- Academic Editor
†These authors contributed equally.
§These authors contributed equally.
During aging, the immune system (IS) undergoes remarkable changes known as immunosenescence, a multifactorial and dynamic phenomenon that affects both natural and acquired immunity and plays an important role in most chronic diseases in older people. Among the determinants of immunosenescence, we find a low-grade sterile chronic inflammation, known as “inflamm-aging”. This condition of chronic inflammation causes a progressive reduction in the ability to trigger antibody and cellular responses effective against infections and vaccinations. In this review, we wanted to explore the role of immunosenescence and inflamm-aging as determinants of the immunological aging process and predisposing viral infections phenomena, with a particular reference to cytomegalovirus (CMV), varicella zoster virus (VZV), influenza virus (IFV) diseases and SARS-CoV2. IS aging is also reflected in a reduction in the antibody response to vaccinations, hence there is a need to expand trials to elderly patients, in order to identify the most appropriate methods for developing effective and safe vaccination and preventive strategies.
Aging is one of the most difficult biological phenomena to understand, with the ability to influence the functions of many organs and systems, and represents the main risk factor for geriatric diseases [1]. The aging process could be considered a result of the dysregulation of different body systems leading to a decline from the normal (homeostatic) regulation level to an altered state of dyshomeostasis, a reflection of the adaptation of the organism to intrinsic and extrinsic stimuli to which it is subjected during aging [2, 3].
This phenomenon that we could define as “bioaging” is dominated by two distinct processes: immunosenescence and chronic inflammation, known as “inflamm-aging”, which places the human organism in the continuum of an altered state of bioreactivity [4]. The immune system (IS) is characterized, in older age, by a series of pathophysiological variations that go under the term “immunosenescence”, i.e., a multifactorial process that affects both natural and acquired immunity and which plays a fundamental pathogenetic in most of the chronic diseases of the elderly [5].
Inflamm-aging, a systemic state of chronic low-grade inflammation, is one of the hallmarks of immunosenescence; it is characterized by an overproduction of upregulated circulating inflammatory markers and is considered the central pillar of aging [6]. Accumulation of damaged macromolecules is responsible for inflammation and endogenous host cellular debris is the source of chronic tissue damage [7]. This condition of chronic inflammation causes a progressive reduction in the ability to trigger antibody and cellular responses, which are effective against infections and vaccinations [8].
Aging also affects the human hematopoietic system, resulting in a decrease in
bone marrow cellularity, a decline in the adaptive immune response, and an
increase in hematological disorders and malignancies. These changes observed in
hematopoietic stem cells play an important role in the generation of the T and B
cell repertoire. In the case of T cells, this is exacerbated by the involution of
the thymus and the decline of its function [9]. Specifically, in over 65
patients, a progressive decrease in the absolute values of CD4
Virgin T cells from older people also show several functional defects, including significantly shorter telomeres, a restricted receptor repertoire, and some decreased interleukin (IL)-2 production. As a result, their ability to mediate effective immune responses against new antigens diminishes [11].
This progressive loss of naïve T cell function is compensated in about 30%
of the elderly, with the expansion of CD8
Downregulation of CD28 expression due to chronic immune activation of human T cells is one of the signatures of replicative senescence and has been associated with impaired vaccine responses [13].
Changes in the bone marrow microenvironment that occur with aging (e.g., reduced cytokine IL-7) result in decreased pro-B cell survival and a tendency for hematopoietic stem cells (HSCs) to generate myeloid lineage cells rather than lymphoid [14].
Although there is a decrease in the genesis of B cells in older patients, the ability to synthesize and secrete antibodies is unaffected. The downregulation of the transcription factors XBP-1 and Blimp-1, and the upregulation of the transcription factor PAX-5 lead to a decrease in the number of IgM B-1 cells in this category of patients [15].
Frasca D et al. [16] showed that B cells stimulated with anti-CD40/IL-4 from old mice proliferate 2 times less than young controls, while B cells from old mice produce 6 times less IgG1 and 12 times less IgE in culture. Therefore, this evidence suggests that humoral immune responses are dramatically impaired in older subjects, which translates into a reduced response to anti-viral vaccinations.
Aging also has significant effects on all cells of the innate immune system. In elderly subjects, there is an impairment of multiple functions of neutrophils, for example, such as phagocytic capacity, the synthesis of reactive oxygen intermediates, and the efficiency of intracellular killing. Macrophages are also affected by the aging of the individual, causing a progressive loss of effectiveness of their immunological defense functions (e.g., phagocytosis, secretion of cytokines and chemokines, and presentation of the antigen) [17].
