- Academic Editors
Background: Amongst the specific plaque pathogen Aggregatibacter
actinomycetemcomitans (Aa) ATCC 43718 serotype b is one of the
highly virulent bacteria that causes periodontitis. Probiotic therapy is a
treatment in which the lactic acid bacteria in are utilized to impede the
colonization and growth of the pathogenic bacteria to prevent the further
formation of dental plaque. Objective: The present research aimed to evaluate
inhibiting effect of purified bacteria from various commercially available yogurt product containing
bacteria named (Lactobacillus casei strain Shirota;
Lactobacillus bulgaricus and Streptococcus thermophilus;
Lactobacillus reuteri Prodentis) on the growth of Aa. Methods:
The research made use of the diffusion method by fixing Aa on BHIB (brain heart
infusion broth) medium, incubated at 37 °C and 24 hours later
planted on MHA (Mueller-Hinton agar) media. Aa were divided into four
subgroups each with a paper disk; group 1 consists of untreated bacteria (i.e.,
control group), group 2 with purified bacteria from Yakult 0.5
Periodontitis is an oral disease mainly affecting supporting tissue (gingiva, periodontal ligament and alveolar bone) around the tooth with high frequency of occurrence, whereas the percentage of such cases in Indonesia goes as high as 74.1% [1, 2, 3, 4]. Aggregatibacter actinomycetemcomitans, Tannerella forsythia, and Porphyromonas gingivalis were identified as specific periodontal pathogens in periodontal disease. A. actinomycetemcomitans strongly associated with the aggressive forms of the disease [5]. The major cause of periodontitis is the existence of pathogenic bacteria which accumulate and later colonize in the dental plaque [6]. Attempts in controlling the amount of plaque around the teeth is a main stay of treatment of periodontal disease [7].
Various attempts are made to break the chain of bacterial adherence in plaque matrix. Probiotics are live microorganisms that are able to provide advantageous impacts on the health of their hosts when consumed in adequate quantities [8, 9, 10]. Many advantages can be found in probiotics, including helping the immune response, increasing resistance to pathogenic bacteria, reducing harmful bacteria, and maintaining the balance of healthy microbes in the body. Several studies have shown that probiotics can impede the formation of plaque which is a predisposing factor for the diseases in the oral cavity such as; caries, halitosis and periodontal disease [11, 12, 13]. Bacteria in probiotics helps to impede the adhesion and invasion of pathogenic bacteria [14, 15].
This research is aimed at observing the benefits of probiotics for the oral cavity, mainly in the treatment of periodontal disease. Lactobacillus casei strain Shirota bacteria play a beneficial role in reducing gingival inflammation and improving periodontal health due to the fact that this probiotic bacteria can actually cut down the number of A. actinomycetemcomitans bacteria in dental biofilm [14, 16].
Lactobacillus bulgaricus and Streptococcus thermophilus will produce organic acids, e.g., lactic acid, acetic acid, formic acid, hydrogen peroxide, diacetyl and bacteriocin which all have antibacterial properties during the process of fermentation [17, 18]. L. bulgaricus and S. thermophilus exhibits beneficial effects on periodontal tissue by their impeding effect on the growth of the A. actinomycetemcomitans bacteria [19]. The probiotic Lactobacillus reuteri Prodentis has the role of reducing gingival inflammation as well as decreasing the gingival bleeding and healing periodontal tissues after scaling and root planing [20, 21]. L. reuteri produce reuterin which is an antimicrobial compound. Reuterin has been shown to be bioactive against bacteria, viruses, and fungi [22].
The investigating bacteria were divided into three experimental groups to observe and later to conclude their impeding strength against A. actinomycetemcomitans. Based on previous researches, probiotic L. casei strain Shirota, L. bulgaricus and S. thermophilus; L. reuteri Prodentis were capable of inhibiting the plaque bacteria causing periodontitis [14, 23]. In this study researchers aimed to observe the antibacterial efficacy of commercially available probiotics in the market by inhibiting the growth of the A. actinomycetemcomitans that is one of the pathogens in the etiology of periodontitis. The null hypothesis to be tested is that the probiotics will not have inhibitory effect on the growth of Aggregatibacter actinomycetemcomitans (Aa).
