- Academic Editor
Background: The SLC5A8 gene is silenced in various types of cancer, including cervical cancer; we recently demonstrated that the SLC5A8 gene is also silenced in cervical cancer by hypermethylation of the CpG island in the gene promoter. This study aims to analyze whether SLC5A8 could be a tumor suppressor in cervical cancer. Methods: After ectopic expressing SLC5A8 in the HeLa cell line, we evaluated its effects on cell behavior both in vitro and in vivo by Confocal immunofluorescence, cell proliferation, migration assays, and xenograft transplants. Results: Overexpression of SLC5A8 in the HeLa cell line decreased its proliferation by arresting cancer cells in the G1 phase and inhibiting cellular migration. Furthermore, we observed that pyruvate increased the SLC5A8 effect, inducing S-phase arrest and inhibiting the entry into mitosis. SLC5A8 decreased tumor growth in xenograft transplants, significantly reducing the volume and tumor weight at 35 days of analysis. Conclusions: In summary, our results indicate that SLC5A8 has a role as a tumor suppressor in cervical cancer.
The solute carrier (SLC) superfamily comprises 55 families with almost 362 active and passive transporters. The family SCL5 has 12 members of Na/substrate cotransporters; SLC5A8 was identified for the first time in 2002 by Rodriguez et al. [1] as an essential protein related to the iodide uptake in the thyroid. The human gene of SLC5A8 is located on chromosome 12q22-23 and is coded by 15 exons [2]. This protein has 610 amino acids, 13 transmembrane domains, and one PDZ domain. It is related to the transport of sodium/monocarboxylates, including short-chain fatty acids (SCFA), such as propionate, butyrate, and pyruvate, which have histone deacetylase activity [1, 2, 3, 4, 5, 6, 7].
Li et al. [2] proposed SLC5A8 as a tumor suppressor for the first time, showing that it is not expressed in colon cancer tissues and cell lines [8]. Subsequently, several studies reported the SLC5A8 downregulation at transcription level in other cancer types such as glia, thyroid, pancreas, and prostate, which have been associated with the repression of SLC5A8 promoter by methylation [2, 9, 10, 11, 12].
The lost expression and nuclear trans localization of SLC5A8 have been associated with poor survival and worse prognosis in pancreatic cancer patients, so the determination of protein levels of SLC5A8 could be used as a biomarker of the malignity level [13]. In lung cancer, a silenced SLC5A8 expression is also associated with poor survival, and it was demonstrated that Tobacco smoking upregulated the grade of methylation of the SLC5A8 gene promoter and switched off its expression [14].
On the other hand, worldwide, cervical cancer is the second cause of mortality and incidence only below breast cancer; in 2020, it was estimated that 13,800 new cases and 4290 deaths were caused by this type of cancer in the United States. Currently, the strategy for prevention includes the vaccination against HPV and monitoring for the detection of human papillomavirus in Papanicolaou smears; however, access to the vaccine is limited in developing countries, and the benefits of vaccination will be assessed in the coming decades [15, 16, 17].
Previously, we demonstrated for the first time the silencing of SLC5A8 expression in cervical cancer tissues and cell lines; we showed that repression of SLC5A8 gene expression is due to hypermethylation on the CpG Islands of the promoter [18]. In the present work, we also characterized the SLC5A8 function as a tumor suppressor protein in cervical cancer by in vitro and in vivo analysis.
The cell line HeLa was used for SLC5A8 expression assays and was obtained from
the American Type Culture Collection (Cat. No. CCL-2, Manassas, VA, USA). This
cell line was validated by Short Tandem Repeat (STR) profiling and tested
negative for mycoplasma. Cells between the 60th and 90th passage were cultured at
36.5 °C in cell culture flasks/plates in a humidified atmosphere with
5% CO
HeLa cells at 70% confluence were transfected with 1 µg of plasmid pCMV6-SLC5A8-GFP (RG217620, Origene; Rockville, MD, USA) or pCMV6-GFP (PS100010, Origene, Rockville, MD, USA) using Lipofectamine® 2000 (Cat. No. 11668019; Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the manufacturer’s instructions. 48 hrs after transfection, cells were incubated with G418 800 mg/mL (11811023; Invitrogen Corporation; Carlsbad, CA, USA) to select the transfected cell population. After a month, the transfected cell culture was enriched by FACS. The culture was maintained in the selection antibiotic (G418) for 2 more months, at the end of which the different experiments were performed. Stably transfected cells were designated as HeLa SLC5A8-GFP or HeLa-GFP.