Natural Killer (NK) cells also demonstrate a significant susceptibility to aging, which results
in a defect of the cellular cytotoxic capacity as a consequence of a reduced
generation of inositol triphosphate (IP3). IL-2-induced IFN-
“Inflamm-aging” represents one of the main features of the aging process; it is understood as a progressive chronic increase in the low-grade proinflammatory state. Inflamm-aging is a determinant of the speed of the aging process and of the duration of life, and it is highly correlated to some chronic pathologies typical of advanced age such as Alzheimer’s disease, Parkinson’s disease, atherosclerosis, heart disease, type II diabetes, osteoporosis and insulin resistance, cancer and other diseases. Inflammatory aging also increases morbidity and mortality, significantly harming patients’ health and causing a decline in patients’ quality of life [19, 20].
Several possible pathogenetic mechanisms can lead to a condition of chronic inflammation, including the process of accumulation of macromolecules and damaged cells (auto-debris) auto-debris that occurs with aging due to increased production and/or inadequate elimination. Self-debris can mimic bacterial products and function as molecular patterns associated with endogenous “damage”, which activate innate immunity by activating a network of sensors (including the Nlrp3 inflammasome) that recognize them as “danger” signals and initiate immune reactions necessary for physiological repair [21].
Studies about oldest/old subjects have highlighted significant differences between their microbiota and that of younger patients, in particular a rearrangement in the Firmicutes population and an enrichment of facultative anaerobes, specifically pathogens, have been highlighted. The impairment of the microbiota in elderly patients is related to an increase in inflamm-aging, determined by markers such as IL-6 and IL-8. The microbiota of centenarians showed a marked decrease in Faecalibacterium prauznitzii and symbiotic species endowed with anti-inflammatory properties and an increase in Eubacterium limosum and its relatives [22].
In the immunological aging process, there is a mitochondria-mediated over-production of anti-inflammatory mediators such as mitokines (HN, FGF21, GDF15), thus allowing to preserve the balance between inflammatory and specific immune responses. Unsuccessful aging is characterized, however, by an imbalance between the inflammatory and anti-inflammatory immune responses, with a prevalence of the former and a consequent increase in the production of specific inflammation mediators such as reactive oxygen species (ROS) and associated molecular models to danger (DAMP), leading to an increase in procytokines [23, 24].
An increased number of senescent cells during the aging process contributes to chronic low-grade inflammation via the senescence-associated secretory phenotype, or SASP, which includes pro-inflammatory cytokines (e.g., IL-6, IL-1, HMGB1, S100), chemokines (e.g., IL-8, MCP-1), soluble receptors (for example, sTNFR), metalloproteases (e.g., collagenase), some protease inhibitors including SERPIN, and growth factors. Chronic inflammation can induce in turn telomere dysfunction, promoting cellular senescence. This highlights how inflammation and senescence reinforce each other in a sort of vicious circle [25, 26].
Immunosenescence contributes to increased susceptibility to viral infectious diseases, resulting in increased incidence of cytomegalovirus (CMV), varicella zoster virus (VZV), and influenza virus (IFV) diseases [27].
A prospective Canadian study of patients over 65 in 32 nursing homes found that
a high representation of CMV-reactive CD4
Persistent CMV infection has been hypothesized to trigger a pro-inflammatory
environment that enhances T cell differentiation. Indeed, CMV-induced
IFN-
With aging, there is a defect in aspects of the adaptive cellular and humoral response to CMV, resulting in an increased rate of viral replication in older individuals. CMV viral reactivation results in a systemic inflammatory condition, which may be responsible for increased morbidity and mortality in elderly patients with co-existing infections [33].
