1 Department of Pathophysiology, Chengde Medical University, 067000 Chengde, Hebei, China
Abstract
Objective: The present study aims to investigate the effect of Huaier
on oxaliplatin (OXA) resistance in HCT-8 colorectal cancer (CRC) cells.
Methods: Oxaliplatin-resistant HCT-8/L CRC cells were used. The Cell
Counting Kit-8, western blotting, quantitative real-time polymerase chain
reaction, protein extraction kit, immunofluorescence and acridine orange staining
assays were used in the study. The experiment results proved that Huaier has an
influence on the Wnt/
Keywords
- Huaier
- drug resistance
- colorectal cancer
- Wnt/β-catenin
- autophagy
Colorectal cancer (CRC) is one of the major malignant tumours occurring in humans. It has a high morbidity and mortality worldwide [1], and its incidence in China is increasing annually [2]. The drugs used for CRC chemotherapy mainly include 5-fluorouracil, irinotecan and oxaliplatin (OXA) [3]; OXA is a third-generation platinum drug and a standard drug for CRC treatment [4]. However, chemotherapy resistance in CRC has become one of the reasons for treatment failure. Therefore, the causes of chemoresistance failure in CRC need to be explored.
The underlying mechanisms of tumour multidrug resistance are complex and remain unclear [5]. ABCB1, also known as p-gp [6]. Various chemotherapeutic drugs can be expelled from cells by binding to different p-gp sites, resulting in cell resistance [7]. Therefore, by inhibiting p-gp expression, chemotherapeutic drugs in tumour cells cannot be released, which can reverse the cells’ drug resistance.
Huaier is a fungus that grows on the trunks of damp locust trees. Its main
components are proteoglycans [8], and studies have demonstrated that it has an
inhibitory effect on tumour cells. The anticancer mechanism of ear may be
associated with a variety of biological activities, such as inhibition of cell
proliferation, anti-metastasis, interference with tumour angiogenesis and
tumour-specific immunomodulatory effects [9]. Specific mechanisms, such as
Huaier, can inhibit cutaneous squamous cell carcinoma cells by regulating the DNA
methylation of CDKN2A and TP53 [10]. Huaier can also affect the process of tumour
cells through the NF-
Huaier was provided by Gaitianli Pharmaceutical (Qidong, Jiangsu, China); the Huaier grain was dissolved in RPMI-1640 medium (Procell Company, Wuhan, China) and sterilised with a 0.22 µm filter (Merck Millipore, Darmstadt, Germany).
The Wnt agonist 1 was purchased from the MedChemExpress Company (Monmouth Junction, NJ, USA), and chloroquine (CQ) was obtained from the Sigma Company (St. Louis, MO, USA).
The HCT8 cells were purchased from the Procell Company, and the resistant cells
of HCT8/L were purchased from the Aolu Biological Company (Shanghai, China); all
cell lines were cultured in Dulbecco’s-Modified-Eagle-Medium-High Glucose
supplemented with 10% fetal bovine serum (FBS, Procell Company) and 100 mg/mL of
penicillin/streptomycin/glutamine (Procell Company) at 37 °C with 5% CO
The CCK-8 assay was employed to detect cell viability using a Cell Counting
Kit-8 (MCE, NJ, USA). Specifically, cells at the logarithmic stage were digested
with trypsin and diluted to 1
The cells were seeded in 24-well plates. After 24 h, the culture medium was discarded, and a sensitive strain control group, a drug-resistant strain control group and a drug-resistant strain plus different concentrations of Huaier were cultured for 24 h. Next, the cells were repeatedly washed with phosphate-buffered saline (PBS), added with a 500 µL acridine orange (AO) mixed solution in each well, stained for 15 min, washed twice with PBS and immediately analysed using a fluorescence microscope (Olympus, Tokyo, Japan). The proportion of positive AO staining was determined by counting the number of fluorescent cells.
The cells were seeded on coverslips. After treatment with Huaier or 10%
complete medium, the cells were fixed with 4% paraformaldehyde; 5% bovine serum
albumin (BSA, Solarbio, Beijing, China) was used to block for 1 h, and LC3-B
antibody (Abclonal, Woburn, MA, USA) was applied for incubation overnight at 4 °C.
Next, the cells were incubated with Alexa Fluor 488 (Bioss, Beijing, China) for 1
h and stained with 4
The cells were digested with trypsin and diluted in 6-well plates containing
10% complete medium. Next, the cells were cultured in a 5% CO
Prior to use, phenylmethylsulfonyl fluoride was added to the cytoplasmic protein extraction reagent A and the nuclear protein extraction reagent at a working concentration of 1 mM. The PBS-washed cells were used 2–3 times, then scraped, blown with a pipette gun and transferred to a centrifuge tube for centrifugation; the supernatant was discarded every 20 µL. For precipitation, cytoplasmic protein extraction reagent A containing PMSF was added, shaken vigorously for 5 s, and soaked in ice for 15 min. Then, 10 µL cell protein extraction reagent B was added into the precipitate, shaken well for 5 s and soaked in ice for 1 min before vigorous shaking again for 5 s at 4 °C. Subsequently, the mixture was centrifuged for 5 min at 15,000 g. A pipette gun was used to transfer the extracted cytoplasmic protein supernatant to a new Eppendorf (EP) tube for analysis. A nuclear protein extraction reagent was added to the remaining precipitate and shaken vigorously for 5 s to completely disperse the cell precipitate. Under the condition of an ice bath, it was shaken forcefully for 30 s/2 min for a total of 30 min and then centrifuged for 10 min at 4 °C and 15,000 g. The extracted supernatant of the nuclear protein was transferred to a new precooled EP tube with a pipette gun for standby.