HeLa cells were fixed with 100% methanol at –20 °C for 20 min and then blocked with Bovine serum albumin (BSA) 2%; the remaining protocol was performed following a previously described protocol [19, 20]. Slides were mounted with Vecta shield with DAPI (cat. no. H1200; Vector Laboratories, Inc; Maravai Life Science, Newark, CA, USA). Images were captured Using a confocal microscope (Leica TG5 SP8; Leica Microsystems GmbH, Wetzlar, Germany) at 63X objective.
2000 cells/well were cultured in 96-well plates and incubated at 37 °C for the indicated time. Then, the cell counting kit-8 (CCK-8) assay (ab228554, Abcam, Cambridge, UK) was performed according to the manufacturer’s instructions. A measure of 10 mL of WST-8 reagent was added per 100 mL cell media and incubated for 2 hrs at 37 °C. Absorbance was then measured at 450 nm.
5.0
Confluent monolayers grown on 6 well plates. After 2 hrs, the media was retired,
and the monolayers were wounded with a pipette tip. Then, the monolayers were
washed twice with PBS and incubated with DMEM; the wound healing was monitored at
different times, images were captured at 40
6
All the statistical analyses were performed using GraphPad Prism v8.0 (GRAPH PAD
Software Inc., Los Angeles, CA, USA), and the results are presented as the mean
Studies were done in cervical cancer HeLa cells to analyze the effect of the SCL5A8 transporter as a possible tumor suppressor protein. Previously, we analyzed the expression of the SLC5A8 gene in several cervical cancer cell lines, showing that the HeLa cell line does not express this gene and that this negative regulation was due to promoter hypermethylation, which is reversed by treating these cells with 5-Azacytidine [18].
Therefore, we generated a stable transfected HeLa cell line to explore the role of SCL5A8 as a tumor suppressor protein in cervical cancer. Once we obtained the stable cell line with the expression of SLC5A8-GFP, we analyzed the subcellular localization of the SLC5A8 protein by confocal microscopy. We observed the localization of SLC5A8 in the plasma membrane, apparently in the cell-cell junctions, a site in which it normally exerts its function (Fig. 1); this location of SLC5A8 was previously described in Madin-Darby canine kidney (MDCK) cells [21]. In addition, we observed the SLC5A8 protein localized close to the cell nucleus; this localization is like that of proteins found in the Golgi apparatus and used in the cellular secretion system; however, further studies are necessary to verify this assumption. These results motivated us to continue investigating the effect of the ectopic expression of SLC5A8 on different cellular functions, such as the proliferation in the HeLa cell line.
 Fig. 1.
Fig. 1.Ectopic expression and localization of SLC5A8 protein in HeLa cell line. Ectopic expression of SLC5A8 shows localization at the plasma membrane and the cell-cell junction. HeLa cells were stably transfected with SLC5A8-GFP plasmid or empty vector. The immunofluorescence was mounted and analyzed by confocal microscopy. Images show the localization of the transfected protein, SLC5A8-GFP (green), located in the plasma membrane (arrow), cell-cell junctions (asterisk) accumulated near the nucleus (arrowheads), and the nucleus was stained with Vecta Shield DAPI API (blue).
Next, we analyzed the effect of SLC5A8 overexpression over HeLa cell proliferation by comparing cells transfected with SLC5A8-GFP or empty vector for 6 days using the Cell Counting Kit-8 (CCK8) protocol. We did not observe significant differences on the first two days, but on the third day, the cells overexpressing SLC5A8 showed lower proliferation than the control cells (Fig. 2). Moreover, this difference was maintained until the last day of the experiment, where control cells (transfected with empty vector) proliferated twice more than cells transfected with SLC5A8. Since the decrease in cell proliferation may be due to several factors, we decided to investigate the cause, so we first performed cell cycle characterization assays using propidium iodide.
 Fig. 2.
Fig. 2.The expression of SCL5A8 inhibits HeLa cell proliferation.