CMV seropositivity is not only associated with modified CD8
Elevated CMV antibody levels have been associated with an increase in the
cytokines IL-6 and TNF-
VZV reactivation increases during aging, predominantly manifesting in the skin
(shingles). In elderly patients, the number of circulating VZV-specific CD4
Burke BL et al. [38] conducted an in vivo study of the immune
function of elderly patients in response to VZ antigen and phytohaemagglutinin
(PHA) skin testing, mitogen (PHA)- and antigen (VZ)-induced lymphocyte
stimulation, and antibodies to VZ. The study demonstrated that the stimulation of
lymphocytes with PHA in vitro supported the hypothesis of a progressive
decline in reactivity with advancing age. In contrast to this decline in cellular
immune responses to VZ with aging, humoral immunity remains relatively intact. In
cases where the viral infection becomes chronic, i.e., a persistence of viral
antigens, there is typically a failure in the development of memory CD8
Shahbazi M et al. [42] highlighted that in patients with severe forms
of COVID-19, there is an increasing number of CD8
Age-related changes in adaptive immunity are also seen during influenza virus
infections. In fact, during aging, there is a progressive impairment of the
signaling capacity of the T cell receptor (TCR). CD4
The decline of B-cell precursors in old age is associated with the preferential loss of lymphoid-polarized hematopoietic stem cells. With aging, there is a decrease in germinal center formation during influenza infection and B cells produce lower quality antibodies. Furthermore, the increase in senescent B cells with aging is negatively associated with a protective response after influenza vaccination [47].
In addition, memory CD8
Interestingly, although the total number of CD4
Fig. 1 shows the main functional alterations that occur in some of the immune cells (B and T cells, alveolar macrophages - AM, and neutrophils). These immunological changes predispose the elderly patient to viral infections, resulting in increased morbidity and mortality.
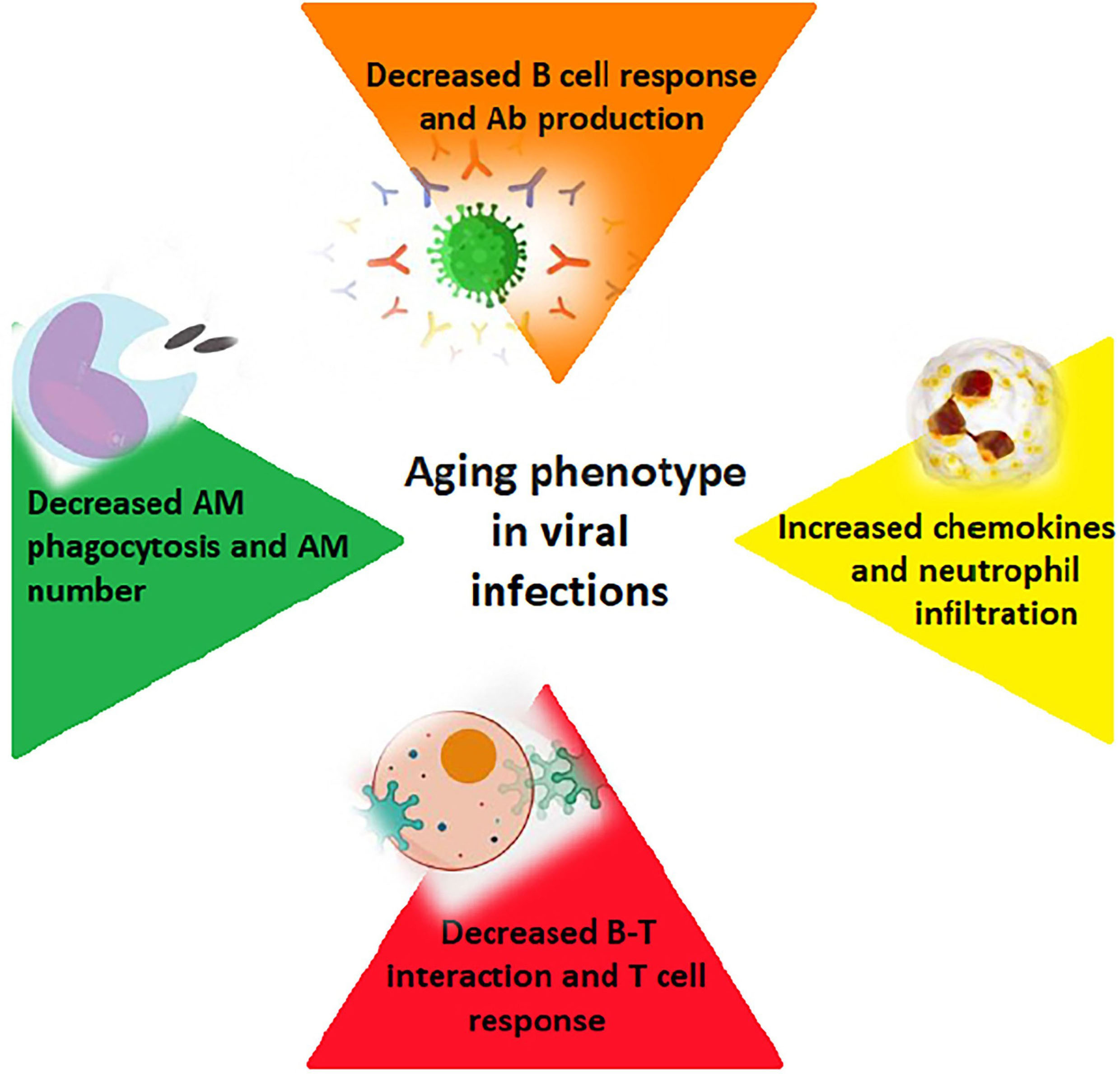 Fig. 1.