Cells were lysed using radio-immunoprecipitation assay buffer (Solarbio) to
collect proteins. The BCA protein assay kit (Solarbio) was used determined the
protein concentration, and the proteins were subsequently separated using sodium
dodecyl sulphate-polyacrylamide gel electrophoresis. The proteins were
transferred onto polyvinylidene fluoride membranes, 5% BSA was used to block the
membranes for 2 h, and the mixture was incubated with primary antibody to
p-gp (1:1000, CST, MA, USA), LC3B (1:2000, Abclonal), P62 (1:2000,
Abclonal), Wnt 3a (1:1000, Bioss),
All data were shown as mean
A comparative plot showed the cell inhibition rates of HCT-8 and HCT-8/L strains
in response to different concentrations of OXA. As shown in the
Supplementary Fig. 1, the IC
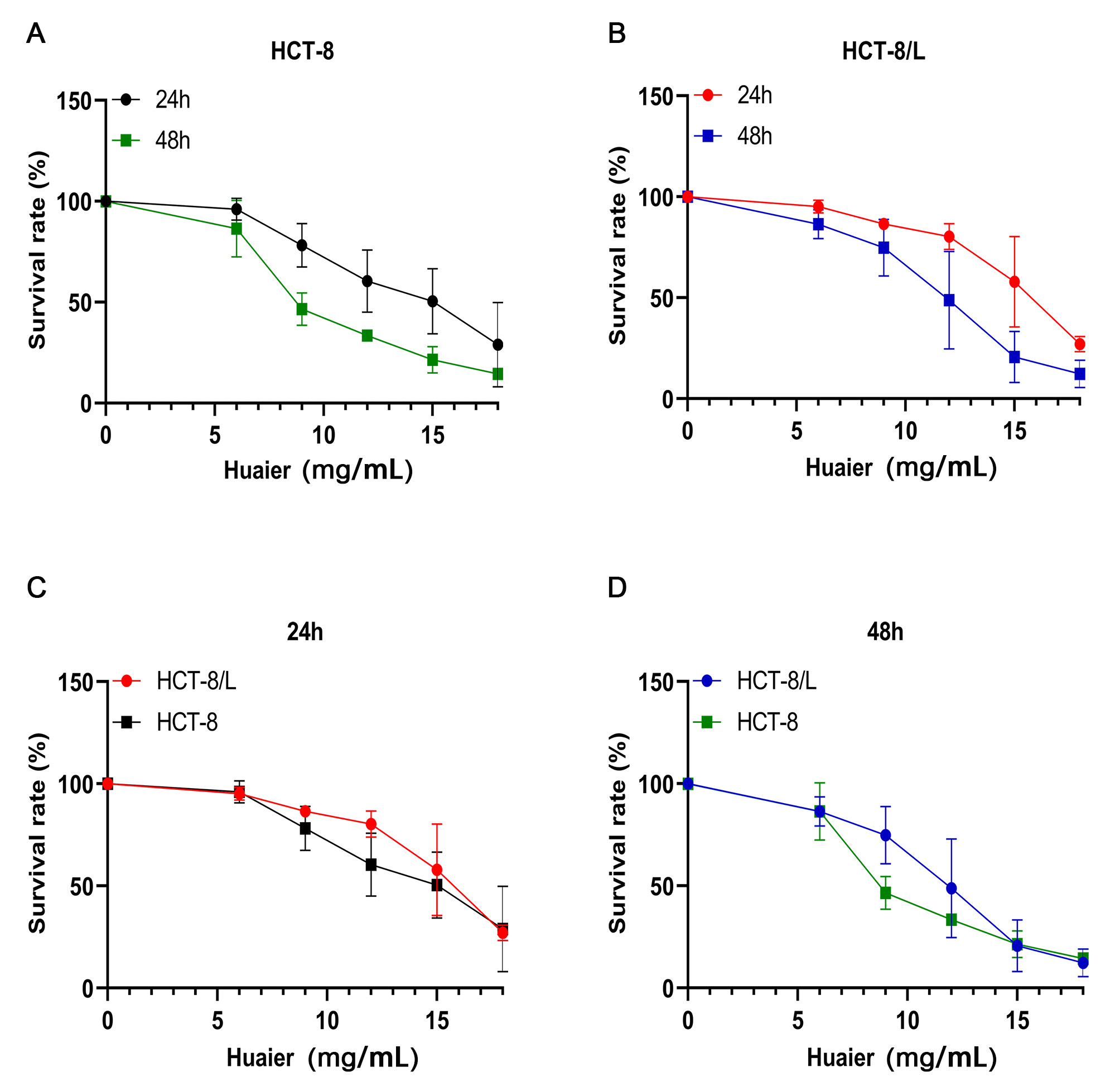 Fig. 1.