The minimum sample amount proper for further analysis was determined with Federer formula, in which t stood for the groups in the research treatment, and r stood for the number of replications in each group of petri dishes used in the research.
(t–1) × (r–1)
(4–1) × (r–1)
3 × (r–1)
3r – 3
3r
r
Thus, based on the calculation above, the least number of the required samples were 6 [24].
The samples in the research were divided into 4 disk diffusion procedural groups. These groups consisted of, group 1 containing the pathogenic bacteria A. actinomycetemcomitans ATCC 43718 serotype b untreated with any probiotics (i.e., control group); group 2 was that which treated with L. casei strain Shirota bacteria; group 3 was that which was treated with L. bulgaricus and S. thermophilus bacteria; group 4 was that which was treated with L. reuteri Prodentis bacteria.
Reference strain of A. actinomycetemcomitans ATCC 43718 serotype b. The
bacteria were replaced in a reaction tube which has been provided with BHIB
(brain heart infusion broth). The outcome of the culture was then placed in an
anaerobic jar and incubated at 37 °C for 24 hours in anaerobic
environment. Later the opacity was observed in order to be balanced with the
standard of 0.5 McFarland (1.5
The following is the method of probiotic purification.
Product A contained the L. casei strain Shirota in the Yakult drink
manufactured by Perseroan Terbatas (PT), (Jakarta Selatan, Indonesia). Yakult Indonesia Persada. This probiotic
was placed in a reactive tube which had been given the BHIB media. The reactive
tube was then placed in an anaerobic jar incubated at 37 °C for 24
hours, which further incubated for 24 hours in anaerobic environment could be
seen in Fig. 1. The resulted opacity was then observed with the 0.5 McFarland
(1.5
 Fig. 1.
Fig. 1.Probiotic was placed in a reactive tube then placed in an anaerobic jar and incubated at 37 °C for 24 hours, which further incubated for 24 hours in anaerobic environment.
Product B contained the L. bulgaricus and S.
thermophilus obtained from Cimory Yogurt Drink produced by PT. Cisarua Mountain
Dairy Tbk. This probiotic was placed in a reactive tube which had been given the
BHIB media. The reactive tube was then placed in an anaerobic jar and incubated
at 37 °C for 24 hours, which further incubated for 24 hours in anaerobic
environment could be seen in Fig. 1. The resulted opacity was then observed with
the 0.5 McFarland (1.5
Product C contained the L. reuteri Prodentis contained within
BioGaia Prodentis lozenges produced by BioGaia. This probiotic was placed in a
reactive tube which had been given the BHIB media. The reactive tube was then
placed in an anaerobic jar and incubated at 37 °C for 24 hours, which
further incubated for 24 hours in anaerobic environment could be seen in Fig. 1.
The resulted opacity was then observed with the 0.5 McFarland (1.5
A. actinomycetemcomitans ATCC 43718 serotype b bacterial colonies were
taken from BHIB media with sterile cotton swabs. The bacteria were then planted
on MHA using 3
 Fig. 2.
Fig. 2.
A. actinomycetemcomitans ATCC 43718 serotype b were
then planted on MHA using 3
 Fig. 3.
Fig. 3.Paper disk was placed on MHA.
The diameter measurement of the inhibition zone in each testing group was conducted with a Vernier caliper in millimeters could be seen in Fig. 4. The inhibition zone is the transparent zone surrounding the tested probiotics. The measurement was performed from the inner circle of the paper disk to the outer periphery of the disk [14].
 Fig. 4.
Fig. 4.The diameter measurement of the inhibition zone in each testing group was conducted with a Vernier caliper in millimeters.
