- Academic Editor
†These authors contributed equally.
Cardiovascular diseases rank as the leading cause of death worldwide and are a major contributor to disability, posing a significant threat to human health. Organoids offer a partial simulation of the structure and function of the tissue of origin. It is a promising model that can supplement the disadvantages of two-dimensional culture and animal models. Due to the complexity of heart development, the research of cardiac organoids is still maturing. The advancement of technology has helped address certain challenges, but it has also unveiled new issues and complexities. This paper summarizes the application of organoids technology in the cardiovascular field, the common construction methods of cardiac organoids, and the latest progress of cardiac organoids in the fields of disease model construction, cardiac development research, drug research, and regenerative medicine. The future development and challenges of cardiac organoids are also addressed.
The prevalence and mortality of cardiovascular diseases have gradually increased from 1990 to 2019, ranking first in the global mortality rate. Cardiovascular diseases also the main factor leading to disability, which has brought a serious burden to the global medical economy [1]. Research has revealed variations in the incidence of cardiovascular diseases across countries with different income levels, as well as differences observed between genders [2, 3]. With the in-depth study of cardiovascular diseases, these complex issues demand effective solutions.
Organoids are structures comprised of organ-specific cells that originate from
stem cells or organ progenitors. They can self-organize and assemble through
processes like cell sorting out and spatially restricted lineage differentiation
within a living organism. Unlike other multicellular 3D tissue models such as
gastruloids and embryoid bodies, organoids represent only a single organ (or
parts thereof), while gastruloids and embryoid bodies include precursor cells of
endoderm, mesoderm and ectodermal lineages. In gastruloids, as with embryos, the
trilineage precursors organize spatiotemporally relative to each other [4, 5, 6]. In
this review, we only discuss organoid technology. In 2008, Eiraku et
al. [7] used embryonic stem cells (ESC) to produce cortical neurons within
a 3D structure in cell aggregation cultures. In 2009, Sato et al.
[8] found that adult stem cells, namely small intestine crypt Lgr5
| Organoids | Cell resource | Cultivation duration | Essential signaling pathways | Factors | Ref. |
| Brain organoids | hiPSC | 20–30 days | FGF/WNT/BMP signaling | SAG, SB431542, IWR1 | [9] |
| mESC | 10 days | FGF/WNT/BMP signaling | SAG, SB431542, IWR1 | [7] | |
| Lung organoids | hPSC | 50–80 days | FGF/WNT/BMP signaling | SB431542, CHIR99021, SAG, FGF4, FGF10 | [10] |
| Xenopus laevis foregut | 19 days | FGF/WNT/BMP signaling | - | [17, 18] | |
| Heart organoids | hPSC | 7 days | WNT/BMP signaling | CHIR99021, BMP4, IWR1, XAV939 | [11] |
| hiPSC | 20 days | - | CHIR99021, Y-27632, IWP4 | [19] | |
| Cardioids (a gastruloid model) | hiPSC | 7 days | WNT/BMP signaling | CHIR99021, BMP4, HGF, IGF-1, VEGF, Y-27632, FGF2 | [20] |
| Liver organoids | Lgr5+stem cells of mice | Several months | FGF/BMP signaling | EGF, HGF, BMP7, FGF19 | [10] |
| hiPSC | 20 days | - | FGF2, VEGF, EGF, CHIR-99021, A83-01 | [21] | |
| Kidney organoids | hiPSC | 20 days | FGF/WNT signaling | CHIR99021, FGF9 | [13] |
| hiPSC | 14 days | FGF/WNT signaling | CHIR99021, FGF9, Y27632, BMP4 | [22] | |
| Blood vessel organoids | hPSC | 18 days | BMP/WNT/NOTCH signaling | BMP4, VEGF-A, SB43152, CHIR99021, FGF2 | [14] |
| hiPSC | 30–50 days | BMP/WNT/NOTCH signaling | SB43152, CHIR99021, BMP4, Y27632, VEGF-A, FGF2 | [23] | |
| Retina organoids | hESC | 126 days | WNT/BMP signaling | IWR1, CHIR99021, SAG | [15] |
| hiPSC | 18 days | - | IWR1, SAG | [24] |
hiPSC, human induced pluripotent stem cells; mESC, mouse embryonic stem cells;
hPSC, human pluripotent stem cells; hESC, human embryonic stem cells; SAG,
Hedgehog/Smoothened agonist; SB431542, a TGF-
At present, two-dimensional culture and animal models are the main models for studying cardiovascular diseases. Two-dimensional culture models have been used in the field of life science for decades, enabling researchers to study the physiological and pathological activities of cells in vitro. However, this method typically only involves a single cell type and lacks the interaction and information exchange between cells and the cell matrix [25]. The growth environment of this cell is far from that in vivo, resulting in a discrepancy between two-dimensional cell experiments and those done in animal or outcomes of human clinical trials [26, 27]. Animal models have been instrumental in advancing our understanding of diseases. However, the limitations posed by the use of different species and the high costs associated with animal modeling restrict their application [28, 29, 30]. Comparing the two models, the organoids produced by three-dimensional culture have the following advantages:
 Fig. 1.