Fig. 1.Huaier reduced the viability of colorectal cancer (CRC) cells. (A) Effect
of Huaier on the activity of HCT-8 cells. (B) Effect of Huaier on the activity of
HCT-8/L cells. (C) Huaier treatment for 24 h. (D) Huaier treatment for 48 h. Data
means
Subsequently, qRT-PCR and western blotting (WB) were used to detect the effect of Huaier on the expression of resistance-associated protein p-gp in HCT-8/L cells (Fig. 2A,B). At the protein level, the expression level of p-gp in HCT-8/L cells decreased with increasing Huaier concentrations; the expression level of p-gp mRNA was significantly lower in HCT8 cells than in HCT8/L cells, whereas the expression of p-gp mRNA decreased with the increase in Huaier concentrations (Fig. 2C). The above results showed that Huaier could reverse the drug resistance of HCT-8/L cells by decreasing the p-gp expression.
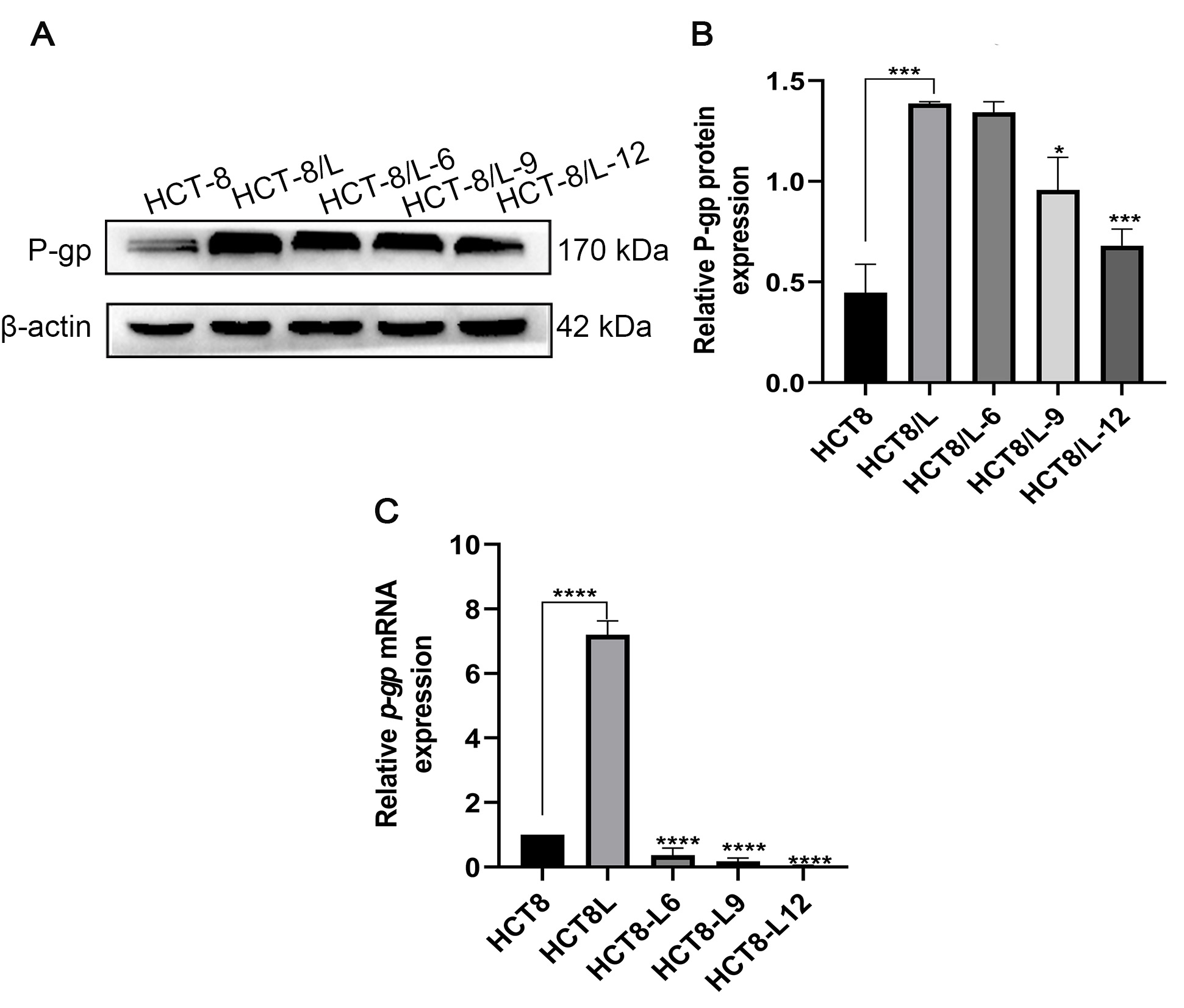 Fig. 2.
