Isochrysis is commercially available marine algae used for animal feed, human nutrient supplements, and biodiesel. The Isochrysis species is one of five genera of haptophytes that produces unique, long-chain lipids known as alkenones that are promising new ingredients for green cosmetics, personal care products and pharmaceutical delivery. However, there is a lack of toxicity data for alkenones in animals, thus limiting their use in humans. In this study, we performed acute oral, acute dermal, and repeated 28-day dermal toxicity studies, using female SAS Sprague Dawley Rats. Our behavioral studies indicated that the specific alkenones had no overt behavioural effects at oral doses up to 4000 mg/kg. In the acute and chronic dermal toxicity studies, the alkenones produced less irritation and did not significantly damage the skin based on the Draize skin reaction scale and trans-epidermal water loss readings compared to the positive control, 1% sodium lauryl sulfate. Overall, our results indicated that alkenones are safe in Sprague Dawley rats, suggesting that they could be used for both oral and dermal formulations, although additional studies will be required.
Due to the increasing shift in consumer demand for products with organic ingredients, personal care industries are expanding their products to include natural, green, and sustainable ingredients (1, 2). Natural ingredients, such as mushrooms, wheat, green tea, bacteria, yeast, marine algae, and certain microorganisms (3-5) have been shown to promote skin elasticity, activation of skin cell renewal and metabolism, and have anti-inflammatory efficacy (3, 4, 6, 7). The extracts of bioactive substances derived from these natural ingredients have been reported to have anti-inflammatory and antioxidant efficacy, as well as inhibiting NF-κB-meditated gene expression and a low likelihood of producing significant toxicity (8-10). For example, extracts from microalgae genera such as Arthrospira, Anacystis, Chlorella, Dunaliella, Halymenia, Nannochloris, and Spirulina, enhance collagen synthesis, preventing wrinkle formation, and contain amino acids and carotenoids that provide protection against sun damage (11). Interestingly, no independent studies have been performed or reported toxicity/safety for many of these marine extracts, perhaps due to lack of adverse clinical reports among consumers.
Marine microalgae-derived extracts from harvested seaweed are used as animal feed, wastewater treatment, biofuels, pharmaceuticals (11), and for nutraceutical and personal care applications (12). Because marine microalgae must adapt to new environments when exposed to extreme conditions (13), they typically produce diverse secondary, biologically active metabolites that are not present in terrestrial plants (14). There are an estimated 200,000 to several million species of microalgae, (11) and their unique metabolites could be of importance in the development of drug delivery or personal care formulations. Some of these compounds could be incorporated into drug/personal care formulations, including mycosporine-like amino acids, glucosyl glycerols, polysaccharides, sulfated polysaccharides, polyphenols, and lipids, which can be further biotransformed into alkenones. Alkenones are long-chain lipids, consisting of 35-41 carbons (15), with a high melting temperature (~70 °C), (16) thereby making them potential compounds for use in pharmaceuticals formulations when developing topically delivered drugs. Pavlova salina and Isochrysis galbana are the best-known species of the phylum Haptophyta and are produced globally (11). The Isochrysis sp. is a non-toxic food source for cultivated marine animals (17) as it produces essential polyunsaturated fatty acids, such as eicosapentaenoic acid and docosahexaenoic acid for the growth and development of fish larvae (18).
Four strains of Isochrysis sp. have been reported to produce alkenones (19). Pure alkenones can be isolated from Isochrysis using a 5-step process (15). Alkenones have historically been used as reliable palaeothermometers (20). However, more recently alkenones have been investigated as a source of biofuel (21) and as an ingredient in topical formulas. For example, the alkenones physical characteristics were previously reported (16) to be compatible with commonly used waxes (e.g., microcrystalline wax and ozokerite) in lipstick and lip balm products. Furthermore, the alkenones simplified molecular-input line-entry system (SMILES) were entered into Formulating for Efficacy™ (ACT Solutions Corp, Kirkwood, DE, USA), to generate Hansen Solubility Parameters (16). The Formulating for Efficacy™ was used to calculate the molar volume (MVol), Ingredient-Skin Gap value (ISG), LogK and Solubility in Skin (SolS). The smaller the ISG value is, the more compatible that ingredient is with the outer layer of the epidermis, the stratum corneum, and the greater tendency for that ingredient to penetrate the skin. Similarly, the smaller the MVol value, the greater likelihood that ingredient will penetrate the skin. The alkenones had high MVol and ISG values and low SolS value of 0.3 which is ideal for topical skin applications. However, the toxicity of the alkenones has not been assessed in animals, which is essential information for these applications. In this study, female SAS Sprague Dawley (SD) rats were given p.o. alkenones acutely and repeatedly and the major organs were removed and analyzed for damage. Furthermore, blood chemistry protein levels were measured in whole blood taken from the rats after the oral acute treatment. Finally, we determined the effects of an alkenone-containing paste (applied once a day for 28 days) on the dorsal skin sites of rats.
The marine microalgae Isochrysis was purchased from Necton S.A. (Olhão, Portugal). Alkenones were isolated, purified and characterized from the Isochrysis biomass as previously described (15, 22, 23). Briefly, dried Isochrysis was put through a Soxhlet extraction process and the resulting extract saponified (allowing for separation of fatty acids and neutral lipids), and finally alkenones were isolated and purified from the neutral lipids by crystallization (15). Sodium chloride (0.9%) was purchased from BioVision Incorporated (Milpitas, CA, USA). The following items were received as gifts: natural glycerin, (Spectrum, New Brunswick, NJ, USA); sodium lauryl sulfate (SLS) (PCCA, Houston Texas, USA); hydroxypropylmethylcellulose (HPMC) (Spectrum Chemical, Gardena, CA, USA). All ingredients were of pharmaceutical grade.
Vehicles used for the alkenones and other treatments are later described 3.3.1 and 3.8 in this article.