Fig. 1.Advantages and disadvantages of two-dimensional culture, three-dimensional culture, and animal model. Two-dimensional culture, three-dimensional culture, and animal models play an important role in biomedicine.
Organoids are an important supplement to the research model of heart disease. At present, organoids are expected to be applied to many research fields, such as establishing disease models, studying the molecular mechanism of disease, drug research and regenerative medicine [34, 35, 36]. The emergence of organoids is of great significance. Although many technical difficulties and problems remain to be solved, organoids have the potential to bridge the transition from basic cell biology research to clinical medicine.
In the initial stages of research, scientists commonly generated embryoid bodies
through the suspension culture of ESC or pluripotent stem cells. Subsequently,
they induced the differentiation of these cells into cardiomyocytes. Chen
et al. [37] developed a polymerized suspension culture system for
human-induced pluripotent stem cells (hiPSCs). This system effectively guides the
differentiation of hiPSCs into cardiomyocytes by modulating the WNT pathway and
utilizing suspension culture techniques, resulting in myocardial cells with a
purity of up to 90%. The cardiomyocytes produced by this method showed typical
morphological and electrophysiological characteristics of cardiomyocytes.
Likewise, by using patch clamp to analyze electrophysiological properties,
cardiomyocytes show action potential, representing nodal-, atrial-, and
ventricular-like cardiomyocytes. Electrophysiological analysis also showed that
from day 8 to day 28, cardiomyocytes’ key action potential characteristics
gradually matured (or increased). The maximum rise rate (Vmax) expressed by V/s
was 34.2
The strategy employed in organoid culture primarily relies mimicking the growth signals that occur during the development of the target organ, as well as replicating the requirements for tissue environment homeostasis. In recent years, the advancement of tissue engineering technology and the deepening understanding of organoid construction methods, have led to various approaches in constructing cardiac organoids. These methods predominantly include tissue engineering techniques, self-organization/assembly methods, 3D printing and organ-on-a-chip technologies (Fig. 2). Tissue engineering includes regulating metabolism [38], matrix rigidity [39] and tissue patches [40]. Self-organization/assembly includes cardioids [11] and other heart organoids [41]. Some 3D printing studies include cardiac patches [42], collagen [43] and vascular conduits [44]. Organ-on-chips include microfluidic devices [45] and miniature cell culture chambers [46].
 Fig. 2.
Fig. 2.Construction methods of cardiac organoids. Cardiac organoids are mainly induced by human pluripotent stem cells. Common construction methods include tissue engineering, self-organization/assembly, 3D bioprinting, and heart-on-a-chip technologies.
Many methods for constructing heart organoids in vitro have been reported, but there are some differences in the methods applied and the cell types used to generate them. The main cell types used to construct cardiac organoids include hiPSCs, embryonic stem cells, and cardiac progenitor cells. Ergir et al. [47] uses hiPSCs to differentiate into a cardiovascular lineage and further aggregate them in a low-adhesion dish in 3D. The resulting human organotypic cardiac microtissues contain various cell types and can beat without external stimuli for over 100 days. Lee et al. [48] optimized the method for culturing human cardiac organoids from embryonic stem cells in the presence of high concentrations of laminin-entactin and fibroblast growth factor 4. The resulting human heart organoids have a unique cardiac morphology, with atrium- and ventricle-like chambers composed of cardiomyocytes, and express the integral proteins of gap junctions and ion 20 channels [48]. Ho et al. [49] successfully obtained human cardiovascular progenitor cells by utilizing hiPSCs and inducing targeted differentiation to generate cardiomyocytes. They used human cardiovascular progenitors to differentiate chambered cardiac organoids by regulating WNT/ß-catenin signaling, as well as several growth factors necessary for the expansion of human cardiovascular progenitors. It is worth mentioning that these are cardiac organoids derived from different cells and different construction methods and thus have different applications.