Fig. 2.Effect of Huaier on the expression of p-gp. (A)
Western blotting results of p-gp in HCT-8 and HCT-8/L cells under the
effect of different concentrations of Huaier. (B) Effect of Huaier on the
relative quantification protein expressions of p-gp. (C) On the gene
level, the change of p-gp and the change of the expression level of
p-gp in HCT-8/L cells under the effect of different concentrations of
Huaier. Data means
To investigate the effects of Huaier on autophagy, AO staining assay was performed. Acridine orange is a specific dye that can penetrate acidic organelles, such as autophagic lysosomes. Owing to its sensitivity to pH, AO emits red fluorescence when the pH is low. The AO staining results are presented in Fig. 3A,C. Compared with HCT-8/L cells, HCT-8 cells had more acidic vesicular organelles, which were stained red by AO. After treatment of HCT-8/L cells with different Huaier concentrations, the number of acid vesicular organelles stained red with AO increased significantly.
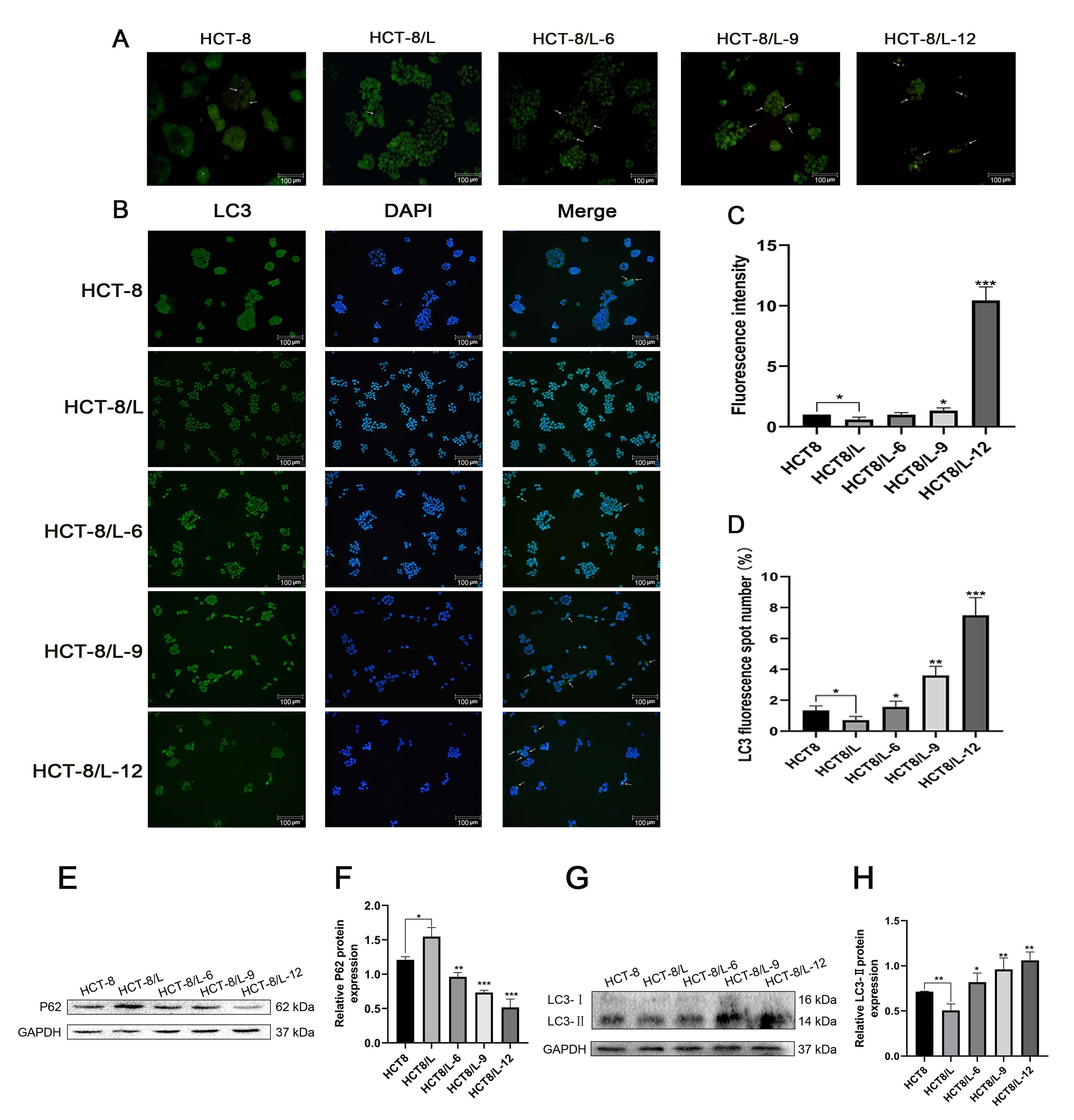 Fig. 3.
Fig. 3.Effect of Huaier on cell autophagy. (A,C) Acridine
orange staining was used to observe the changes of acidic vesicular organelles
induced by Huaier (white arrow: acidic vesicular organelles). (B,D) Detection of
Huaier-induced autophagy via immunofluorescence staining. (E,F) Western blotting
for P62 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) treated with Huaier.