Proliferation analysis was performed with the CCK8 assay using a 96-well. 2000
cells/well were seeded with HeLa SLC5A8-GFP or HeLa-GFP, analyzed for 6 days, and
measured at 450 nm. Each point represents the mean
After we observed a decrease in cell proliferation in HeLa cells transfected with SLC5A8-GFP, we decided to analyze whether this effect is due to cell cycle arrest. By analyzing the cell cycle in HeLa SLC5A8-GFP cells, we observed an accumulation in the G1 phase and decreased entry into mitosis (Fig. 3A). This is a hallmark of cell cycle arrest since we observed the SLC5A8 protein in the plasma membrane. Therefore, we assumed that it could be functional, so we analyzed whether treating these cells with pyruvate (Fig. 3B), a substrate of this transporter, and it has been reported that pyruvate decreased proliferation [22]. We found that pyruvate treatment increased cell arrest in the G1 phase and induced accumulation in the S phase, decreasing mitosis phase entry of HeLa SLC5A8-GFP cells compared with cells without treatment.
 Fig. 3.
Fig. 3.Cell cycle profiles in HeLa cells with over-expression of
SLC5A8. 500 thousand cells/wells were seeded and treated (A) or not with
pyruvate 1 mM (B). After 24 hrs, the cell cycle profile was made with propidium
iodide and analyzed by cytometry (Beckton Dickinson FACS Calibur). For each
analysis, 10,000 events were evaluated. The data were analyzed with the Modfit
software. **** p
Another critical parameter in the function of a protein as a tumor suppressor is the inhibition of the migration capacity of cancer cells, which is a step before invasion. Therefore, we performed wound healing assays to evaluate the role of SLC5A8 in cell motility. Interestingly, we observed that cancer cells expressing the SLC5A8 transporter migrate less than control cells, which at 72 hrs have already completely closed the wound (Fig. 4A,B). Based on the results indicating that SLC5A8 inhibits proliferation, produces cell cycle arrest, and inhibits migration of HeLa cancer cells, we decided to take the next step in characterizing this protein as a potential tumor suppressor in an in vivo model.
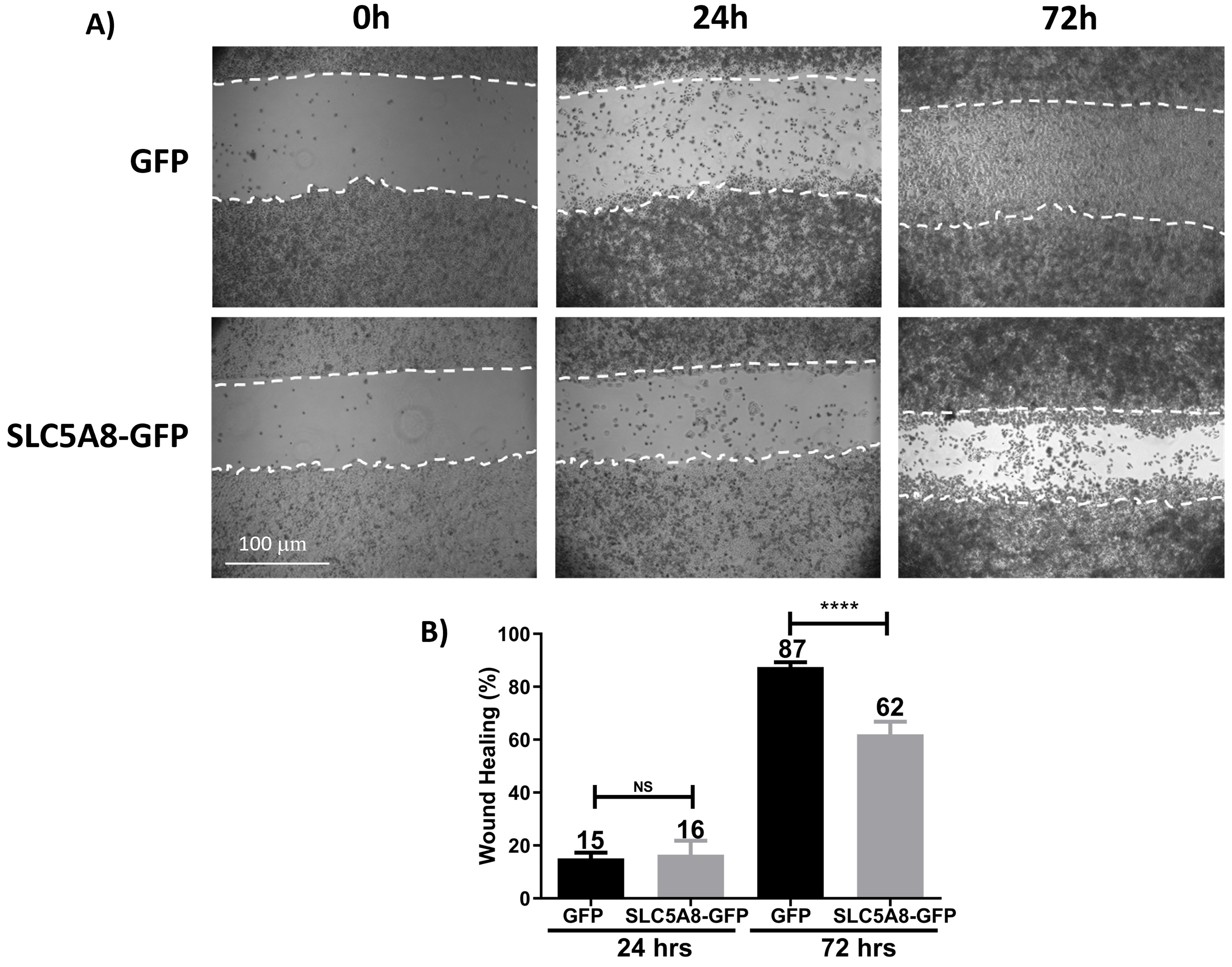 Fig. 4.
Fig. 4.SLC5A8 overexpression decreased migrated cells at 72 hrs in HeLa
(about 25%). (A) Representative images of contrast microscopy at different
times of the analysis. (B) Quantification and statistical analysis of the
percentage of wound healing at 24 and 72 hrs. The cells were seeded at 100%
confluence; then, a wound healing assay was performed and monitored at different