Induced pluripotent stem cell-derived cardiomyocytes show great potential in the
research of human heart disease. However, the maturity of cardiomyocytes obtained
by traditional methods is low, which seriously restricts their use as a disease
model and their application in drug research. Immature cardiomyocytes have a low
resting potential (approximately –60 mV) and the current is mediated by
inward-rectifying potassium channel Kir2.1. Meanwhile, mature ventricular
cardiomyocytes have a resting potential of approximately –90 mV. The action
potential of immature cardiomyocytes rises much more slowly than in mature
cardiomyocytes, and repolarization begins soon after depolarization. The rapid
repolarization phase is mainly mediated by rapid delayed-rectifier potassium
currents, while mature ventricular cardiomyocytes have a plateau phase [50].
Sarcomere is the basic unit of cardiomyocyte contraction. Monitoring the
expression levels of troponins such as cardiac troponin T, cardiac troponin I,
In adult cardiomyocytes in a relaxed state, the ganglion length is approximately
2.2 µm, while the sarcomere length of immature cardiomyocytes is
approximately 1.65 µm [51]. Tissue engineering is an emerging field that
integrates the principles of cell biology and materials science to construct
functional tissues or organs. The application of tissue engineering methods in
the cardiovascular field can mitigate the issue of cardiomyocyte immaturity.
Researchers mainly combine the induced cardiomyocytes with biomaterials such as
hydrogel or extracellular matrix to generate a series of myocardial tissues with
similar structure and contraction characteristics to adult myocardium. Feyen et al. [38]
developed a medium culture formula different from the traditional cardiomyocytes
culture medium to maintain the fatty acid
The contractile performance of myocytes was evaluated using dynamic monolayer
force microscopy, and the intracellular tension was significantly increased from
2.01
With the development of tissue engineering technology, more research is devoted not only to cell research but also the use of disease animal models as well as drug testing applications. Querdel et al. [40] applied tissue engineering to produce a myocardial tissue patch and transplanted it into the guinea pig model of hypothermic injury. The experiment showed that it could maintain the mechanical and electrophysiological characteristics of myocardial tissue and improve guinea pigs’ left ventricular function through the muscle. Goldfracht et al. [53] combined cardiomyocytes with chitosan-enhanced extracellular matrix hydrogel from an acellular pig heart. The effect of myocardial tissue produced by this method on drugs such as isoproterenol, carnitine, E-4031, ATX2, Uben, and quinidine is similar to that observed in adult cardiac tissue. However, this method carries certain limitations. There is still a gap between the maturity level of this kind of myocardial tissue and adult cardiac myocytes. The force it generates is at least one order of magnitude lower than that of adult cardiac myocytes. With the rapid development of tissue engineering, the cardiac organoids constructed are increasingly complex and more reflective of normal cardiac tissue. This is an effective supplement to the traditional culture mode.
The process of cardiac development involves complex morphological construction events, including the formation an early cardiac tube, a cardiac tube ring, an atrioventricular cavity, the cardiac septum and valve, connection of outflow tract, the conduction system and coronary circulation [54]. Although 3D organoid technology based on tissue engineering can construct cardiac organoids, it cannot reflect the heart development and formation process. This is so because in the process of embryonic development, the formation of tissues and organs is not controlled artificially but is formed by cell self-organization/assembly.