(G,H) Western blotting for LC3-II and GAPDH in HCT-8 and HCT-8/L cells treated
with Huaier. Data means
The findings of the immunofluorescence staining assay further support the AO staining assay results. Although the HCT-8 cytoplasm exhibited LC3 green fluorescence, the staining intensity was weak. Meanwhile, in the Huaier group, the LC3 staining intensity increased with increasing Huaier concentrations (Fig. 3B,D).
Based on the above assays, the effect of Huaier on autophagy was further researched. After 24 h of treatment of HCT-8/L cells with Huaier, the P62 protein expression level significantly decreased, whereas the LC3-II protein expression significantly increased in each group. The expression of LC3-II was higher in the HCT-8 group than in the HCT-8/L group (Fig. 3G,H). However, the P62 protein expression was lower in the HCT-8 group than in the HCT-8/L group (Fig. 3E,F). The above results showed that Huaier can affect the viability of HCT-8/L cells through autophagy.
To determine whether Huaier affected the Wnt/
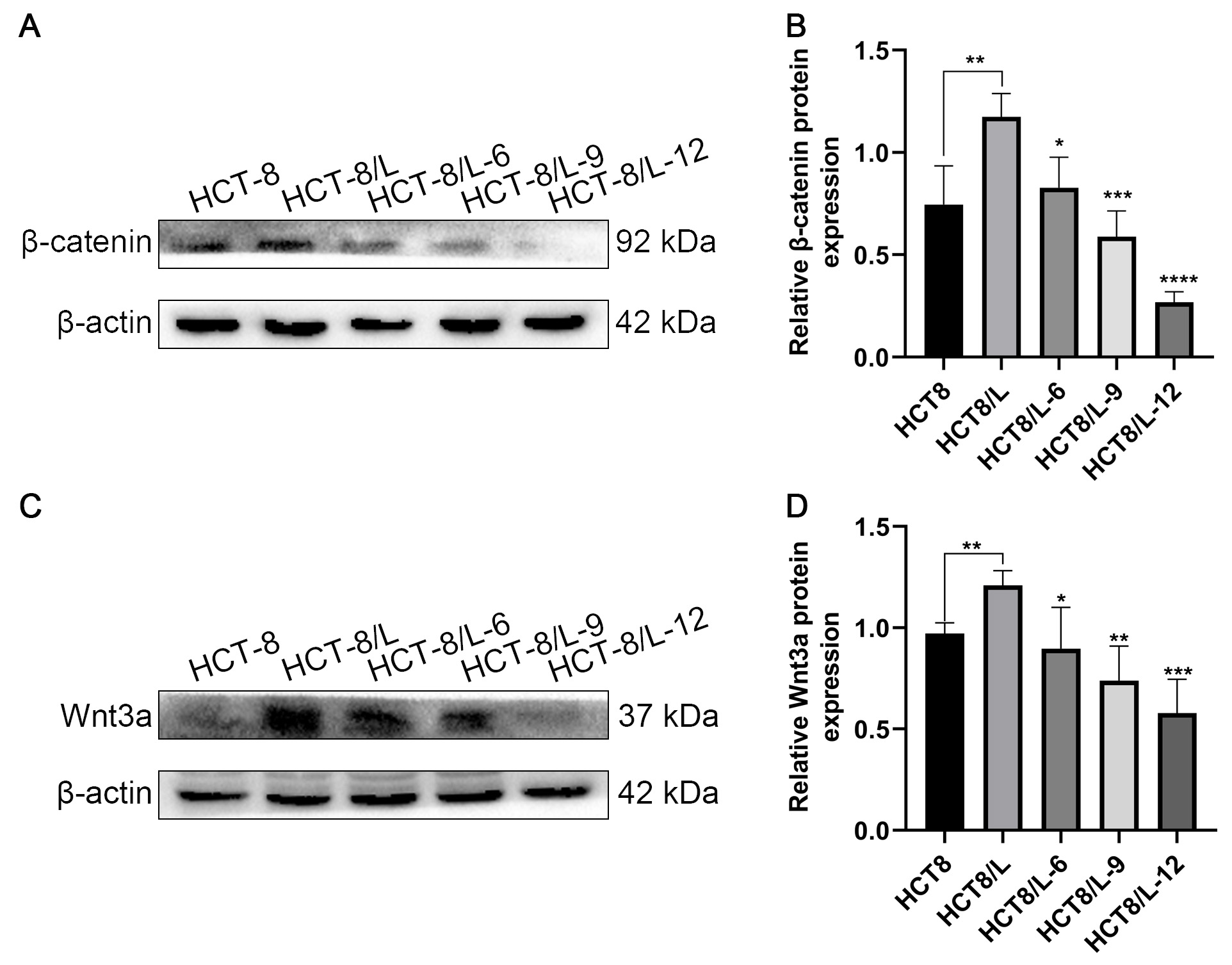 Fig. 4.