times, taking the image of the wound at time zero as control of each condition,
and the closure percentage was calculated. **** p
Since our results indicate that SLC5A8 inhibits proliferation, induces cell cycle arrest, and inhibits migration of HeLa cancer cells, we decided to take the next step in characterizing this protein as a potential tumor suppressor in an in vivo model. We analyzed the effect of the SLC5A8 transporter on the growth of cervical cancer tumors in a mouse xenograft model. HeLa cells transfected with the empty vector (control) or HeLa cells transfected with SLC5A8-GFP were used to induce tumor growth in Nu/Nu mice. Tumor growth was monitored for 7 weeks; we observed that HeLa SCL5A8-GFP tumors grew slower than the control after 20 days; the growth of SLC5A8 tumors was 2-fold lower and became even more significant at 35 days, where, on average, it was 3-fold (Fig. 5A). Furthermore, once the planned time for this experiment was completed, the mice were sacrificed, and the tumors were removed; the weight of the tumors formed with HeLa SLC5A8 cells was even 2-fold lower than the control (Fig. 5B,C).
 Fig. 5.
Fig. 5.SLC5A8 decreased tumor growth. Tumor growth was induced with
HeLa-SLC5A8 or HeLa-GFP cells; (A) Quantification and comparison of tumor volume
concerning the time from induction to the end of the experiment (35 days). (B)
Representative image of the dissected tumors at the end of the experimental
period. (C) Tumor weight quantification and comparative statistical analysis. 6
million cells were injected subcutaneously into the back of male Nu/Nu mice in
the right flank cells without SLC5A8 (control), and on the left side, the cells
with SLC5A8, the growth of the tumors was monitored for 7 weeks in which they
were measured. The volume was calculated using the formula V = (Width (2)
In previous work, we demonstrated the transcriptional inactivation of SLC5A8 by epigenetic silencing in cervical cancer tumors and cell lines, specifically by methylation in the gene promoter [18]. In the present study, we focus on characterizing the role of SCL5A8 as a tumor suppressor in cervical cancer. We decided to use the HeLa cell line as a model because, in our previous study, we observed that SLC5A8 is not expressed in these cells, making them a suitable model for investigating the effects of SLC5A8 ectopic expression; also, the HeLa cell line is positive for human papillomavirus 18, a high-risk HPV type and one of the most frequent HPV types in cervical cancer together with HPV16 [23].