Hofbauer’s team [11] induced hPSC to proliferate and differentiate by activating six known signal pathways involved in embryonic heart development. The experiment found that with the activation of the development signal pathway, the mesoderm induced by hiPSC self-organized/assembled into a single closed chamber that could beat autonomously, while the sarcomere and intercalated disc could be observed under the ultrastructure. It [11] includes common cell types at this stage of development, such as myocardial cells, endothelial cells, fibroblasts and epicardium. In response experiments to tissue damage, researchers observed that fibroblasts in cardiac organs immediately recruited and migrated to the injured site. These cells also synthesized certain proteins to repair the damaged tissue. Compared with tissue engineering, the cardiac organoids constructed by this method do not require external matrix scaffolds. Rather, they rely only on the mechanism of cardiac development; the process is similar to the track of spontaneous growth of the human heart [55].
Lee et al. [41] applied the self-organization/assembly method to construct cardiac organoids and used its mechanical contraction amplitude analysis to confirm that high-dose nifedipine treatment in clinical trials may cause cardiac arrest. They also proved for the first time that inhibition of the hERG channel could lead to QT prolongation or early and late depolarization of arrhythmia in cardiac organoids. Other studies have shown that using self-organizing/assembling methods to construct cardiac organoids can aid in studying the influence of diabetes conditions on the development of cardiac organoids [56]. These data show that cardiac organoids constructed by the self-organization/assembly method are a useful tool for studying the mechanism of cardiovascular diseases and lay a foundation for pharmacological research on the treatment and prevention of these diseases.
The method of producing cardiac organoids by self-organization/assembly has significant advantages in revealing the process of organogenesis and development in vivo. However, it has some limitations. Organoids represent single organs (or parts of them) that lack stimulation from other tissues, such as nerve cells, that are important for heart development. Further, gastruloids can be used to establish an advanced model for human cardiogenesis with multilineage and multisystem integration that includes neurons toward the goal of organ innervation [20]. Amadei constructed an embryonic model using embryonic stem cells and extra-embryonic stem cells, including trophoblast cells, embryonic ectodermal stem cells, and induced embryonic ectodermal stem cells [57]. These models demonstrate self-organizing/assembling ability to reconstruct mammalian development through gastrulation, neurulation, and early organogenesis. These methods have broadened our thinking. Presently such models are representative of the fetal heart. In an organoid study with cardioid organoids [11] and human heart organoids [56], attempts to achieve further developmental growth progress by a longer incubation time under those conditions was insufficient to progress along a normal development path.
3D bio-printing technology is a technology that combines computers and biomaterials that can realize the personalized spatial layout of cells and biomaterials, thus building the desired model. Noor et al. [42] extracted omental tissue from patients, reprogrammed them into hiPSCs, and differentiated them into cardiomyocytes and endothelial cells. They then combined these two cells with personalized hydrogels to produce cardiac parenchyma and blood vessels, and used them as a biological ink. The printed autologous tissue has the same anatomical structure as the original tissue and all cellular components and is not expected to produce a rejection reaction if transplanted back into the patient. This functional vascularized tissue, printed according to the patient’s anatomical structure, is expected to repair or replace damaged/diseased organs. Lee et al. [43] successfully constructed a newborn human heart using 3D bio-printing technology, pulmonary valve, and aortic valve. Wang et al. [44] constructed arterial and venous tissues. These 3D bioprinted arterial and venous catheters display the basic characteristics of blood vessels, including related mechanical properties, barrier function, and the expression of vascular-specific markers. The authors anastomosed the printed blood vessels with different diameters to mice’s aorta and vena cava through in vitro transplantation. They observed that blood flow passed through the transplanted blood vessels normally and without obvious leakage.
3D bioprinting is a promising method, providing a fine basis for building a complete organ. However, some challenges still affect its application, including the need to obtain a large number of cells and achieve the physiological distribution of relevant cell types to obtain the engineering of large and functional organs. Constructing a perfusion vascular network in thick tissue remains an additional challenge [58].
Organ-on-a-chip is a new technology that combines lab-on-a-chip and organoids. It can build and simulate the microenvironment of human tissue and form a micro-physiological system akin to the human body. Grosberg et al. [45] designed a specific microfluidic device to collect myocardial contractility and electrophysiological data. The cytoskeletal structure of multiple tissues can be observed in the same experiment. This technology can collect organization-scale data and effectively reduce the error caused by the traditional test of multiple single samples.