Fig. 4.Effect of Huaier on the Wnt/
In this study, the autophagy inhibitor CQ was used to further clarify whether Huaier leads to the transformation of cellular drug resistance by enhancing the degree of autophagy. Acridine orange and immunofluorescence staining assays were performed to determine the effects of Huaier and CQ on autophagy. Chloroquine treatment decreased autophagy in HCT-8/L cells, which was significantly increased in HCT-8/L cells after Huaier treatment; furthermore, when used alongside Huaier, it did not reduce its effect on autophagy in HCT-8/L cells (Fig. 5A–D). The effects of Huaier and CQ on autophagy and drug resistance were detected using WB (Fig. 5E–J). The results showed that Huaier treatment significantly enhanced autophagy. After addition of the autophagy inhibitor CQ, the amount of LC3-II increased. Some studies have indicated that an increase in LC3-II is related to the fusion of autophagosomes and lysosomes, and/or lysosome degradation [15]. As demonstrated in Fig. 5B,D, CQ significantly inhibited autophagy, and the combination of Huaier and CQ promoted autophagy. Huaier reduced the expression of p-gp, CQ enhanced the expression of p-gp, and the combination of Huaier and CQ reduced the expression of p-gp (Fig. 5K).
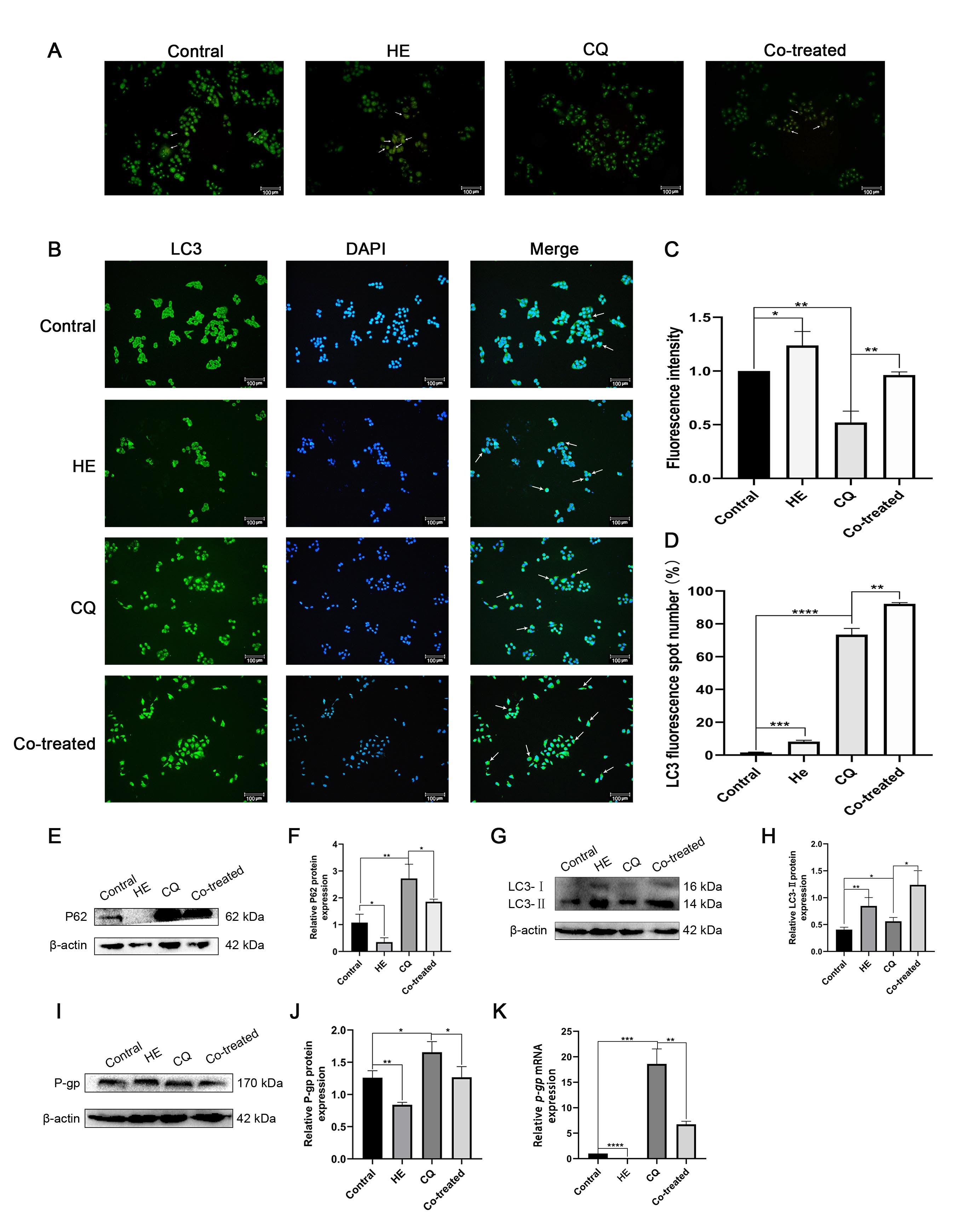 Fig. 5.