The data analysis of the inhibition zone of each tested group was done according
to the statistical analysis test based on Shapiro-Wilk test, Levene’s test, One
Way Anova with the assistance of SPSS software (version 21, IBM Software, Armonk,
NY, USA). Results were considered statistically significant at p
Inhibition Zone Diameter of 3 Types of Probiotics Against A.
actinomycetemcomitans stp. b: Bacteria growth after conducting the research, the
inhibition data obtained among product A with L. casei strain Shirota,
product B containing L. bulgaricus and S. thermophilus, as well
as product C containing L. reuteri Prodentis, the researchers concluded
that the probiotics worked against the growth of A.
actinomycetemcomitans bacteria. The average value of inhibition zone diameter in
product C was 19.60
| Replication | Inhibition Zone (Diameter, mm) | |||
|---|---|---|---|---|
| Product C | Product B | Product A | Control (-) | |
| 1 | 19.60 | 16.80 | 13.20 | 0.00 |
| 2 | 19.80 | 17.30 | 13.15 | 0.00 |
| 3 | 19.60 | 16.70 | 13.30 | 0.00 |
| 4 | 18.93 | 15.88 | 11.95 | 0.00 |
| 5 | 19.38 | 15.90 | 12.90 | 0.00 |
| 6 | 20.25 | 17.05 | 11.70 | 0.00 |
| Average | 19.60 |
16.60 |
12.70 |
0.00 |
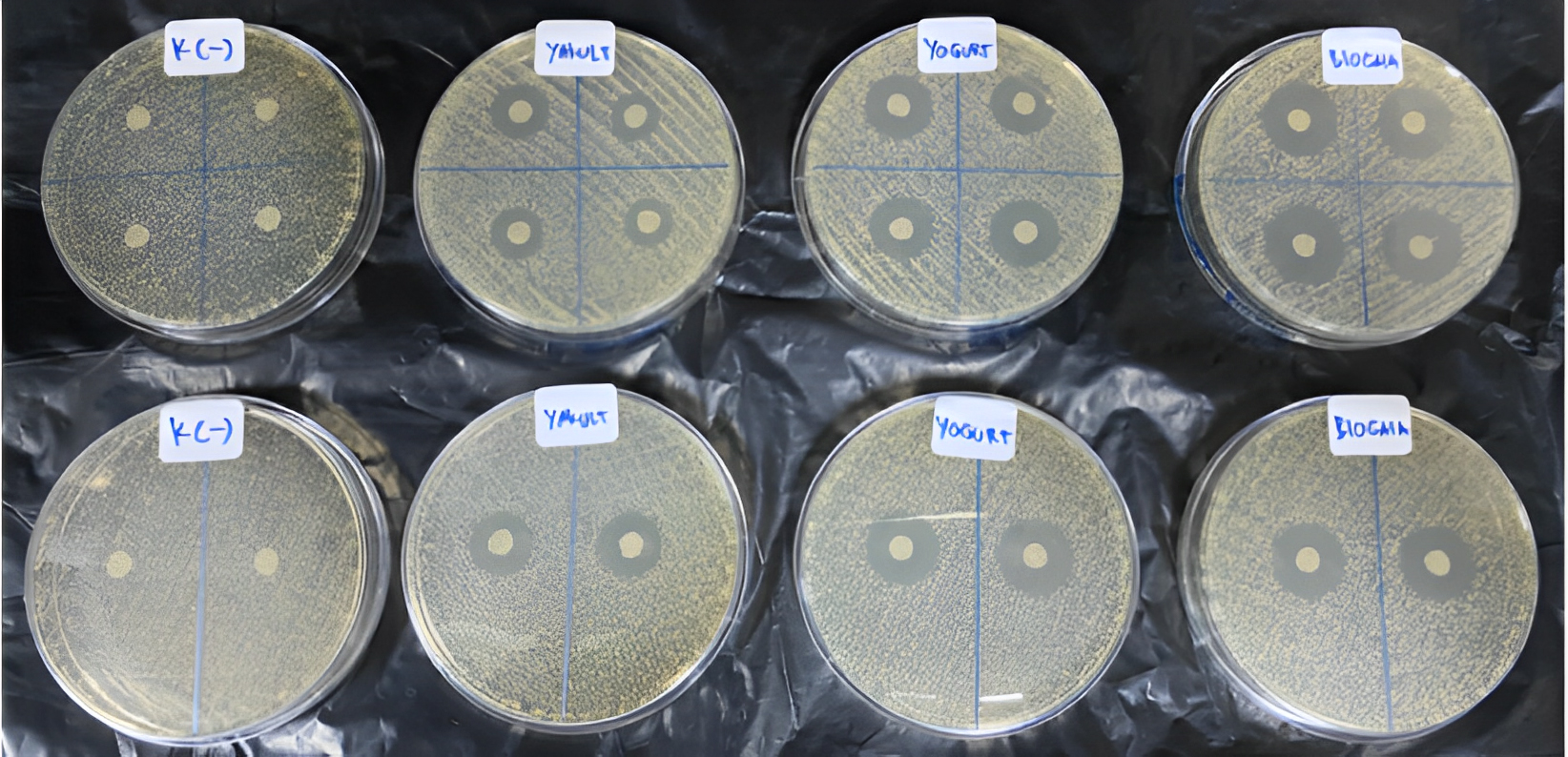 Fig. 5.