Other scholars have built a miniature cell culture chamber using a microfluidic device. The lateral channel of the device can precisely adjust the flow rate of the culture fluid in the culture chamber, mimicking the passing of blood through local areas in the capillaries under physiological conditions. The mouse cardiomyocytes planted in this device can closely simulate the physiological state of cardiomyocytes [46]. Because of the physiological characteristics of myocardial cells, such as contractility and electrophysiological response, it is extremely difficult to replicate the real internal environment of heart tissue in vitro.
Microfluidic technology makes it possible to closely simulate the internal environment, which is also the core value of organ chips. This technique has been applied to the construction of disease models, drug testing and toxicological research. Myocardial infarction and heart failure models were included in the basic and preclinical research [46, 59]. In drug testing and toxicological research, a doxorubicin toxicological model was constructed [60]. Organ-on-a-chip can also be used to determine the partial pressure of oxygen [61]. This technology has been used to explore the effects of different culture conditions on cardiomyocyte proliferation, morphology, and arrangement [62].
Cardiac organoids are a highly promising technology. With the deepening of research, more and more reports have shown its extensive application, such as construction of heart disease model, heart development, drug research, and regenerative medicine. In the construction of disease models, such applications include models of myocardial infarction [63], heart failure [64] and arrhythmia [65]. Researchers have also looked at cardiopulmonary co-development, and congenital heart disease [66, 67, 68]. Some drug studies include tacrolimus (calcineurin inhibitor), sirolimus (proliferative signaling inhibitor), and doxorubicin [63, 69]. In regenerative medicine, this includes the transplantation of cardiac damage repair models and printed cardiac tissue [70, 71] (Fig. 3).
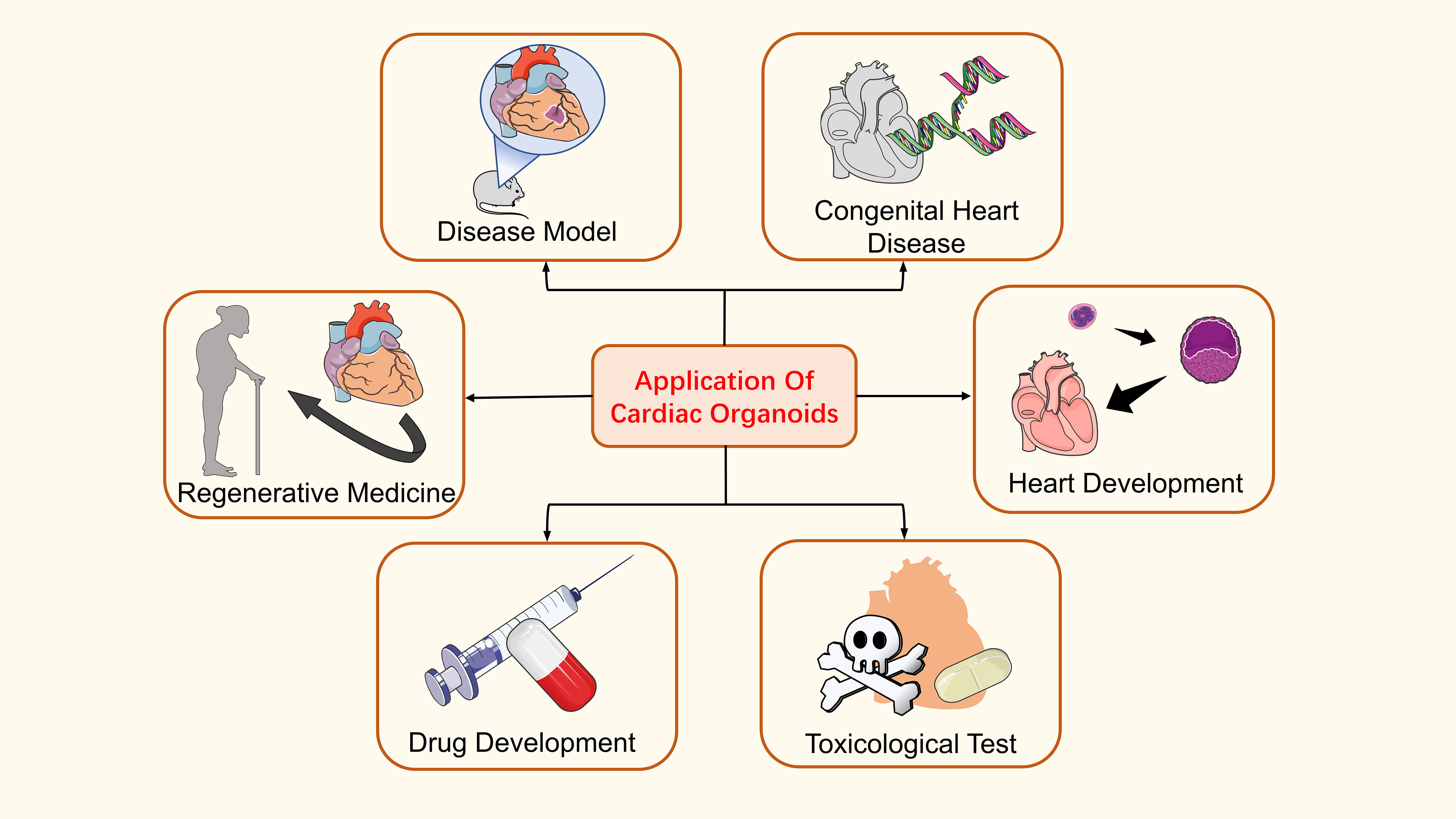 Fig. 3.