Fig. 5.Effect of Huaier and CQ on HCT-8/L. (A,C) Acridine
orange staining was used to observe the changes of acidic vesicular organelles in
HCT-8/L cells induced by Huaier and CQ. Control group. (B,D) Detection of Huaier
and CQ induced autophagy by immunofluorescence staining. (E,F) Western blotting
for P62 and
The Wnt agonist 1 was used in the present study to further elucidate that Huaier
affected autophagy through inhibiting the Wnt/
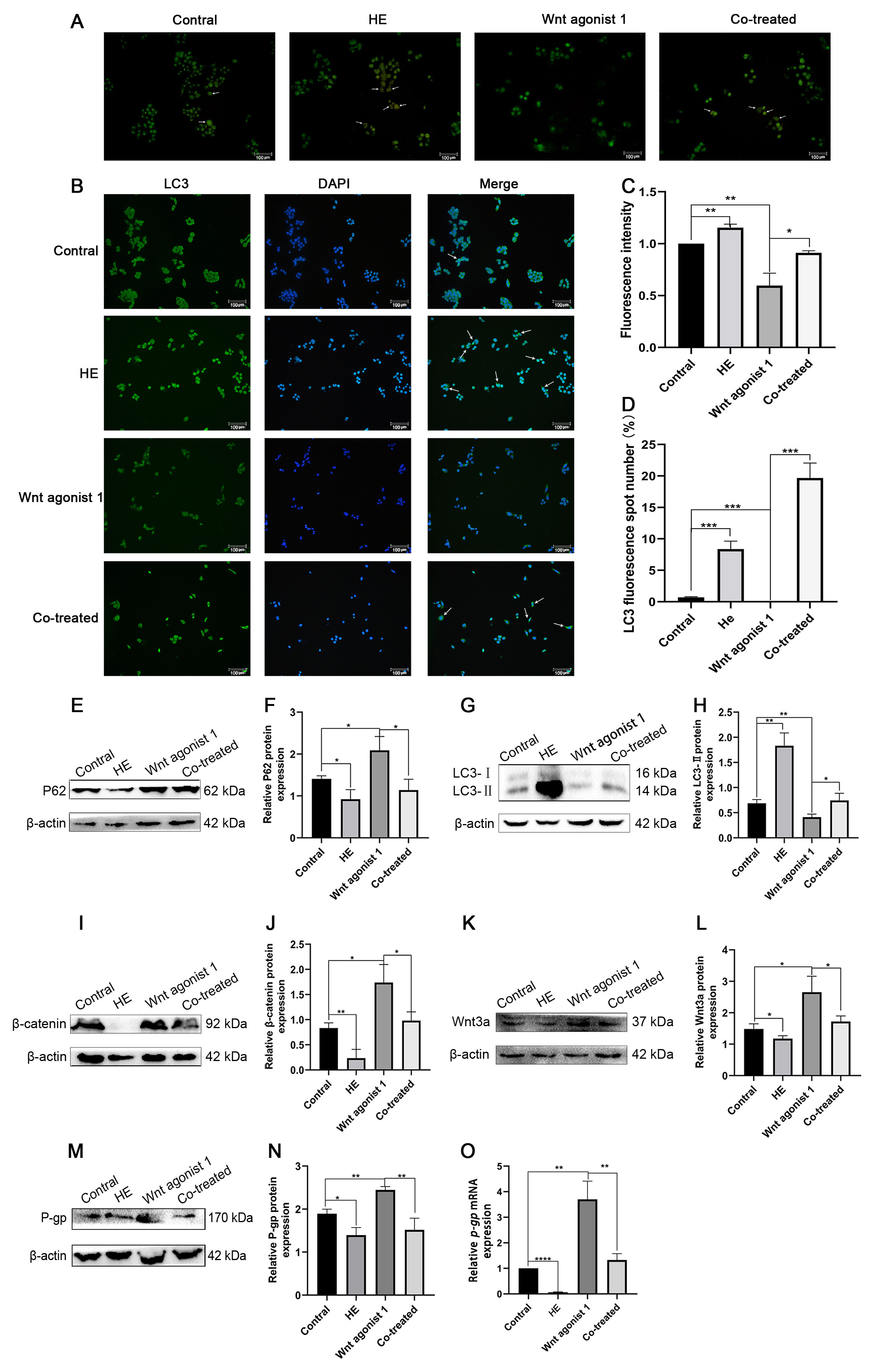 Fig. 6.