Fig. 1.How aging affects the immune response to viral infections (B and T cells; alveolar macrophages – AM; Ab - antibodies).
Coronavirus disease 2019 (COVID-19) is a viral disease affecting the upper and lower airways caused by the infectious agent severe acute respiratory syndrome coronavirus 2 (SARS-CoV2), which can cause a large variety of clinical forms ranging from almost asymptomatic cases to critical and potentially lethal forms [52, 53]. The aging of the immune system and the state of systemic inflammation contribute significantly to the development of clinically severe forms of COVID-19 disease, predisposing over 65 subjects to a higher rate of complications and mortality than younger subjects [54].
The increased susceptibility to SARS-CoV2 infection in elderly subjects is primarily related to age-related alterations in the cellular receptor of angiotensin-converting enzyme 2 (ACE2), through which the virus penetrates inside the target cells [55]. For example, elderly patients with severe aortic stenosis had a four-fold higher circulating level of ACE2 than in hypertensive patients. This increase could be justified by a combined effect of reduced left ventricular systolic function, increased pulmonary pressure, and age in this patient population, who are therefore more at risk for SARS-CoV2 infections [56].
Hematopoietic stem cells (HSC cells) also undergo qualitative alterations during
aging (e.g., insufficient DNA repair capacity, increase in HSCs with a
simultaneous decrease in their homing efficiency, and myeloid bias of their
differentiation potential), resulting in a downregulation of lymphoid
differentiation genes in favor of an upregulation of myeloid differentiation
genes. This proneness to myeloid differentiation would seem to contribute to the
deterioration of immune system competence in elderly individuals, thus
influencing the increased susceptibility to SARS-CoV2 infection in this
particular category of patients [57, 58]. COVID-19 patients display a typical
picture of lymphopenia, with marked reductions in the absolute number of
circulating CD4
Macrophages localized in the lungs of elderly patients have also been observed
to have a more pronounced production of IL-6 and other pro-inflammatory cytokines
in response to stimuli [62]. IL-6 inhibits the production of
Interferon-
In addition to macrophages, neutrophils in patients with severe forms of COVID-19 also show changes in number, phenotype, and function, in particular, an increase in the number of immature neutrophils characterized by the surface marker CD10LowCD101 has been demonstrated to associate with clinically severe forms of respiratory disease. Furthermore, COVID-19 patients who developed ARDS had significantly higher neutrophil counts and this factor may be implicated in the development of cytokine storm syndrome [65, 66].
In older patients, there is typically a preponderance of CD56dim NK cells,
endowed with distinctly cytotoxic properties and secretion of IFN-
Taken together, these results indicate that senescence is associated with a progressive loss of functional NK cells, partially compensated by an increase in the number of mature cells NK cells. Longitudinal studies have shown that the reduction in terms of number and/or NK cell function in the elderly is associated with serious infections and increased risk of mortality; they support the significance of these cells in the control of infectious diseases in the elderly [69].
Changes in the immune system that occur with aging can affect the antibody response to vaccination. Among the various changes, the most significant is the picture of inflamm-aging with consequent macrophage activation, capable of creating a harmful environment for the generation of an adequate immune response to a vaccine. This picture of low-grade inflammation is associated with impaired antigen presentation by dendritic cells (DCs), which are unable to efficiently process and present antigens to T cells with aging. Changes seen in lymph nodes with aging contribute to impaired vaccine response [70].
Antigen-presenting cells (APCs) also show changes in older individuals, particularly, this category of patients displays a decrease in the number of naïve cells mainly in the subpopulation of CD8 T cells, thus precluding priming by new antigens [71].