Fig. 3.Application of cardiac organoids. Cardiac organoids are widely used, including in the construction of disease models, the pathogenesis of congenital heart disease, cardiac development, regenerative medicine, drug development, and toxicological detection.
Myocardial infarction is an acute disease that may be life-threatening due to acute coronary artery obstruction and cardiac muscle necrosis due to lack of blood supply. Richards et al. [63] used the iPSC to build cardiac organoids and built a model combining oxygen diffusion gradient. Norepinephrine stimulation can simulate the heart’s “infarct edge remote region” of the heart after myocardial infarction. The disease model reproduced the characteristics of myocardial infarction (such as metabolic changes after myocardial infarction and pathological fibrosis) at the transcriptome, structure, and function levels. Notably, due to the lack of inflammatory cells, this model cannot reflect the immune system’s role in vivo. Heart-on-a-chip can be used to build myocardial infarction models. Using serum-free medium and common medium to regulate heart-on-a-chip perfusion precisely can simulate the low perfusion state when myocardial infarction occurs. It can be observed that myocardial cells are separated from each other in space, actin filament is decomposed, and cell volume is gradually reduced. The above changes are positively related to the degree of hypoxia. The device can regulate and measure the extracellular microenvironment of myocardial cells in physiological and pathological conditions, greatly promoting myocardial infarction research [46].
Heart failure is common at the end stage of cardiovascular disease, manifested by blood stasis in the venous system and insufficient perfusion in the arterial system. Tiburcy et al. [64] used cardiomyocytes cultured with hiPSCs to study heart failure under specific serum-free conditions. They found that the toxic response of these kinds of organoids to chronic catecholamine was characterized by systolic dysfunction, cardiomyocyte hypertrophy, cardiomyocyte death, and the release of heart failure markers. These phenotypes are typical features of heart failure. Although the maturity of the organoids obtained by the method is only at the level of the 13-week fetus in the unbiased global transcriptome profiling [59], the cardiac organoids still show great potential for modeling heart failure.
Arrhythmia is a common heart disease caused by abnormality of the cardiac
electrical conduction system. Lee et al. [72] used mouse ESC to
construct cardiac organoids with atrial and ventricular structures, including
myocardium, smooth muscle, endothelial cells, and conductive tissue. They
evaluated the electrophysiology of this model and found that it showed normal
excitability. After using the K
Between 20% and 25% of patients with novel coronavirus pneumonia have acute
heart injury during hospitalization. Arhontoulis’s team [73] utilized IL-1
The process of hiPSC self-organizing/assembling to form cardiac organoids can partially stimulate the growth and development of the heart under natural conditions. As such, the process provides new avenues for studying organ growth and development and the pathogenesis of congenital heart disease. To study the interaction between the heart and lungs during human embryogenesis, Ng et al. [66] used hiPSCs to cultivate heart organoids and lung progenitors. The heart-lung co-culture micro-tissue was established through three-dimensional suspension culture technology, and it was found that the alveolar maturation was accelerated in the presence of the heart. How the human body separates different tissues and organs in close contact and maintains the boundary between organs remains unclear. After stopping the WNT agonist, the heart and lung tissues were effectively separated. This work provides a new model for studying the molecular and cellular mechanisms of the co-development of the human heart and lung and the formation of tissue boundaries.