Fig. 5.The inhibition zone diameter dissimilarities in each tested group.
The prerequisite to the ANOVA test was conducted with Shapiro-Wilk test to
determine the normality of data distribution, which yeilded the significance
(Sig.) of
| Probiotics | Shapiro-Wilk test | |||
|---|---|---|---|---|
| Statistic | df | Sig. | ||
| Inhibition Zone | Produc C | 0.974 | 6 | 0.917 |
| Product B | 0.885 | 6 | 0.292 | |
| Product A | 0.814 | 6 | 0.078 | |
The homogeneity test was performed with Levene’s test. In turn the significance of (Sig.) 0.917, 0.292 and 0.078 were obtained for product C, B and A, meaning that the variant data were homogenous. The results of inhibiting zone diameter homogeneity of each treated group was in accordance with Levene’s test with the significance of (Sig.) 0.260.
ANOVA test was used to find out the difference between mean inhibition zone of three treatment groups. The result of the test revealed a significant difference (Sig.) 0.000, suggested that the mean inhibition zone of three treatment groups was significantly different.
In the post hoc test (Tukey HSD), the mean value of the group treatment which had been given product C was 2.98833 higher than that of in product B and 6.89333 higher than that the treatment given product A. The results of post hoc test (Tukey HSD) can be seen in Table 3.
| 95% Confidence interval | ||||||
|---|---|---|---|---|---|---|
| Probiotic | Mean Difference | Std. Error | Sig. | Lower Bound | Upper Bound | |
| Product C | Product B | 2.98833 | 0.33748 | 0.000 | 2.1117 | 3.8649 |
| Product A | 6.89333 | 0.33748 | 0.000 | 6.0167 | 7.7699 | |
This study was intended to determine the effects and the differences in the
probiotic inhibition, namely L. casei strain Shirota in product
A (i.e., Yakult), L. bulgaricus and S. thermophilus in product
B (i.e., Cimory Yogurt Drink), and L. reuteri Prodentis in product C
(i.e., BioGaia Prodentis) in reducing the growth of bacteria named A.
actinomycetemcomitans. In this study the statistical results obtained by ANOVA
test were further supported the finding of the influence of probiotic in product
A, product B, and product C compared to the control group. In addition, there was
a notable difference in the inhibition zone diameter among the three probiotics.
The anti-bacterial effect possessed by the aforementioned probiotics was capable
of, indeed, inhibiting the growth of A. actinomycetemcomitans. This was
influenced by the mechanism of the action of probiotic bacteria. Probiotic
bacteria are lactic acid bacteria with strong inhibitor effect against
gram-negative bacteria, and they also work as an antimicrobial agent to impede
the activity of pathogenic bacteria. Additionally, the decrease of the
intracellular pH of the organic acids contained within the probiotic bacteria can
cause cell death of pathogenic bacteria. Probiotic bacteria produce antimicrobial
peptides, including bacteriocins [25, 26]. Bacteriocins are formed by
gram-positive bacteria, which kills pathogenic bacteria by destroying target
cells and inhibiting cell wall synthesis [27, 28]. The diameters of the inhibition
zone on the MHA media indicate whether the A. actinomycetemcomitans was
resistant towards the probiotics. In this study, the inhibition zone diameter was
formed in the treatment groups which means that the A.