Seven-week-old female SAS Sprague Dawley (SD) rats (180-200 grams) were obtained from Charles River (Wilmington MA) and used for the acute oral, acute dermal, and repeated dermal toxicity tests. Twelve rats were used for the acute oral toxicity test, whereas ten rats were used for the acute dermal toxicity and ten rats for the repeated dose 28-day dermal toxicity. The toxicity tests were performed according to the Organization for Economic Cooperation and Development (24) test guideline, i.e., OECD Guideline 423 for the acute oral toxicity test (25) and OECD Guideline 404 for the acute dermal toxicity test (26-28), with slight modifications. The injection volume (4mL/kg) was adjusted depending on the body weight of the rat (29). The female rats were nulliparous and non-pregnant. The SD rats were given standard rat pellets (Envigo Teklad) and reverse osmosis water ad libitum. They were acclimatized to the laboratory conditions for 7 days before the experiments. The rats were housed in groups, depending on the weight of the animal, in accordance with IACUC guidelines (three to four rats for acute oral toxicity and two to three rats for acute and repeated dermal toxicity). The animals were housed under a 12-hour light/dark cycle, at temperatures of 23±2 degree Celsius) and a humidity of 55±5%. The animal protocol for this study was approved by the Institutional Animal Care and Use Committee of The University of Toledo and was in accordance with all National Institutes of Health guidelines (No. 8023, revised 1978) for animal research, including the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Sciences, 1996).
To assess the alkenones toxicity in the rats, three independent toxicity studies were performed i.e. oral exposure of alkenones up to 24 hours, one-time dermal exposure for 14 days, and repeated 6-hour dermal exposure for 28 days. The study design is presented in the form of cartoon schematic diagram (Figure 1a). Briefly, the doses used were modified from the OECD Guideline 420 for the oral administration study and the OECD Guideline 434 dosages were used to determine the dose of acute dermal exposure and repeated exposure. For oral exposure, the animals were randomly divided between two groups with N = 6 vehicle and vehicle containing the alkenones. Further, as shown in Figure 1b acute dermal exposure of alkenones were performed in 2 standalone studies, with N = 5 SD rats for each study. The first study used the highest possible test dose of alkenones that could be applied in 6.25 cm2, i.e., that was compared to the nearby exposures of vehicle, positive control (1% SLS) and negative control (saline). We repeated the same procedures in Study 2 with N = 5 SD rats, but instead of using negative control (which was non-toxic in first study), we applied an additional 750 mg/kg dose adjacent to high dose for two reasons: (a) to determine the effect of alkenones on the dermis on a large surface area on the same rat, i.e., a total of 2250 mg/kg exposure in 12.50 cm2 area; (b) to determine if there would be any effects from different concentrations of the alkenones with the vehicle. Later, as shown in Figure 1c, the first repeated exposure study would use a high dose of 1000 mg/kg alkenones that could be applied in 6.25 cm2 and compared to the nearby exposures of vehicle, 1% SLS and saline for 6-hours a day for 28 days. We repeated the same procedures in Study 2 with n = 5 SD rats, but instead of choosing the vehicle and saline, we applied two additional doses of 200 and 50 mg/kg alkenones. This would (a) determine if the dermal exposure of the alkenones on a greater surface area could be tolerated on the same rat, i.e., a total of 1250 mg/kg exposure in an 18.75 cm2 area; (b) determine if there would be any effects of the different concentrations of the alkenones with the vehicle following daily exposure.
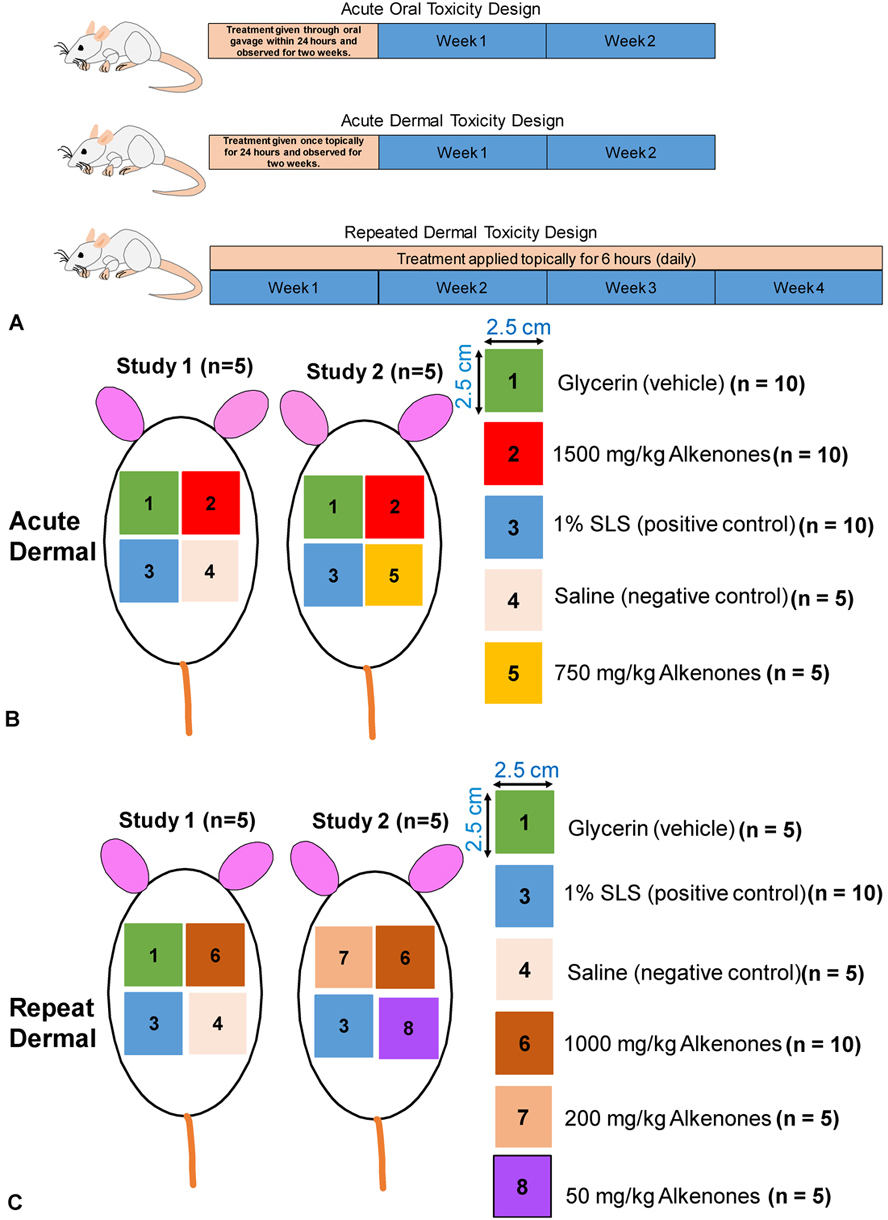 Figure 1
Figure 1(A) Experimental design showing duration of administration of alkenones for three different studies in SAS Sprague Dawley rats. (B) Experimental design showing the site (6.25 cm2) of acute dermal applications of different treatments in two stand-alone studies. (C) Experimental design showing the site (6.25 cm2) of repeat dermal applications of different treatments in two stand-alone studies.
