- Academic Editor
†These authors contributed equally.
Background: Alcohol abuse leads to alcoholic liver disease (ALD), for
which no effective treatment is yet known. Gentiana Scabra Bge is a
traditional Chinese medicine; its extract has a significant liver protection
effect, but its effects on the mechanism of improving alcohol-induced toxicity
remain unclear. Therefore, this study used cell and mouse models to investigate
how Gentiana Scabra Bge extract (GSE) might affect the
TLT4/NF-
Alcoholic liver disease (ALD) refers to liver diseases caused by long-term or heavy drinking. ALD is the leading cause of liver cirrhosis and liver-related death worldwide, accounting for 4% of global mortality [1, 2, 3]. Alcoholic fatty liver often manifests in the initial stage when it develops into alcoholic hepatitis (ASH), liver fibrosis and cirrhosis, and finally, hepatocellular carcinoma [4, 5]. Therefore, controlling ALD at an early stage, such as ALD be controlled before the occurrence of ASH [6], may be of great significance for preventing the development of ALD.
The potential mechanisms of acute alcoholic liver injury relate to oxidative
stress, steatosis, endotoxin, immune disorders, and inflammation. However, recent
research has focused on the inflammatory pathway of ALD. Increasing evidence
shows that ASH is caused by the activation pathway of nuclear factor
Gentiana Scabra Bge is a Gentianaceae medicinal plant with dried roots and rhizomes. It has the traditional medicinal effects of clearing away heat and dampness and purging liver and gallbladder fire. The chemical components of Gentiana Scabra extract (GSE) are mostly iridoid glycosides, such as gentiopicroside, Swertiamarin, and login, which have anti-inflammatory, liver-protecting, and hypolipidemic effects. It has been reported that it can protect the damaged liver by improving mitochondrial dysfunction, reducing P2X7 receptor dependence, resisting oxidative stress, and inhibiting inflammation [14, 15, 16, 17]. However, although GSE is a commonly used medicine that was put into research earlier and widely used in clinics, the academic literature on the pharmacodynamics of its liver-protecting effective substances primarily focuses on the liver-protecting effect of its monomer compound, gentiopicroside. In addition, few studies examine the effect and mechanism of GSE active components in treating ALD. The main active components have not been systematically screened, and the related mechanism remains unclear.
Studying the function and mechanism of active components of traditional Chinese medicine is thus a practical, novel approach to the modern development of ethnic medicine. In this study, the active components of traditional Chinese medicine are used to intervene and prevent non-alcoholic fatty liver disease (NAFLD), providing research ideas for ALD drug development. In addition, GSE is reportedly safe for humans: no apparent side effects have been observed after several months of continuous administration [18]. Therefore, Gentiana’s anti-inflammatory effect may have a broad application prospect in ALD treatment.
Based on previous research, we aim to obtain the active anti-inflammatory
components of Gentiana by extraction and separation; based on the in
vitro and in vivo models of acute alcoholic liver injury, we detect the
protein expression levels of lipid synthesis genes and critical targets of the
NF-
BI fetal bovine serum was obtained from Israel Biological Industries (catalog
No. 2120140, Beit Haemek, Israel); DMEM culture medium was from Gibco BRL (catalog
No. 8121342, Grand Island, NY, USA); trypsin and penicillin-streptomycin double antibodies were sourced from HyClone,
USA (catalog Nos. J210033 and J200012, Logan, UT, USA). MycoLumi™
Mycoplasma Detection Kit (# C0298M, Biyuntian Biotechnology Co., LTD, Shanghai,
China). Aspartate aminotransferase (AST) (catalog No. MM-44115M1), alanine
aminotransferase (ALT) (catalog No. MM-44625M1), leukocyte interleukin
1
Using 3.0 kg of Radix Gentianae, we added water and decocted twice (the ratio of water to medicinal materials was 10:1 and 8:1, respectively), and each time was 1.5 h. The two filtrates were combined and concentrated into a solution with a mass concentration of 1.2 g/mL (based on the crude drug). We diluted the above solution with water into a sample solution with a mass concentration of 0.1 g/mL, added it to D101 macroporous resin column, eluted and purified it with 30% ethanol, and collected eluent. The eluent was distilled and concentrated under reduced pressure at 60 °C and evaporated to dryness to obtain GSE (the yield of GSE was 7%, and the gentiopicroside content was 54.6 mg/g).