Fig. 6.Effect of Huaier and Wnt agonist 1 on HCT-8/L. (A,C)
AO staining was used to observe the changes of acidic vesicular organelles in
HCT-8/L cells induced by Huaier and Wnt agonist 1. (B,D) Detection of autophagy
induced by Huaier and Wnt agonist 1 via immunofluorescence staining. (E,F)
Western blotting for P62 and
The
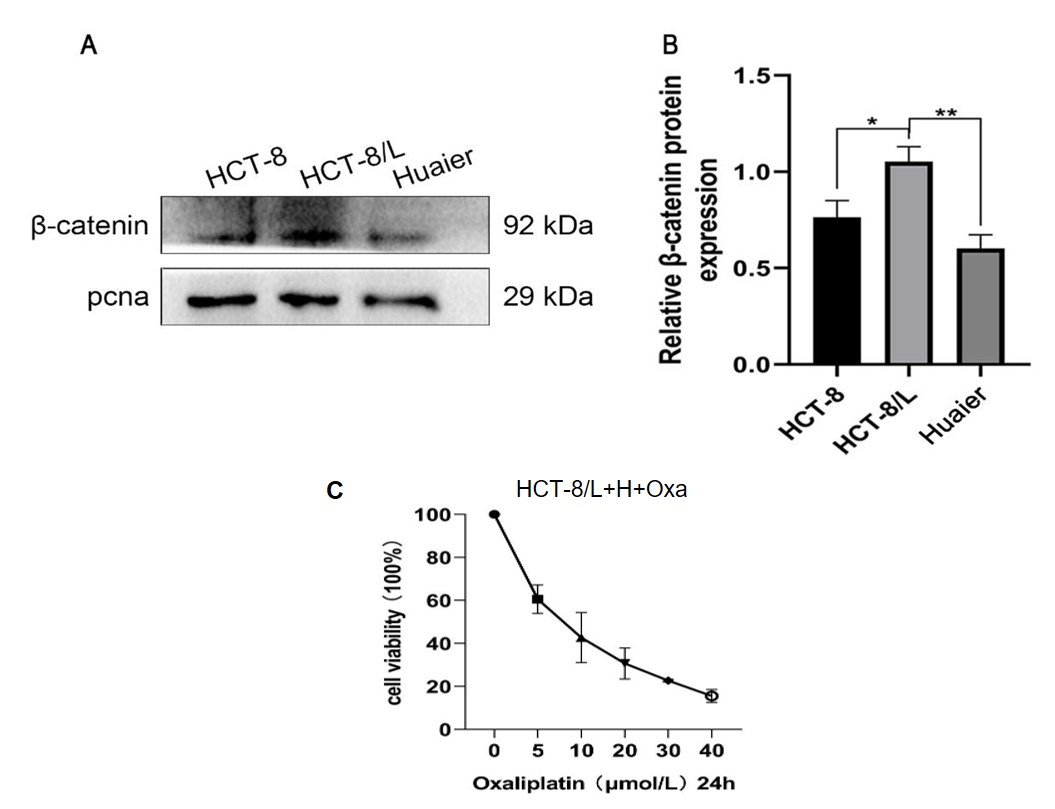 Fig. 7.
Fig. 7.Effect of Huaier on the entry of
Colorectal cancer is the third most common malignant tumour [1]. Currently, OXA is the first-line chemotherapy for its treatment, and patients with CRC also frequently develop OXA resistance. However, the molecular mechanism of OXA resistance is still unclear. At present, with the continuous increase in research on traditional Chinese medicine (TCM), more TCM methods have been reported to have the effects of treating cancer and reversing the drug resistance of tumours.
Huaier plays an anti-tumour role in the treatment of many cancers [12, 16], and
studies have indicated that Huaier inhibits tumour cell proliferation and
invasion [17]. Moreover, Huaier extract could prevent pancreatic cancer through
inhibition of the Wnt/
Wei et al. [19] reported HES as one of the effective anti-tumour components of Huaier, stating that it can induce apoptosis and autophagy in gastric cancer cells. At the same time, the authors found that mammalian target of rapamycin signalling and ERK signalling may be involved in the anti-gastric cancer effect of hydroxyethyl starch. Zhang et al. [20] reported that the use of Huaier in patients with sorafenib-resistant liver cancer significantly enhanced the sensitivity of liver cancer cells to sorafenib. Autophagy plays a two-way role in tumour development [21], and an increasing number of experiments demonstrate that autophagy is related to tumour cell apoptosis and multidrug resistance [22, 23]. The present study examined autophagy induced by Huaier through AO staining, immunofluorescence staining and WB. The results suggested that the level of autophagy increased with an increase in Huaier concentration; this was mainly indicated by the dark colour in AO staining, higher number of autophagic spots in IF staining, higher expression of LC3-Ⅱ and lower expression of P62 in WB.
Furthermore, it was discovered that under the effect of Huaier, the Wnt3a and
Zou et al. [25] found that Huaier inhibited DSS-induced intestinal
tumour production in a mouse model by inhibiting pro-inflammatory cytokine levels
and signal transducer and activator of transcription 3 stimulation. In this
study, autophagy inhibitors (CQ) and pathway agonists (Wnt agonist 1) were used
to test whether Huaier can reverse cell resistance by inhibiting the
Wnt/
However, there are still some limitations to the present study. Firstly, only
in vitro studies were performed, and to further confirm the experimental
results, subsequent in vivo studies will be performed on experimental
animals. Secondly, this study found that Huaier could affect colon cancer cells
by affecting the Wnt/
In conclusion, the present study proves that Huaier can regulate the level of
autophagy, inhibit the Wnt/
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conception and design of the work: LWW, XLY; Data collection: QF, MYH; Supervision: QF; Analysis and interpretation of the data: QF, MYH; Statistical analysis: JNZ; Drafting the manuscript: QF, MYH, JNZ; Critical revision of the manuscript: LWW, XLY; Approval of the final manuscript: all authors. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
Not applicable.
The present study was supported by the Project Funding of Hebei Provincial Education Department (grant no. ZD2020137), the Key Discipline Construction Project of Hebei Provincial Universities (grant no. Ji Jiao Gao-2013-(4)-2012-37) and basic scientific research business expenses of Chengde Medical College for outstanding students.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.