Due to the long chain structure of the alkenones, finding a suitable solvent to test it with in vitro test methods can be problematic (30, 31). The alkenones were not soluble in solvents such as DMSO, Tween 80, Tween 20, Sesame seed oil, and Dulbecco’s modified Eagle’s medium (DMEM) media. Therefore, we used a suspension formulation to administer the alkenones to rats using oral gavage. After weighing, the alkenones were pulverized using a mortar and pestle, and glycerin was added as a wetting agent to form a paste. Hydroxypropylmethylcellulose (HPMC) was placed in water heated to 80 ºC to yield a 3% w/v solution. The HPMC solution was vortexed and allowed to cool to room temperature. The 3% w/v of HPMC was added to the paste. The resulting suspension was poured into a large test tube and withdrawn using an oral gavage stainless steel needle (3 inches length and ball diameter of 2.25 mm) before dosing the rats.
The alkenones suspension and vehicle suspension alone were administered to female rats by oral gavage in divided doses of 1 mL/kg body for a total of 4000 mg/kg body weight. The starting dose of 1000 mg/kg of the alkenones in the suspension vehicle and the vehicle itself was used on two individual rats (four total) as a starting dose as recommended by Whitehead and Stallard (32), with modifications. When that dosage did not produce a loss of more than 20% body weight, change in physical appearance, or change in behavioral pattern after 48 hours, two more animals recieved 2000 mg/kg of the alkenones. After 48 hours, the 2000 mg/kg dose did not appear to be toxic to the animals. Twelve female SD rats were divided into two groups (6 rats per group). Group 1, over a period of up to 24-hours, was given a total of 4000 mg/kg of the alkenones derived from Isochrysis sp. that was prepared in a suspension of glycerin and 3% HPMC. Group 2 received the vehicle only with the same dosage. The rats were observed for general behavioral changes using the body condition scoring scale, based on the following typical signs: failure to eat or drink, ruffled fur, runny eyes, respiratory distress, abdominal pain (hunched posture), or weight loss in excess of 20% (weighed daily). In addition, the facial expression of the rats was observed and measured based on the Grimace Scale, as suggested by Sotocinal et al. (33), for changes in position for ears and whiskers, for orbital tightening, and nose/cheek flattening. Symptoms of toxicity (and mortality) were noted after each divided treatment for the first 1 h, followed by observations made within 30 minutes of treatment and then at 1, 6, 12, 24 hours and in parallel, rats in group 2 were treated with vehicle (Glycerin and 3% HPMC) to establish a comparative negative control group according to the guideline (25). All animals were observed at least once during the first 30 min in the first 24 hours and then again at 4 hours and 24 hours following vehicle or alkenones suspension administration and then once a day, at approximately 10 AM, for 14 days, as indicated in the (28) guidelines. All behavioral and physical observations, including changes in skin, fur, secretions from eyes and mucous membrane, and behavioral pattern changes such as posture, response to handling, motor activity, grip strength and sensory reactivity to stimuli were recorded for each rat. Food consumption was recorded daily, and the body weight of each animal was recorded immediately before the administration of the test substance and daily throughout the length of the study.
At the end of the 14-day observation, animals were sacrificed (N = 12) using CO2 asphyxiation, followed by cardiac puncture. Subsequently, 1mL of blood was obtained and placed in a 1.3mL K3E Micro tube. Five hundred microliters of blood were placed in a test tube containing lithium heparin. Subsequently, in whole blood samples, the number of RBCs, WBCs, PLTs, HGB, and monocytes were determined using a VetScan HM5 instrument (Abaxis, Union City, CA, USA).
In a separate lithium heparin test tube with collected blood, 120 μL was withdrawn and added to the comprehensive diagnostic profile plate (Abaxis, Union City, CA, USA) and run in a VetScan 2 device (Abaxis, Union City, CA, USA). A panel of functional parameters, consisting of albumin, total protein, globulin, sodium (Na+), potassium (K+), alkaline phosphatase (ALP), alanine aminotransferase (ALT), amylase (AMY), total bilirubin (TBIL), bilirubin urea nitrogen (BUN), total calcium (CA), phosphorus (PHOS), creatinine (CRE), and glucose (GLU), were analyzed in whole blood samples using the VetScan 2 instrument.