Mouse Mononuclear Macrophages Cells (RAW264.7) were purchased from the Institute
of Biochemistry and Cell Biology, Chinese Academy of Sciences, and retained by
the laboratory for preservation. The study was carried out in accordance with the
guidelines of the Declaration of Helsinki and approved by the Ethics Committee of
Changchun University of Traditional Chinese Medicine (Protocol No. 2021220). All cell lines were verified by STR analysis and tested negative for mycoplasma. RAW264.7 cells were inoculated in DMEM medium (hereafter “DMEM”) containing
10% fetal bovine serum and 1% penicillin/streptomycin and cultured in a cell
incubator with 37 °C and 5% CO
Forty male C57BL/6J mice weighing 20
The CCK-8 assay was used for testing. First, the range of GSE toxicity to cells
was investigated. RAW264.7 cells in the logarithmic growth phase were inoculated
into 96-well plates at 1
RAW264.7 cells in the logarithmic growth phase were inoculated, grouped,
modeled, and administered according to the above-described method and cultured
for 24 h. The culture medium was collected and centrifuged at 3000 RPM for 15
min, and the supernatant was taken. Following the last gavage, blood was
collected from the orbit of the mice, left undisturbed for 30 min, and
centrifuged at 3000 RPM for 30 min to separate the serum. The microplate
method and ELISA kit instructions directed measurements of AST, ALT,
IL-1
The examination was performed using the hematoxylin and eosin (H&E) staining. The liver tissues of mice were cut at the same site of the left hepatic lobe and fixed in 4% paraformaldehyde for 24 h. Routine dehydration, paraffin embedding, histological sectioning, H&E staining, and pathological changes in the liver tissues were observed under a light microscope. Mouse primer sequences used for RT-q-PCR experiments in Table 1.
| Primer name | Primer sequence |
| F-5 | |
| R-5 | |
| TLR4 | F-5 |
| R-5 | |
| NF-κB | F-5 |
| R-5 |
TLR4, toll-like receptor 4; NF-
Real-time quantitative PCR (RT-qPCR) was used for detection. Total RNA was
obtained from cell samples and mouse liver tissues using an RNA extraction kit,
reverse-transcribed into cDNA according to the transcription kit method, and
amplified by PCR. PCR reaction conditions were pre-denaturation at 95 °C
for 30 s for 1 cycle, denaturation at 95 °C for 3 s, and annealing at 60
°C for 30 s for 40 cycles. According to the relative quantification
method,
Detection was performed by Western blotting. Total protein was extracted from
100 mg of liver tissue from RIPA lysate at 1:9, which was lysed thoroughly on ice
for 30 min and centrifuged at 12,000 r/min for 15 min at 4 °C. The
supernatant was then assayed for protein concentration by BCA. After boiling and
denaturing, the protein was subjected to sodium dodecyl sulfate-polyacrylamide
gel electrophoresis; the membrane was transferred and blocked with 5% skimmed