Congenital heart disease is caused by abnormal cardiovascular development in the fetal period. Marini et al. [67] used hiPSCs from patients with Duchenne muscular dystrophy (an X-chromosome recessive disease) to construct cardiac organoids and observed cardiac hypertrophy or expansion caused by myocardial cell degeneration, followed by fibrosis and adipose tissue formation. At the same time, they also identified five microRNAs considered to play a key role in this gene network. This model permits a deeper understanding of the pathogenesis of hypertrophic/dilated cardiomyopathy associated with Duchenne muscular dystrophy. Lewis-Israeli et al. [56] used GSK3 and PORCN inhibitors to optimize the multi-step operation conditions of standard WNT signals and constructed self-assembled cardiac organoids. Diabetes in pregnant women is one of the common causes of neonatal coronary heart disease. The team used glucose and insulin at the level of diabetes in the culture medium, proving the influence of diabetes conditions on the development of cardiac organoids. They found that the cardiac organoids in this medium had a large volume; at the microscopic level, mitochondria were reduced, while lipid metabolism dysfunction and structural tissue damage were observed. Studies have also been published on the use of cardiac organoids for congenital heart diseases, such as Ebstein syndrome [68].
Animal models are widely employed for preclinical drug efficacy research and toxicological studies. Still, the characteristics of species differences have great limitations, while the translation of numerical data obtained from animal experiments to reflect real human conditions is challenging. The advent of organoids is an important supplement. Organoids possess certain structural and functional characteristics of the original tissue, thus typically meeting the needs of drug researchers. Sallam et al. [69] studied the cardiovascular effects of tacrolimus (calcineurin inhibitor) and sirolimus (proliferation signal inhibitor) using the cardiac organoid model produced by hiPSC. After treatment with tacrolimus, the markers of cardiac fibrosis response were significantly up-regulated. Compared with tacrolimus, sirolimus has no such effect. Cardiomyopathic and cardiac allograft dysfunction generally pose problems of increased fibrosis and extracellular matrix deposition. These findings explain the long-term benefits of proliferative signal inhibitors on adverse remodeling after heart transplantation. However, calcineurin inhibitors may be related to the fibrotic phenotype in cardiac tissue, leading to poor cardiac remodeling. Richards et al. [63] used the iPSC to build cardiac organoids, developing a tissue-level human myocardial infarction model for drug screening. The authors found that doxorubicin expressed more vimentin in the myocardial infarction group, consistent with the results of anthracycline drugs leading to myocardial fibrosis previously reported. This myocardial infarction model simulates the process of hypoxia enhancing doxorubicin cardiotoxicity. In addition, some teams use cardiac organoids to study the impact of environmental exposure on cardiovascular diseases, explore the mechanism of gene-environment interaction, and understand the impact of individual susceptibility to the environment [74].
To summarize, cardiac organoids derived from hiPSCs provide a platform for future drug research that may be more useful to study targeted relationships between drugs and specific human organs. The application of cardiac organoids alleviates the limitations of animal models that are non-anthropogenic, require a lengthy period to model, and cannot be modeled in large quantities simultaneously. However, the maturity of cardiac organoids still presents a challenge.
Unlike Zebrafish, human cardiomyocytes are permanent cells and do not have the ability to regenerate and repair without intervention [75, 76]. After a myocardial injury, they are usually repaired with fibrous tissue [77]. Heart transplantation is still the gold standard for the treatment of heart failure. Although the heart transplantation rate has improved in the last decade with economic development, we have observed the aging of the donor population and the increase in the prevalence of diabetes, hypertension, obesity, and other diseases in the donor population. These donor risk factors are significantly related to the short-term mortality rate, long-term mortality rate, and the occurrence of vascular diseases in heart allograft transplantation [78]. Therefore, heart transplantation is still limited by the lack of donor organs.