The animals were euthanized at the end 14 day after sub-acute oral administration using CO2 asphyxiation, followed by a cardiac puncture for collecting blood. Immediately following sacrifice, the major organs of each rat (except the brain) were evaluated by gross necropsy. Parameters such as abnormal growth of lesions, necrosis, changes in overall organ size and color were evaluated. The brain tissue was not evaluated as alkenones, which consist of long carbon chains between 35 and 41 (21), do not cross the blood-brain barrier. The liver, kidneys, spleen, and heart were removed, examined for the presence of abnormal lesions, weighed, and collected for further histopathological examination. The lungs, stomach, intestine, and colon (data not shown) were examined as described above and weighed, but not collected for histopathological examination. Subsequently, the organs were immediately fixed in 10% neutral buffered formalin for 24 hours, then dehydrated in 70% ethanol, and embedded in paraffin. The block of embedded tissue was cut into 5 µm sections using a microtome (Reichert-Jung Biocut 2030 Microtome, Rankin; Holly, MI, USA). The resulting tissue sections were subjected to a modified protocol from www.aladdin-e.com (Aladdin, 2018) for haematoxylin and eosin (H&E) staining. Images of the tissue sections were taken using a VS 120 Virtual Slide Microscope (Olympus, Pittsburgh, PA, USA.) The H&E-stained images were examined using OlyVIA Ver.2.9 software.
Twenty-four hours before treatment, fur was removed by closely clipping the dorsal area of the trunk of the animals according to the guideline (26-28). On the treatment day, for Study 1 animals, the alkenones were weighed for the administration of dermal doses of 750 mg/kg or 1500 mg/kg. The doses were based on the OECD guideline 434 fixed dose flow chart, where the highest dose that could be given was 2000 mg/kg, with 1000 mg/kg as the next highest dose. The 2000 mg/kg dose could not be formulated as a suspension that could be applied to a defined skin site area (approximately 6 cm2). This was modified to the highest dosage that could be orally administered, which was 1500 mg/kg, with a second dose of 750 mg/kg in case of severe irritation manifestations during the 24-hour contact exposure of the high dose. The alkenones were pulverized in a mortar using a pestle. The powder was mixed with a fixed amount of glycerin to form a paste-like substance. Five rats in Study 1 were exposed to 1500 mg/kg of alkenones, the positive control, the negative control and the vehicle, on four different skin sites of each individual animal. Another five rats in the Study 2 were given the positive control, the vehicle, 1500 mg/kg, and 750 mg/kg at different skin sites on the shaved backs of the rats. The test compound (300 milligrams of the paste or 300uL of liquid treatment) was applied to a small area (approximately 6 cm2) of skin and covered with a gauze patch that was held in place with non-irritating tape. Other treatments applied at different test sites were a negative control (0.9% sodium chloride), a positive control (1% sodium lauryl sulfate (SLS: dissolved 0.9% saline solution) and glycerin. The rats had ad libitum access to standard rat food pellets that were kept in small paper cups and water throughout the study. To further prevent the rats from removing the covered gauze patch, an Elizabethan collar was placed on each rat and kept in a separate cage for the 24-hour test period. At the end of test period, the covering was removed and 1) images of the skin sites were taken; 2) the reaction of the skin was graded based on the Draize skin reaction numerical scale (34) for severity of the skin sites from each application and 3) a Tewameter reading from each skin site was recorded before washing the skin sites with water. Following the above tests, the rats were returned to their cages.
The clipping of the fur was done as described above. On the treatment day, the animals were weighed for the dermal application of 1000, 200, and 50 mg/kg of alkenones. These doses were also based on the OECD guideline 434 fixed dose flow chart. Since we could not reach use the 2000 mg/kg dose, we chose to use the other three doses for our sub-chronic study. In Study 1 five rats were given the positive control, 1000 mg/kg, the negative control and the vehicle on four different skin sites. In Study 2 five rats were given the positive control, 1000 mg/kg, 200 mg/kg and 50 mg/kg on four different skin sites. The procedure for preparing and applying the treatments, the housing of animals, and tests performed after treatment were the same as those mentioned for the acute dermal administration of the alkenones. However, in the sub-chronic studies, the treatment time was lowered to a 6-hour period. For the repeated dermal toxicity study 6-hour post-application, each site was gently wiped with a dry gauze pad to remove any remaining paste residue and was allowed to breathe for 2-3 minutes before reading with the probe occurred. Afterwards, the skin sites were washed with autoclaved water for any remaining substances on the skin and wiped down again gently with a dry gauze pad. This process was done once a day for 28 consecutive days, after which the animals were euthanized, organs dissected and weighed for future histological analysis.
A Tewameter® TM 300, a non-invasive device, was used to measure the amount of water loss through the skin. The measurement unit for reading the rate of loss is known as trans-epidermal water loss (TEWL) (35). It has two pairs of sensors in the probe to receive corresponding information on the temperature and moisture readings of each TEWL-value during the complete measurement (36). The Tewameter® TM 300 has been accepted for measuring TEWL worldwide and has been used in both animals (37) and humans (38, 39). The open chamber probe was held in the middle of each skin site application for 20 seconds before and after treatment. The open cylinder chamber probe of the Tewameter® TM 300 was held against each skin with light pressure for at least 20 seconds to get an average reading. TEWL values ranged from 0-10 (very healthy conditions) to above 30, indicating critical skin conditions. If alkenones produce skin damage to the skin, then similar to the positive control, it should yield high TEWL values.
Experimental results were expressed as the mean ± standard deviation (SD). Statistical analysis of the data was performed using GraphPad Prism (Version 7.0, GraphPad Software, La Jolla, CA, USA). For the body weight and food consumption for the acute oral, dermal, and sub-chronic test, the data were not normally distributed and were expressed as the modes and analyzed using the Mann-Whitney U test. The data from the Vet Scan HM5 and VS2 values were all normally distributed except for TBIL, BUN, and potassium. Therefore, the TBIL, BUN, and potassium data were analyzed using the Mann-Whitney U test, whereas the other blood chemistry data were analyzed using Welch’s t-test. The post-mortem organ weight data were analyzed using Welch’s t-test. The Tewameter data for acute dermal skin site application was analyzed using a mixed effect ANOVA with the treatment and baseline measurements as the fixed effect and ID as the random effect. For the repeated Tewameter data, a mixed effects model was used with treatment, baseline and days (treated as a categorical variable) as the fixed effect and ID as the random effect. Tukey’s post hoc analyses were used for the pairwise difference for the dermal Tewameter values. The a priori significance level was P < 0.05.