milk powder for 1.5 h. TLR4, I
Statistical analyses were performed with SPSS 22.0 software (IBM SPSS
statistics, Chicago, IL, USA). The results were expressed as
The effect of different GSE concentrations on the viability of RAW264.7 cells
was determined, with the results shown in Fig. 1A. Except for the 4 mg/mL group,
the cell survival rate of each treatment group was more than 90%, indicating
that the cell viability was unaffected and there was no significant toxicity in
the concentration range below 2 mg/mL. We also determined GSE’s effect on the
viability of RAW264.7 cells exposed to LPS, which Fig. 1B shows. Compared with
the control group, the viability of RAW264.7 cells in the model group was
significantly reduced (p
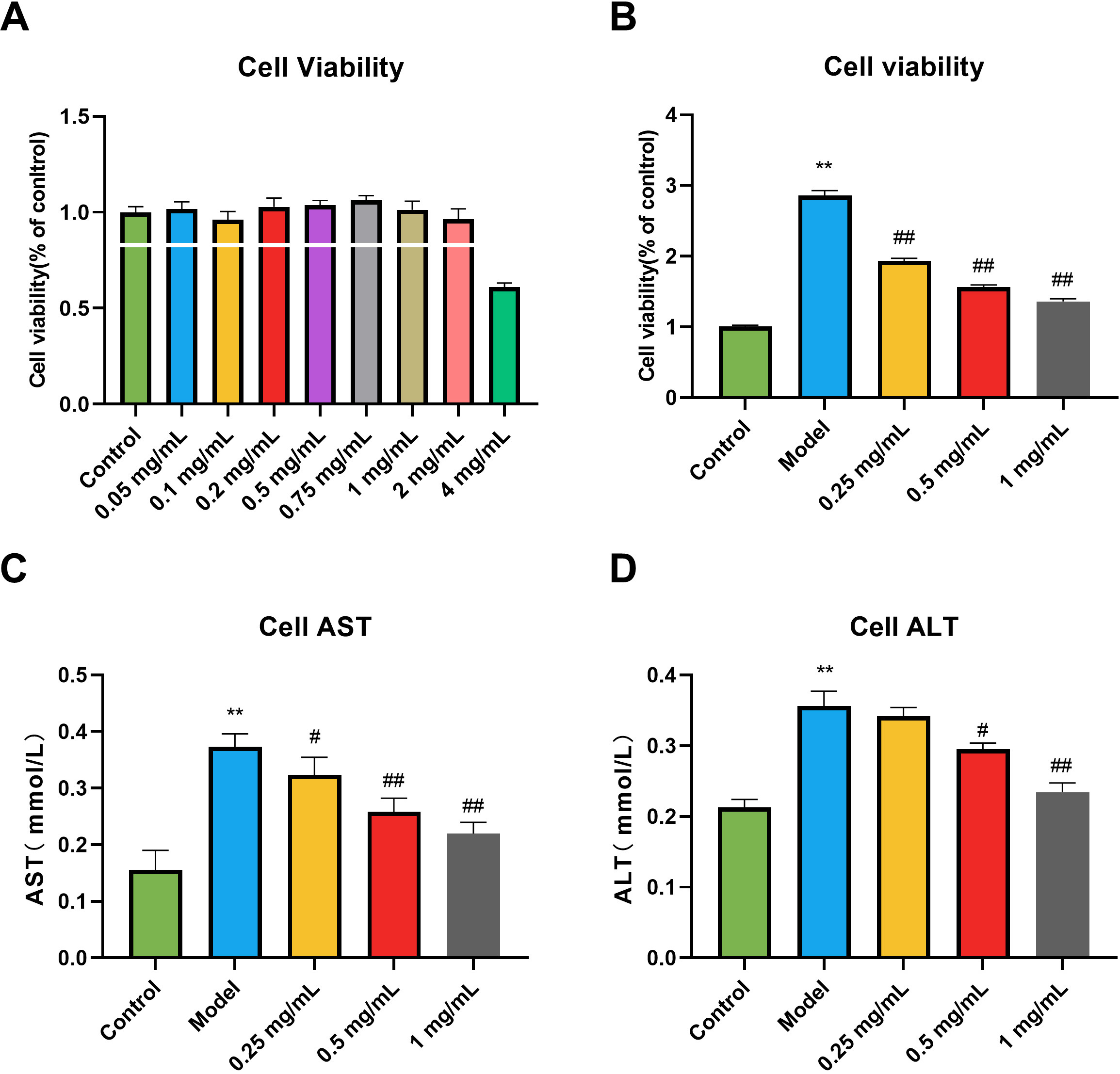 Fig. 1.
Fig. 1.Effects of Gentiana Scabra Bge Extract (GSE) on the cell
viability and liver function of RAW264.7 cells induced by lipopolysaccharide
(LPS). (A) The range of GSE toxicity. (B) Activity screening of GSE. (C) Alanine
aminotransferase (AST) in cell supernatant. (D) Aspartate aminotransferase (ALT)
in cell supernatant. All data are presented as the means
We investigated the effect on liver function in RAW264.7 cells exposed to LPS.
As shown in the results (Fig. 1C,D), compared with the control group, the
contents of alanine aminotransferase (ALT) and aspartate aminotransferase (AST)
in the cells of the model group were significantly increased (p
As shown in the results (Fig. 2), compared with the control group, the contents
of interleukin-1 (IL-1
 Fig. 2.
Fig. 2.Effects of GSE on the levels of inflammatory factors of RAW264.7
cells induced by LPS. (A) IL-1
As shown in the results (Fig. 3), compared with the control group, the TLR4 and
NF-
 Fig. 3.
Fig. 3.Effects of GSE on the levels of TLR4, NF-
Observation of ALD mice from appearance and morphology revealed that mice in the
control group had a smooth coat color and animated activity levels. Compared with
the control group, the mice in the model group had slow movement, unkempt hair,
poor gloss, and murmuring in the lungs. The treatment group’s mice were observed
with slightly smoother coats, higher activity levels, and improved mental status
compared to the model group. Based solely on an isolated comparison of the mice’s
body weight (Fig. 4A), no significant difference in body weight was found between
the groups. However, over time, the body weight of mice in the model group tended
to be lower than that in the control group, and the body weight of mice in the
treatment group tended to increase. As the changes in the liver index (Fig. 4B)
show, compared with the control group, the liver index of mice in the model group
became larger, reflecting the liver swelling to a certain extent; compared with
the model group, the liver index of mice in the GSE treatment group and the
positive group was significantly reduced (p
 Fig. 4.