The transfection of SLC5A8 on HeLa affected cell viability (data no show), which is consistent with a previous study in liver cancer cell lines, in which ectopic expression of SLC5A8 was evaluated, resulting in decreased cell viability [24]. We also analyzed the expression and localization of SLC5A8 by confocal microscopy. The ectopic expression of SLC5A8 in HeLa cells localizes to the cell membrane similar to the previously reported in MDA-MB-231 breast cancer cells transfected with SLC5A8 and in the non-cancerous cell line, MDCK, a model polarized cell line [21] suggesting that it is a functional protein [25]. Notably, the apical localization of the transporter is essential for tissues in the physiological uptake of the substrate from the luminal space [6]. It is related to preventing lactate loss into the urine at the proximal end portion of the kidney [26], a monocarboxylate used as energy in a wide type of cells, such as neurons, which is the main energy source [26].
On the other hand, we observed the localization of SLC5A8 apparently in the junctions between two neighboring cells, as previously reported in a monolayer of non-cancerous epithelial cells such as MDCK [21], which is surprising because the HeLa cells are characterized by loss of epithelial cell polarity and alterations in intercellular adhesion [27]. Therefore, the localization of SLC5A8 in the plasma membrane on HeLa cells, delimiting the cell’s perimeter in a polygonal shape [27], could contribute to its activity as a tumor suppressor, possibly participating in the cell junction. In this sense, the protein-protein interaction between SLC5A8 and PDZK1. PDZK1 is a tight junction protein previously observed at the plasma membrane and has recently been shown to bind SLC5A8 at this location. The SLC5A8-PDZK1 interaction plays an important role in the filtration and reabsorption of lactate and the reabsorption of urate in the human kidney, both indispensable substrates for the organism, so it is of utmost importance that the SLC5A8 protein localizes to this cellular portion [28]. As mentioned, we also observed localization of SLC5A8 near the nucleus, apparently in the Golgi apparatus, as has been observed for the SLC35D2 transporter [29]. This localization is typical on proteins that use the secretory pathway to access the plasma membrane as a final destination, suggesting again that SLC5A8 could be functional as in normal cells; however, further studies will be necessary to confirm and characterize the Golgi localization of SLC5A8 in transfected HeLa cells [30, 31, 32].
Since the main objective of our study was to evaluate SLC5A8 as a probable tumor suppressor in cervical cancer, the first feature aimed to address this question was the evaluation of proliferation. We demonstrated that SLC5A8 inhibits cell proliferation, even for a short period (72 hrs). It is worth mentioning that this result is similar to that observed in a hepatocarcinoma cell line [24]. We also observed that the cells were not able to recover their normal proliferation rate since this effect was more noticeable at the end of the analysis at 6 days compared to the control cells. This is a relevant result because the HPV type of the HeLa cells (HPV 18) is observed in more aggressive and faster-evolving cervical cancer [33]. This proliferation-decreasing effect resembles that of overexpression of the tumor suppressor protein MAGI1 in glioma cancer cell lines, a very aggressive cancer. In addition, it should be highlighted that the MAGI1 protein is localized in the tight junctions of well-polarized cells, where it is part of the tight junctions. As mentioned before, SLC5A8 is also localized in the cell-cell junction protein complex. The canonical function of MAGI1 is limiting the diffusion of molecules in the paracellular space and as an anchoring protein to the actin cytoskeleton. Its function as a tumor suppressor protein has been taken with great interest; likewise, we can now observe that the SLC5A8 protein, as well as MAGI1, has a role in decreasing proliferation in the HeLa cervical cancer line [34]. For proliferation analysis we used an assay that assesses Formazan formation. This approach has also been used to characterize other tumor suppressors in cervical cancer using HeLa cells, such as the RASSF1A protein, a member of the GTPase family, which also decreases cell proliferation when expressed ectopically [35].
It is widely known that proliferation is controlled by different mechanisms related to cell cycle regulation; for this reason, we analyzed whether SLC5A8 affects the cell cycle in HeLa cells, and we showed a cell cycle arrest in this cell line, specifically in the G1 phase. Since the ectopic expression of the SLC5A8 protein was in the plasma membrane, we assumed it could be functional. Therefore, we analyzed the effect of SLC5A8 overexpression in combination with pyruvate treatment, which is a substrate of this transporter. As expected, pyruvate further increased the effect of cell cycle arrest, confirming that the transporter is functional for the uptake of this molecule. Furthermore, in addition to increasing cell arrest in the G1 phase, S phase arrest was achieved, further decreasing the entry into the mitosis phase of HeLa SLC5A8-GFP cells. The importance of this effect is highlighted by the fact that pyruvate is an inhibitor of HDACs, which are proteins that are elevated in several types of cancer compared to normal tissue [36]. So, this effect strengthens the idea that SLC5A8 could be a tumor suppressor in cervical cancer. A fact worth mentioning is that pyruvate had an effect at a concentration 5-fold lower than the treatments previously used in this cell line without SLC5A8 overexpression (5 mM versus 1 mM) [22]. This effect on the cell cycle is characteristic of the control of the G1/S checkpoint, as occurs with other tumor suppressors in HeLa cells, in addition to the fact that this effect is almost always related to the activation of apoptosis, as occurs with the effect of miRNA miR-34a, miR-449a and miR-16 [37].