Statistical analysis indicated that the administration of 4000 mg/kg of the alkenones formulation did not significantly alter the body weight compared to animals treated with vehicle (Figure 2A). Furthermore, there was no significant difference in food consumption between the alkenones- and vehicle-treated animals Mann-Whitney analysis (Figure 2A).
 Figure 2
Figure 2Determination of (A) body weight, and (B) food consumption, following acute oral administration of alkenones (4000 mg/kg) compared to vehicle in female SAS Sprague Dawley rats (n=6 per group).
The oral administration of 4000 mg/kg of the alkenones formulation did not significantly alter behavior based on the facial grimace scale, the body postures or signs of stress compared to animals treated with the vehicle after 1-2-hour administration (data not shown). There was no significant difference in grip strength between both treated groups (data not shown). The gross necropsy results indicated that neither the administration of 4000 mg/kg of the alkenones formulation nor vehicle produced apparent signs of pathology (Figure 3). No histopathological changes were present in the liver, kidneys, spleen, heart, stomach, intestine and colon of animals treated with 4000 mg/kg of the alkenones or vehicle group (data not shown). The brain tissue was not evaluated as the alkenones, which consist of long 37 hydrocarbon chain, would not cross the blood brain barrier intact. However, the metabolites of the alkenones could potentially cross the blood-brain barrier via fatty acid transport proteins (FATPs) (40). Further studies must be done to verify this hypothesis.
 Figure 3
Figure 3Histopathological evaluation of the (A) liver, (B) spleen, (C) heart, (D) kidney and (E) skin of SAS Sprague Dawley rat after acute oral exposure to vehicle control and alkenones (4000 mg/kg) after 14 days. (C1) Represents vehicle control rat 1, while (T1) represents treated rat 1. Treated samples were stained with haematoxylin and eosin (H&E) stains and observed at a magnification of 20× (n=6). There were no significant pathological changes in the various tissue sections between the vehicle and treated groups.
The acute administration of the 4000 mg/kg formulation of the alkenones did not significantly alter the number of WBC, RBC, PLT, HGB, and monocytes or the level of haemoglobin (p < 0.05) compared to vehicle-treated animals (Figure 4). There was no significant difference between the alkenones- and vehicle-treated animals in the whole blood levels of albumin, total protein, globulin, Na+, K+, ALP, ALT, AMY, TBIL, BUN, CA, PHOS, CRE, and GLU (P < 0.05) (Figure 5).
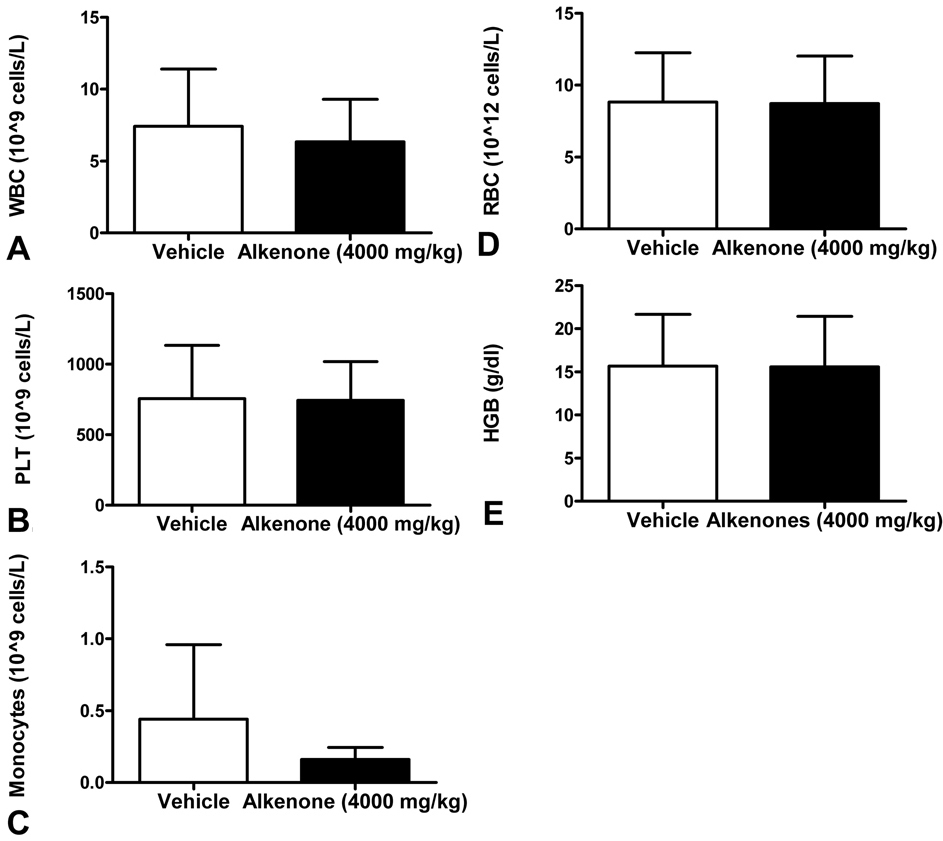 Figure 4
Figure 4Blood chemistry profile to determine immune functions in SAS Sprague Dawley rats after acute oral exposure to vehicle control and alkenones (4000 mg/kg) for 14 days. The levels of (A) white blood cells (WBC), (B) platelets (PLT), (C) monocytes (D) red blood cells (RBC), and (E) haemoglobin (HGB) were measured from rats that were treated with vehicle or alkenones suspensions at the day 14.