actinomycetemcomitans bacteria were not resistant to probiotic bacteria. All
three probiotics in product A, product B, and product C can slow down the growth
of the bacteria. However, there was a significant difference in the inhibition
effect among the three probiotics. The results of this analysis were different
from the hypothesis due to the size of the inhibition zone formed not only by the
strains of probiotic bacteria, but also by the concentration of antimicrobial
compounds or substances, types of microbes, the number of microbes, pH,
temperature and contact time. In this study there were differences in the types
and numbers of microbes in the three probiotics. Therefore, the results of
statistical analysis show an important difference in the aforementioned zone of
inhibition [14]. Of the three treatment groups given probiotics, probiotic in
product C had the largest inhibition zone diameter compared to product B and
product A. This was because product C contains L. reuteri Prodentis
bacteria. According to Jaffar et al. [29] L.reuteri as
potential candidates, are effective in the treatment of oral diseases by
repressing the development of periodontal pathogens. They serve as a good
alternative due to the benefits that these organisms have to counteract
pathogenesis by periodontal pathogens. As a matter of fact their ability to bring
about an immunomodulatory response by an increase in cytokine production, an
antiviral response against vesicular stomatitis by way of an interaction with
macrophages, and also the induction of nitric oxide synthesis might give a strong
effect against pathogens which tend to be virulent toward immune cells, as
reported for A. actinomycetemcomitans previously. These bacteria include
the Lactobacillus heterofermentative type, which later causes the
glucose fermentation process, which in turn not only produces lactic acid, but
also manufactures other components in equal amounts such as acetic acid, carbon
dioxide (CO
The probiotics in product B and product A contain homofermentative lactic acid bacteria. They in turn perform the glucose fermentation process which only produces lactic acid as the main component. However, the group treated with probiotic in product B had a larger diameter of the inhibition zone than the group treated byproduct A because the product B contained two lactic acid bacteria, namely L. bulgaricus and S. thermophilus. L. bulgaricus bacteria that produces amino acids and peptides as a source of nitrogen to form bacterial cells, so that more bacterial cells are produced. While S. thermophilus bacteria, apart from producing lactic acid, also produce formic acid. Albeit it is solely produced in small amount, it has a strong antimicrobial effect. Lactic acid and a little formic acid that are formed capable of inhibiting the growth of pathogenic bacteria because the presence of non-dissociating acid molecules that are able to penetrate cell walls and disrupt the metabolic process of pathogenic bacteria, furthermore a decrease in pH below the optimum pH for the growth of pathogenic bacteria [37]. The group treated with probiotic in product A had the smallest inhibition zone diameter compared to the group treated with probiotic in product C and product B. Although product B and product A both contain homofermentative types of lactic acid bacteria, product A only contains one lactic acid bacteria, that was L. casei strain Shirota. Thereof, the inhibition force of probiotic in product A was the smallest among the other two group treatments. Nevertheless, probiotic in product A could still inhibit pathogenic bacteria because L. casei strain Shirota contained was capable of lowering the local pH, inhibiting the growth of pathogenic bacteria because it produces antibacterial substances, that is bacteriocin [38, 39].
The results of the current study revealed that all the purified bacteria from three probiotic treatment groups have growth inhibiting property on the periodontopathic bacteria A. actinomycetemcomitans ATCC 43718 serotype b starin. However, amongt the three treatment groups, L. reuteri Prodentis showed the highest inhibiting effect on the A. actinomycetemcomitans ATCC 43718 serotype b starin. Futher clinincal studies are required to support the findings of the current in-vitro study.
The data supporting the finding is available with the corresponding author on the personal request to author.
CPS, MS, and DFR designed the research study. MS, NU, SKS, and DFR performed the research. NU, SKS, IKEW, and SSA provided help and advice on data collection. SSA, CPS, IKEW, and PD analyzed the data. CPS, MS, NU, SKS, and SSA wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
This study received a Certificate of Ethical Eligibility from the Faculty of Dentistry, Universitas Airlangga Indonesia. Ethical clearance for the research was obtained from the Health Research Ethical Clearance Commission (No. 295/HRECC.FODM/VI/2020). All the experiments were conducted according to the Declaration of Helsinki.
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University, Abha, Saudi Arabia for funding this work through the Large Research Group Project under grant number (RGP-2/234/1443).
This study was funded by Universitas Airlangga, grant number (RGP-2/234/1443).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