We also evaluated the effect of overexpression of SLC5A8 on the capacity of cell migration in the HeLa cell line. The cells expressing the SLC5A8 transporter migrate less than control cells, which can completely close the wound at 72 h. A previous study showed similar results when transfected SLC5A8 in liver cancer cell lines [24]. Currently, the SCL5A8 protein has only been performed on in vitro models of different cancer cells; based on our results also obtained in vitro, we went a step further in the characterization of this protein as a potential tumor suppressor in an in vivo model.
It is worth highlighting the importance of confirming in an in vivo model the observations in cell cultures, even more so in the context of tumor development that requires an environment rich in nutrients, oxygen, and other very particular conditions now widely known as the tumor microenvironment. In this sense, we analyzed the effect of the SLC5A8 transporter in an in vivo tumor xenograft model. SLC5A8 overexpression significantly decreases the capacity to form tumors in the HeLa cell line. This result is also similar to that observed when MAGI1 is overexpressed in colorectal cancer cells; in other words, SLC5A8 behaves like MAGI1, which, as previously mentioned, has already been characterized as a tumor suppressor in glial and colorectal cancer, and which incidentally MAGI1 also decreases its expression in high-risk HPV positive cell lines, since it is targeted for degradation by the viral E6 oncoprotein present in the HeLa cell line [38]. On the other hand, SLC5A8, whose function is instead that of a solute transporter and not as such a tumor suppressor; however, all our findings highlight the idea of the multiple functions that a protein can exert in different cellular mechanisms [39]. Thus, by considerably decreasing the size of tumors with only the ectopic expression of the SLC5A8 transporter, we highlight the importance of the correct function of this protein, taking into account that the development of cancer involves the sum of more than one genetic alteration [40].
Moreover, we observed that tumors formed with HeLa cells without SLC5A8 expression apparently have higher formation of new blood vessels; this could explain in part why these cells grow more than HeLa cells overexpressing SLC5A8, suggesting that SLC5A8 might affect angiogenesis; a process necessary for the nutritional maintenance of the tumor, which requires an ample supply of nutrients such as glucose to grow [41]. However, further experiments and analysis are needed to confirm this observation.
In summary, we conclude that the SLC5A8 protein is a tumor suppressor protein in cervical cancer; this opens a new panorama in the study of this type of cancer and in the design of new treatment strategies, such as the use of therapies to restore the expression of this important transporter.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conceptualization, JDC, PG, LAH and OVS; Formal analysis, OVS, JHJ, PYUU and GDG; Sample management, OVS, JHJ, PYUU and GDG; Writing-original draft preparation, JDC, PG, LAH, OVS; Writing review and editing, JDC, PG, LAH, OVS, JHJ, PYUU and GDG; Project administration, JDC and PG; Funding acquisition, JDC and LAH. All authors have read and agreed to the published version of the manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The animal study protocol was approved by the Research Unit for Laboratory Animal Care Committee (UPEAL-Cinvestav-IPN, México; NOM-062-ZOO-1999).
Mexican Council of Science and Technology (CONACYT), Mexiquense Council of Science and Technology (COMECyT), and The Mexican Center for Cultural and Social Studies (CEMECUS A.C.).
This research was funded by the Mexican Council of Science and Technology (CONACYT); Orlando Vargas-Sierra, recipient of doctoral fellowships from CONACYT (243103), also received from a fellowship of Mexiquense Council of Science and Technology (COMECyT) (16BEPD0021-11) and doctoral fellowships from The Mexican Center for Cultural and Social Studies (CEMECUS A.C.), RICARDO J. ZEVADA FUND 2016.
The authors declare no conflict of interest. Given his role as Guest Editor, José Díaz-Chávez had no involvement in the peer-review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Graham Pawelec.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