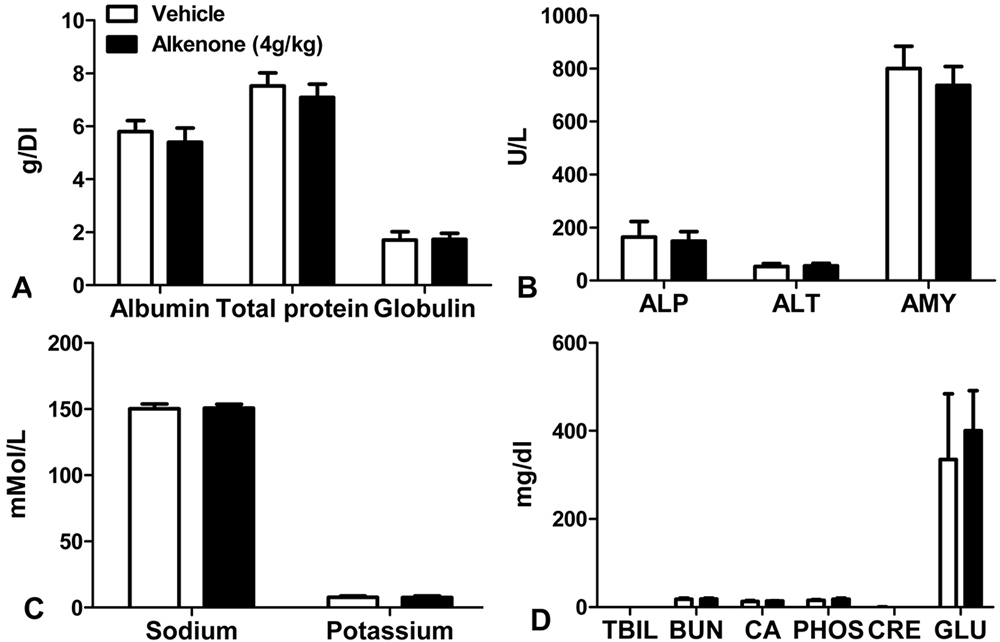 Figure 5
Figure 5Blood chemistry profile to determine organ toxicity in SAS Sprague Dawley rats after acute oral exposure to vehicle control and alkenones (4000 mg/kg) for 14 days. (A) Albumin, total protein, and globulin levels in grams per deciliter (g/dL), (B) alkaline phosphatase (ALP), alanine aminotransferase (ALT) and amylase (AMY) levels in units per liter (U/L), (C) sodium and potassium levels, millimoles per liter (mMol/L), (D) total bilirubin (TBIL), blood urea nitrogen (BUN), total calcium (CA), phosphorus (PHOS), creatinine (CRE) and glucose (GLU) levels in milligrams per deciliter (mg/dl) collected from rats treated acutely with oral vehicle and alkenones suspensions.
We conducted experiments to determine the effects of topical administration of the either 1500 mg/kg or 750 mg/kg of the alkenones formulation for 24 hours to rats over 14 days. The body weights, as well as the food consumption, of the animals in Study 1 and Study 2 were not significantly altered (P > 0.05 for both measurements, Mann-Whitney U test) compare to animals treated with vehicle (Figure 6). Furthermore, the topical application of the alkenones did not significantly alter the relative weights of the liver, kidneys, spleen, heart, stomach, intestines and colon (data not shown). The exposure of skin to the positive control, 1% SLS, for 24 hours significantly increased (Sidak’s multiple comparison, P < 0.0001) skin water loss compared to the alkenones (1500 mg/kg) and glycerin (Figure 7). The open cylinder chamber probe of the Tewameter® TM 300 was held against each skin with light pressure for at least 20 seconds to get an average reading. TEWL values ranged from 0-10 (very healthy conditions) to above 30, indicating critical skin conditions. If alkenones produce skin damage to the skin, then similar to the positive control, it should yield high TEWL values. Four out of ten rats had elevated TEWL readings compared to their positive controls, while all other topical application sites had TEWLs ranging from 0-10 (i.e., very healthy skin condition) (Figure 7).
 Figure 6
Figure 6Determination of (A) body weight, and (C) food consumption, following acute dermal exposure of Study 1 compared to Study 2 in female SAS Sprague Dawley rats (n=5). Determination of (B) body weight, and (D) food consumption, following repeated dermal exposure of Study 1 compared to Study 2 in female SAS Sprague Dawley rats (n=5).
 Figure 7
Figure 7The trans-epidermal water loss (TEWL) was measured before and 24 hours after acute dermal application in (A) Study 1 group treated with 1% SLS (positive control), saline (negative control), glycerin (vehicle) and 1500 mg/kg alkenones (AK); (B) Study 2 group treated 1% SLS (positive control), vehicle (glycerin), 750 mg/kg and 1500 mg/kg alkenones (AK); (C) Combined data from Study 1 and 2 to show any significant change in TEWL measurements. (D) The trans-epidermal water loss (TEWL) was measured before and after acute application of 1% SLS (sodium lauryl sulphate), alkenones (1500 mg/kg) and vehicle (glycerin) on dermal skin in SAS Sprague Dawley rats. Two-way ANOVA analysis followed by Tukey’s multiple comparisons tests indicated the changes between baseline and 24 hours TEWL readings.
The different repeated dermal treatment applications in Study 1 or Study 2 for 6 hour per day for 28 days did not significantly alter body weight (Mann-Whitney test) and food consumption (Mann-Whitney test) between both study groups (Figure 6). Furthermore, the repeated dermal application of the alkenones did not significantly alter the relative weights for the liver, kidneys, spleen, heart, stomach, intestines and colon (data not shown).
Following the 28-day exposure to the alkenones, the 1000 mg/kg dose of the alkenones only increased the TEWL value to 10 in one rat on day 1 of Study 1 (Figure 8). In addition, the application of saline (the negative control) also increased the TEWL in the aforementioned animal. The TEWL values for the positive control (1% SLS) were the highest on day 2, with eight out of ten rats having TEWL values greater than 10. Overall, the 1000 mg/kg paste formulation of the alkenones significantly (Sidak’s multiple comparison, P < 0.0001) lowered the TEWL values throughout the 28-day period compared to the positive control (Figure 9). The positive control always significantly increased water loss.