Fig. 4.Effect of GSE on general inspection and liver function in ALD
mice. (A) Body weight. (B) Liver index. (C) AST in serum. (D) ALT in serum. All
data are presented as the means
Changes in liver function parameters were investigated to determine the effects
of AST and ALT levels on liver function in the serum of mice with alcoholic liver
injury (Fig. 4C,D). Compared with the control group, the enzyme activities of ALT
and AST in the serum of mice in the model group were significantly increased
(p
The results of H&E staining (Fig. 5) showed that the hepatic lobule structure of the control group was intact, the hepatic cords were arranged regularly, and no deformation or necrosis occurred. In addition, the hepatic lobule structure of the alcohol model group was blurred and disorganized, and there was obvious cell nuclear pyknosis and inflammatory cell infiltration of different sizes around the central area. However, in both low and high-dose GSE treatment groups, alcohol-induced pathological changes were alleviated, hepatic cords were arranged neatly, and few inflammatory factors and fat vacuoles were observed. Among them, inflammatory cell infiltration was significantly reduced in the liver group of mice treated with a high dose, and the degree of repair was close to that in the positive group.
 Fig. 5.
Fig. 5.Pathological sections of liver tissues (H&E staining
ELISA measured serum inflammatory factor levels to observe the effect of GSE on
liver inflammation in mice with alcoholic liver injury (Fig. 6). Compared with
the control group, the levels of IL-1
 Fig. 6.
Fig. 6.Effect of GSE on IL-1
We detected the levels of TLR4 and NF-
 Fig. 7.
Fig. 7. Effect of GSE on TLR4 and NF-
We also investigated the levels of TLR4, I
 Fig. 8.
Fig. 8.Effect of GSE on the expression of TLR4, I
GSE acts in various biological functions, including hepatoprotective, cholagogic, and anti-inflammatory, and it has long been studied as therapeutic for chemical and drug-induced liver injuries [21, 22]. However, studies on the mechanism of regulating ALD are rare and remain to be clarified. Accordingly, this study established a macrophage inflammation model by LPS induction in mice to explore the mechanism of action of GSE in ameliorating alcohol-induced inflammatory response. Determination of cell viability revealed that GSE at concentrations below 4 mg/mL was non-significantly toxic, while concentrations of 0.25–1 mg/mL GSE had good anti-inflammatory and hepatoprotective activity. We also measured biological parameters, with results showing that liver function and inflammatory factor levels were abnormally increased in the cell supernatant of the LPS model group.
The liver is the primary organ responsible for metabolism and is subjected to more tissue damage than other organs due to the accumulation of LPS [23]. ALT and AST are common indicators to reflect whether the liver is damaged; ALT is the most abundant in hepatocytes in the body. When hepatocytes undergo inflammation or necrosis, ALT rapidly increases—a sensitive indicator of hepatocyte damage [24, 25]. AST is mainly found in the mitochondria of hepatocytes and increases when hepatocytes are destroyed or severely necrotic, while the rise in pro-inflammatory factors also indicates an increase in the inflammatory response [26]. Thus, our results indicate that the study successfully constructed a classical inflammatory cell model, and after GSE treatment, the abnormal increase in liver function and inflammatory factors was significantly reduced. Thus, GSE may be anti-inflammatory and hepatoprotective in mice.
Second, a mouse model of alcoholic liver injury disease was established by
alcohol induction. The researchers observed that mice’s mental status and body
weight changed, and abnormal increases in serum liver function and inflammatory
factor levels were detected. However, the AST and ALT liver function parameters
and the IL-1
TLR4 is an innate immune receptor expressed on the surface of macrophages that
effectively recognizes pathogen-associated molecular patterns and is the primary
receptor for LPS [30, 31, 32], which binds to TLR4 and activates nuclear
factor-
Accordingly, to further clarify its mechanism of action, this study measured
TLR4, p-NF-
In this study, LPS-induced activation of RAW264.7 cells and alcohol-induced TLR4
expression in the liver of mice were significantly increased. We also find that
TLR4 may be involved in macrophage polarization in ALD. Thus, this study reveals
that TLR4 regulates the development of ALD by regulating NF-
In this study, we studied the potential effect of GSE on alcoholic liver injury. First, we found that GPE significantly improved LPS-induced macrophage inflammatory response. Secondly, using the in vivo model, the results show that GPE can reduce the infiltration of inflammatory cells in mouse liver caused by alcohol. In addition, the mechanism study shows that GPE down-regulates the levels of inflammatory genes and proteins, which is the potential mechanism of GPE in treating ALD. The current work has laid an important foundation for the relationship between GPE and ALD.
Data are available upon reasonable request to the corresponding author.
LLW and YQ designed the research study; LJW and YXJ conducted the research and wrote the manuscript. CPX advised and guided the research in cell experiments and data interpretation. QY and JS analyzed the data. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
All animal procedures were reviewed and approved by Changchun University of Traditional Chinessse Medicine’s Ethics Committee (Approval No. 2021220).
Not applicable.
This research was supported by the Jilin Provincial Department of Science and Technology’s Science and Technology Project, Grant No. [YDZJ202201ZYTS636].
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
