antitumor immunity by boosting the secretion and recruitment of cytokines and enzymes, such as arginase one or IDO1, and by increasing the recruitment of Treg cells [119, 120]. In animal models, the survival rate was notably increased by CAR targeting CD70, presented on the putative M1 macrophages and CD4+ T cells. Therefore, targeting the CD70/CD27 axis could be an effective poly therapeutic strategy for treating both the GBM and the immune TEM [84].
Another potential approach is redirecting or circumventing the endogenous
response to suppressive cytokines and inhibitory signaling pathways.
Immunosuppressive cytokines, such as TGF-
Engineering CAR T to secrete proinflammatory soluble factors can modify the TME,
leading to an antitumor response. CAR T engineered to overexpress the
IL-12
CAR T cell holds a great promising therapeutic strategy for GBM, however, there are still several challenges that need to be considered. One of the major challenges is selecting a target antigen that can selectively eliminate cancer cells while sparing healthy brain tissue. Another challenge is the immune escape mechanisms applied to cancer cells, which can limit the effectiveness of CAR T cells. This includes downregulating the target antigen, inhibiting T cell function, and recruitment of immunosuppressive cells. Furthermore, optimizing CAR T cell delivery to the brain is another significant hurdle to overcome. Several approaches, such as local delivery, disruption of the BBB, and modification of CAR T cells, are being explored to improve CAR T cell delivery to the brain. In addition, developing combinatorial strategies for enhancing the CAR T cells’ efficacy and stimulating the innate immune system is essential. Combining CAR T cells with checkpoint inhibitors, vaccines, and other immunomodulatory agents is an approach that can enhance the activity of CAR T cells and solve immune escape mechanisms.
In the latest research, UniVec-CAR is a new approach that utilizes a single
lentiviral vector that expresses accessory molecules to induce the production of
an immunostimulatory cytokine, an antibody that ameliorated cytokine release
syndrome, and regulatory T cell transcription factors [131]. Meanwhile, several
recently published T cell immunization approaches can be actively utilized in CAR
T research [9]. For example, after cisplatin chemotherapy, the production of
CCL20 and IL-1
Additionally, beyond the conventional CAR T cells, CAR natural killer (CAR NK) and CAR macrophage (CAR M) are potential alternative approaches to CAR T cell therapy for GBM treatment, as they address some of their limitations [134]. Unlike CAR T cells, CAR NK cells do not require prior sensitization to tumor antigens and can identify and eliminate cancer cells without requiring major histocompatibility complex (MHC) matching. Furthermore, they have a low risk of causing graft versus host disease (GvHD) and CRS. A novel cytotoxic CAR NK cells from cord blood by transducing them with an anti-CD19 vector engineered to produce IL-15 for increased efficacy [135]. CAR M therapy involves genetically modifying macrophages to express a CAR on their surface, allowing them to target and kill cancer cells. Similar to CAR NK cells, they do not require MHC matching and are less likely to cause GvHD and CRS. The first published CAR M, which designed antigen-specific phagocytosis and activation of T cells, led to decreased tumor burden in mouse models and enhanced antitumor response in humanized mice, along with induction of a proinflammatory tumor microenvironment [136]. Although CAR NK and CAR M therapies offer several advantages over CAR T cell therapy, they are still in the early stages of development and clinical testing, and their effectiveness in treating GBM and overcoming the limitations of CAR T cell therapy is yet to be determined, and further research is needed.
ALPPL2, alkaline phosphatase placental-like 2; AP-1, activator protein 1; B7-H3,
B7-Homolog 3 protein; BATF, basic leucine zipper ATF-like transcription factor;
BBB, blood-brain barrier; BiTE, bispecific T cell engager; CAIX, carbonic
anhydrase IX; CAR, chimeric antigen receptor; CDK4/6, cyclin-dependent kinases 4
and 6; CLTX, chlorotoxin; synNotch, synthetic notch; CNS, central nervous system;
CSF1R, colony stimulating factor 1 receptor; CTLA4, cytotoxic T lymphocyte
antigen 4; ECM, extracellular matrix; EGFR, epidermal growth factor receptor;
EGFRvIII, EGFR variant III; GBM, glioblastoma; GPC2, heparan sulfate proteoglycan
glypican-2; HER2, human epidermal growth factor receptor 2; HCC, hepatocellular
carcinoma; IDO1, indoleamine 2, 3-dioxygenase 1; IFN
SZ: Investigation, Writing – original draft, Writing – review & editing. HS: Investigation, Writing – original draft, Writing – review & editing. SIC: Conceptualization, Investigation, Visualization, Writing – original draft, Writing – review & editing. JY: Conceptualization, Supervision, Funding acquisition, Writing – review & editing. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
Not applicable.
This research was funded by the National Natural Science Foundation of China, grant number 82173228.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.