 Figure 8
Figure 8The trans-epidermal water loss (TEWL) was measured before and after 6 hours repeated dermal exposure every day for 28 days on skin area exposed to (A) 1% SLS (positive control), (B) 200 mg/kg alkenones, (C) normal saline (negative control), (D) 1000 mg/kg alkenones, (E) 50 mg/kg alkenones and (F) glycerin. The rats were divided into two groups. Rats 11, 12, 15, 16, and 19 were treated with 1% SLS, 50 mg/kg alkenones, 200 mg/kg alkenones, and 1000 mg/kg alkenones). Rats 13, 14, 17, 18, and 20 were treated with 1% SLS, saline, glycerin (vehicle) and 1000 mg/kg alkenones.
 Figure 9
Figure 9The trans-epidermal water loss (TEWL) measurements before and after 6 hours repeated dermal exposure of different treatments each day at (A) Day 1, (D) Day 2, (B) Day 8, (E) Day 14, (C) Day 21, and (F) Day 28.
In order to determine how toxic alkenones are in case of accidental ingestion or exposure, sub-acute oral toxicity was performed in animals. This is the first pilot toxicity study with the alkenones as there have been no previous published reports on the evaluation of alkenones toxicity in animals. Other studies have reported that Sprague-Dawley female rats have greater mechanical allodynia and sensitivity to oral toxicity (41, 42) compared to males. When determining the doses, we followed OECD Test No. 420 and 423 guidelines with modifications of starting with one animal at high dosage of 1000 mg/kg and observing the outcome. There are five toxicity categories with the class 1 being the most toxic at amounts up to 5 mg/kg. Compounds tested between 2000 mg/kg < LD50 ≤ 5000 mg/kg are labeled as a class 5 toxicity category, based on the criteria of the Globally Harmonized Hazard Classification and Labelling Scheme. This facilitates the identification of substances that produce toxicity at low doses (24). Although the class 5 toxicity category is optional, we included this category because the alkenones, if used to formulate personal care products or topical drugs, would come in contact with human skin. The rats treated with alkenones suspension solution produced no overt toxicity at 4000 mg/kg given within 24 hours as compared to the vehicle suspension solution which lacked the alkenones. This high dosage was achieved by giving smaller dosages 1000 mg/kg every five hours to not overload the stomach capacity. Because daily exposure to the alkenones through oral gavage would have been stressful on the animals; we decided not to conduct chronic oral toxicity. If future requirements require chronic oral toxicity data, we will need to prepare the dosage of alkenones needs to be prepared in food pellets form so that the animals are exposed voluntarily and without stress.
In this study, the alkenones derived from commercial Isochrysis algae were found to be in the class 5 toxicity category when given orally to SD female rats. The findings of our study indicate that alkenones have no acute toxicity under the present experimental conditions and its lowest lethal dose is above 4000 mg/kg in female SD rats. There was no significant change in food consumption for the 4000 mg/kg alkenones group compared to the vehicle control group (Figure 2). During the acute oral observation study period, no deaths were recorded in either the control or the 4000 mg/kg group that was not due to accidental oral gavage injury or handling. The observational behavioral data indicated that only grooming and partial eye closure occurred in the first 10 minutes after treatment in both groups, which was considered to be a change attributable to the handling or oral gavage administration. There were no significant changes in the 1) grip strength between both groups and 2) behavioral or physical parameters during the 14-day study.
The oral administration of 4000 mg/kg alkenones suspensions did not induce abnormalities in the gross necropsy after 14-days of observation and did not significantly alter the weight of the organs compared the vehicle control group. The histology data showed no significant changes in the alkenones-treated group compared to the vehicle control group. Thus, even at a high dose, alkenones did not induce any organ-specific damage. The blood analysis results indicated a slight, non-significant decrease in monocytes in the animals treated with 4000 mg/kg alkenones compared to vehicle-treated animals. However, there was no significant difference between the alkenones and vehicle-treated animals in the number of WBC, RBC, PLT or HGB levels or the 14 biochemical parameters tested. Polysaccharides from the Iscochrysis sp. can produce immunomodulatory effects by increasing the levels of IL-1β in macrophages and monocytes (43) which might suggest why the level of monocytes changed with alkenone treated rats. Currently, the explanation for this difference is unknown. Overall, our results suggest that the acute oral administration of alkenones (4000 mg/kg) did not alter the blood chemistry or damage major organs under the experimental conditions used.
Dermal acute and sub-chronic exposures studies were performed to determine alkenones effect on direct exposure to the skin in animals. When determining the fixed doses, we followed OECD Test No. 434 guidelines with modifications for the use of five animals at 1500 mg/kg in Study 1, the highest dose used. A second dose of 750 mg/kg was also included in Study 2 animals in case they were effects with a different amount of alkenones and the vehicle used as well as to find any effects with a larger skin surface area of alkenones exposed directly to the skin. The Draize skin irritation numerical scale was used to record the degree of skin damage for each site. The 1500 mg/kg and the 750 mg/kg doses of the alkenones did not produce erythema, eschar, or edema formation during the 24 hours of contact at the skin sites. Thus, Study 2 rats in the acute dermal test were able to tolerate the alkenones on a large surface area of the skin and the different amounts of alkenones with the vehicle showed no change in effects at a single treatment. The Draize skin reaction numerical scale showed no redness at the other treatment sites when compared to the positive control. It was noted that scabs from the positive skin site application lasted only four days’ post-treatment in some sensitive SD rats. For the sub-chronic repeated treatments, the Study 2 rats exposed to 1000 mg/kg, 200 mg/kg, and 50 mg/kg alkenones did not have erythema, eschar, or edema during the 6 hours of contact per day on the skin sites. There was an increase in the irritation score only on Day 25, as indicated by the reddening of the skin at the 1000 mg/kg dose of the alkenones, but this score was lower than that of the positive control, whose values were higher than all of the other treatments. The positive control, 1% SLS, did produce erythema and eschar formation on the skin in four, but not all rats. In either Study 1 or 2 animals the application of the 1000 mg/kg alkenones formulation for 6 hours per day for 28 days did not produce erythema, edema, or eschar formation during the first two weeks. The positive control produced greater water evaporation after 6 hours. During the third and fourth week, erythema was present in certain rats of Study 2 after 1000 mg/kg of the alkenones formulation was applied on the skin. However, erythema was seen after the application of both the negative control and vehicle, possibly as a result of the bandage being too tight on a specific day. From both experiments, alkenones did not produce a noticeable severe dermal irritation when placed on the skin for a single 24 hours contact or repeated 6 hours contacts for a 28 day period.