Thymic involution involved in impaired immune response to vaccines manifests with a decrease in naïve T cell production by 3% per year. Despite the thymus undergoing a progressive involution starting from puberty, there is a well-preserved number of T cells in elderly subjects; thus its role is assumed by thymic residues or substitute lymphoid organs, which are able to produce and select a large number of T cells every day, up to the very limit of human life. The increase in the number of mature T cells in the periphery and the increase in the ratio of memory T cells to naïve T cells contributes to the maintenance of homeostatic equilibrium in peripheral lymphocyte cell clusters [72, 73].
Age-related thymic involution causes a collapse of the TCR repertoire, which
then becomes a monitoring system of thymic function. In particular, sjTREC -
signal junction T cell receptor excision circles, i.e., a particular TREC that
arises from an intermediate rearrangement at the TCRD/A locus in developing
TCR
Furthermore, there is a progressive loss of lymphocyte proliferative capacity in the elderly as a consequence of the shortening of telomeres, which, once the critical limit is reached, block the ability of the latter to divide, resulting in a cluster of “old” T cells which are no longer able to proliferate, but have limited receptor diversity. This type of aged lymphocytes is characterized by a loss of receptor expressive capacity, as happens, for example, for the costimulatory molecule CD28, which is essential to allow the differentiation of B cells into plasma cells and therefore the production of specific antibodies [75, 76].
In the management of the COVID-19 pandemic emergency, the new messenger RNA (mRNA) vaccines have already played a fundamental role. Once the mRNA for the spike protein is encoded, SARS-CoV2 is injected directly into the host, where the mRNA is translated by ribosomes, resulting in the production of a vital protein responsible for the immune response. The lack of involvement of the infectious agent in their production makes this vaccine technology safer, with a low potential for mutations and, thus a lower risk of degradation of the antigen in vivo [77].
Phase III studies investigating vaccine efficacy in elderly subjects demonstrated that two doses of the Pfizer-BioNTech vaccine produce immunogenicity, regardless of the elderly’s health condition, and provide strong humoral immunity in elderly people who are 80 and 96 years old [78].
Demonstration of post-vaccine immunogenicity in the elderly is an important aspect of the fight against COVID-19, although disease presentation, severity, and mortality are the main outcomes that need to be evaluated in this type of future observational study.
In this perspective, the enrollment of older participants in COVID-19 vaccine trials is essential to understand the vaccine response of this vulnerable population [79].
Immunosenescence and inflammation predispose frail and elderly people to viral infections. As far as COVID-19 is concerned, immunological aging processes favor the development of more serious forms of the disease and complications.
In this area, recent studies have particularly highlighted that skin immunosenescence has a role not only in the onset of dermatological disorders, but also has fascinating system-level implications, whereby respiratory and neurological diseases have emerged as potentially related to skin immunosenescence [80, 81].
Chronic inflammation predisposes the body to acute inflammatory reactions that can be devastating, such as in the case of most elderly patients with COVID-19.
Variations in the efficiency of the immune system and an individual’s inflammatory state can contribute to the severity of the infection by both affecting viral replication and increasing the production of pro-inflammatory cytokines.
Inflammation usually has the ability to suspend itself when it is no longer needed, but if the immune system is confronted with repeated attacks, the inflammatory process can become chronic, becoming latent (symptom-free and hardly detectable) and damaging all tissues.
Since the vaccine response in elderly subjects is altered, with reduced production of neutralizing antibodies, the implementation of vaccine enrollment and trials in patients over 65 is of fundamental importance, in order to identify adequate vaccine formulations able to effectively stimulate the immune system.
This paper has some limitations related mainly to the variability of the type of works that have been analyzed and to the still partial knowledge regarding the immunopathological mechanisms of SARS-CoV2 concerning immunosenescence.
Conceptualization, SG and GMu; methodology FP and GMa; investigation, FP; resources, GMu; data curation, GMu and GMa; writing—original draft preparation, GMa, FP, GMu; writing—review and editing, FP, GMu, GMa, SG; visualization, FP; supervision, SG and GMa; project administration, SG, GMu and GMa. All authors have read and agreed to the published version of the manuscript.
Not applicable.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest. Given their role as Guest Editor or Editorial Board Member, Dr. Giuseppe Murdaca, Dr. Francesca Paladin and Dr. Sebastiano Gangemi had no involvement in the peer-review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Vijay Kumar.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
