Frontiers in Bioscience-Landmark (FBL) is published by IMR Press from Volume 26 Issue 5 (2021). Previous articles were published by another publisher on a subscription basis, and they are hosted by IMR Press on imrpress.com as a courtesy and upon agreement with Frontiers in Bioscience.
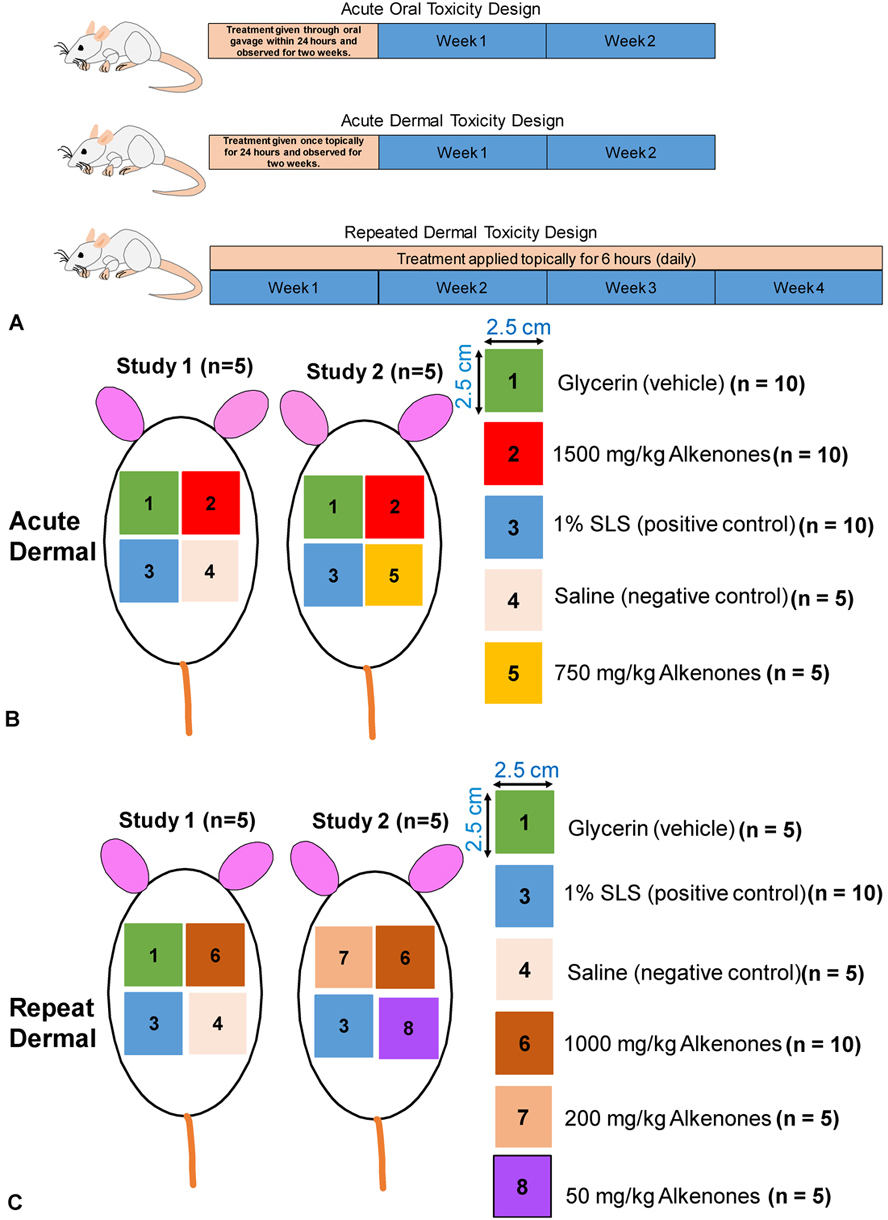
Isochrysis is commercially available marine algae used for animal feed, human nutrient supplements, and biodiesel. The Isochrysis species is one of five genera of haptophytes that produces unique, long-chain lipids known as alkenones that are promising new ingredients for green cosmetics, personal care products and pharmaceutical delivery. However, there is a lack of toxicity data for alkenones in animals, thus limiting their use in humans. In this study, we performed acute oral, acute dermal, and repeated 28-day dermal toxicity studies, using female SAS Sprague Dawley Rats. Our behavioral studies indicated that the specific alkenones had no overt behavioural effects at oral doses up to 4000 mg/kg. In the acute and chronic dermal toxicity studies, the alkenones produced less irritation and did not significantly damage the skin based on the Draize skin reaction scale and trans-epidermal water loss readings compared to the positive control, 1% sodium lauryl sulfate. Overall, our results indicated that alkenones are safe in Sprague Dawley rats, suggesting that they could be used for both oral and dermal formulations, although additional studies will be required.