The Tewameter device was used as a secondary confirmation of skin damage due to water loss. TEWL can be produced by environmental stressors such as ultraviolet radiation, which damages the skin by producing reactive oxygen species (44). In the acute dermal study, there was no significant difference in the TEWL values between the alkenones- and vehicle-treated animals. The Tewameter data indicated that only the positive control, 1% SLS, significantly increased water evaporation before and after 24 hours of treatment (Figure 7). Alkenones applied to the skin sub-chronic 28-day repeated treatment did not produce significant skin damage compared to the positive control, based on TEWL readings taken both before and after the 6-hour daily applications. The alkenones tested did not significantly increase water loss in either single or repeated dermal exposure
One limitation of this study was that we only used SD rats. These alkenones could be used in human epidermis models such as EpiSkin or EpiDerm to further evaluate their effects on the morphology of the skin cells and testing for phototoxicity and corrosivity (45). A second limitation was the alkenones could not be evaluated in vitro due to issues with their solubility. The alkenones would either precipitate out of DMSO, DMEM medium, Tween 20, Tween 80, and sesame seed oil, or the solution became cloudy and opaque after sonication for 15 seconds. Consequently, we could only conduct in vivo studies with the alkenones using a suspension formulation. The solvent dichloromethane completely dissolves the alkenones, but dichloromethane is extremely toxic in cell lines and animals (46). Another limitation was the delivery of the alkenones to the rats through oral gavage. The delivery via water was not feasible as the alkenones are insoluble in water. A suspension of alkenones greater than 200 w/v % was too viscous to pass through the stainless-steel gavage needle. Therefore, a suspension of 100 w/v % was given to the rats four times within a 24-hour period. While corn oil was an option, when mixed with the alkenones, it took a longer time to pass through the needle with more force than should be required to deliver the solution into the stomach. We subsequently used a suspension solution, as suggested by Turner (29), to decrease the stress to the animals.
Glycerin is a common ingredient used in drug formulation and personal care products (47). In addition, it is important to note that glycerin is a humectant which attracts and keeps moisture to the site of application (48). Although it is safe for skin use, it may have some effects of irritating properties when different amount of the alkenones are mixed. Following the application of a high concentration of the alkenones (more than what may be present in a typical skin care product), a large amount of the alkenones were left on the skin site application after 6 (1000 mg/kg) and 24 hours (1500 mg/kg). Interestingly, even when alkenones were applied at a larger surface area of the skin, the treatments were well tolerated with overall no change between the different concentrations of alkenones and vehicle mixtures. Nonetheless, our toxicity study provided new information regarding how well the skin in female SD rats could tolerate alkenones for extended periods of time.
In conclusion, the toxicity studies indicated that the alkenones could be classified as level 5 LD50 toxicant according to globally harmonized hazard classification and labelling scheme (GHS). Our results indicated the alkenones from Isochrysis sp. were practically non-toxic. They were determined to be safe after acute oral and or dermal exposure for up to 28 days in rats in this study. Based on this study, we presume that animals or humans exposed to alkenones should not experience any significant adverse effects. As mentioned previously, Isochrysis sp. has been grown as animal feed for both farm (49) and marine animals (50). Other nutrient ingredients sought after from Isochrysis sp. are eicosapentaenoic acid, an omega-3 fatty acid, (18) and docosahexaenoic acid, another omega-3 fatty acid that is the primary structural component of the human brain and retina (51) Using alkenones derived from the commercially available Isochrysis sp. algae in drug/personal care formulations productions may represent another promising source for pharmaceutical companies. Also, in a previous study (16), the same alkenones tested for toxicity had a low SolS value and high ISG values, indicating the alkenones alone were less compatible with the stratum corneum and thus less likely to penetrate the skin. Although the Federal Food, Drug, and Cosmetic Act (FFDCA) do not require excipient ingredients to be approved by the United States Food and Drug Administration (FDA) before they are marketed, the ingredients must be safe for consumers under the labeled conditions of use. A follow-up study with clinical trials using alkenones should be assessed in healthy volunteers to confirm two things. First, that no other toxic effects are seen that differ from the animal studies. Second, to confirm the safety of using alkenones in human application products. Once the correct formulation for use has been determined, alkenones may prove to be a useful ingredient in products from personal care companies that are constantly searching for natural, green, sustainable ingredients.
The University of Toledo Math Dept. Statistical Consulting Group for their assistance with the Tewameter readings results for both acute and repeat dose dermal toxicity. Dr. Gabriella Baki (University of Toledo) for the use of the Tewameter® TM 300 device. Dr. Zahoor Shah (University of Toledo) for the use of his Grip Strength meter. Allen Schroering for histology services. We also thank members of Tiwari Laboratory, Angelique Nyinawabera, Mariah Pasternak, Noor Hussein, Atul Vij and Hasan Alhaddad, for their assistance with handling the rats and assistance with data analysis. There is no conflict of interests to declare. This work was supported by the Marine Biological Laboratory Woods Hole Oceanographic Institute (WHOI) under grant (N-126665-01, 2017), Washington Research Foundation, and University of Toledo start-up funding under a grant (F110760) to A.K.T. The authors declare no conflict of interest.
ISG
Ingredient-Skin Gap
Molar Volume
Solubility in Skin
Sprague Dawley
Organization for Economic Cooperation & Development
hydroxypropylmethyl cellulose
Trans epithelial Water Loss
Dimethyl sulphoxide
Sodium lauryl sulfate