The emergence of cardiac organoids provides a new strategy for the replacement therapy of myocardial injury. Voges et al. [70] used embryonic stem cells to construct cardiac organoids and study the regeneration ability of immature heart tissue in the injury response. They found that this organoid model is useful in studying repair after hypothermic injury. The tissue repair process showed no pathological fibrosis or unregulated growth resulting in cardiac hypertrophy. Their findings demonstrate that cardiac organoids show great potential for repairing damaged myocardium. Kawai et al. [71] employed cardiac myocytes, human umbilical vein endothelial cells, and human fibroblasts derived from hiPSC to derive cardiac organoids. Combined with 3D printing technology, tubular cardiac tissue without stent was constructed and transplanted to immunodeficient mice’s abdominal aorta and inferior vena cava. As a result, transplanted cardiac tissue was found to spontaneously beat in mice; tissue level changes included myocardial stripes and blood vessel formation. However, the amount of implanted cardiac tissue was insufficient, was not conducive to blood circulation integration and did not restore organ contractility of the heart. Therefore, questions remain on what is needed for full recovery by such methods. The limitation is that the implanted heart tissue is too small and immature, and thus is not conducive to blood circulation. In addition, by not assessing the contractility of the tissue, the authors do not shed light on the maturity of the tissue. Therefore, future research must focus on how to create larger and thicker engineered heart tissue and successfully transplant it into large animal models. Changing the design of the engineered heart tissue or culture system is one of the directions [71]. Despite its limitations, this model is expected to provide a complementary pipeline for patients with congenital heart disease. Other studies point to the importance of multilineage cell types in repair. For example, the simultaneous injection of hiPSC derived cardiomyocytes with patches containing human mesenchymal stem cells on the surface of the heart of myocardial infarction site can significantly improve the function of the heart following injury and can promote the formation of blood vessels [79].
In addition to hiPSCs and embryonic stem cells, mesenchymal stem cells are
worthy of our attention. Lee et al. [80] found that low-frequency
mechanical loading (0.1 Hz, 5% maximal strain) and the addition of
TGF-
To summarize, cardiac organoid technology shows great promise that may include patient-matched tissue repair for transplantation relevant to cardiac repair. However, there is still much progress to be made to ensure consistency and precise conditions necessary for cardiomyocyte replacement, vascular integration, and regular heart contractile function in heart transplantation.
The emergence of cardiac organoids has opened a new door for the research of disease modeling, mechanism research, and precise treatment in the cardiovascular field. The hiPSC used to generate cardiac organoids can be obtained from re-edited patient-derived cells, meaning that the cardiac organoids constructed by this method have the phenotype of patients. It carried the unique advantage of permitting researchers to study the diseases’ occurrence and development mechanisms, especially congenital heart disease, and its application in precision medicine. Precision medicine focuses on using patient data to implement personalized disease management methods. By leveraging the diversity of patient populations and conducting multi-group analyses, researchers can gain deeper insights into the fundamental mechanisms of diseases, going beyond relying solely apparent clinical phenotypes. This approach facilitates the advancement of precision medicine, allowing for a better understanding of individual variations and tailoring treatments accordingly [81].
The replacement therapy of cardiac organoids for the cardiovascular system is another area for future development. Artificial cardiac tissue, even a mature artificial heart, is an important means to treat myocardial injury and heart failure. Current studies have reported a problem with immature cardiomyocytes. The cardiac organoids constructed in vitro can only reach the fetal level, which presently limits applications for full adult organ transplantations. The potential applications in both physiological and pathological aspects of human heart development may be a closer target. Some studies have reported that more mature cardiomyocytes can be obtained by some means, such as prolonging the culture time, transplanting into a human or animal body, mechanical signal, and three-dimensional culture, among other approaches. At the same time, they also pointed out the limitations of using fully mature cardiomyocytes. For example, mature cells could not survive after being transplanted into the recipient’s body. The preferred method might be the maturation of cardiomyocytes after transplantation [50]. These achievements have brought new thinking about how to induce mature cardiomyocytes and the application of cardiac organoid transplantation in the future.
Here, we should also point out the challenges cardiac organoids face. First, as mentioned above, there is a general problem of immaturity in cultured cardiac organoids. How to obtain mature cardiac myocytes is still the focus and primary challenge of current research [11]. Second, there are numerous methods of cultivating cardiac organoids with different standards. An efficient method of obtaining high-quality cardiac organoids should be standardized. Third, cardiac organoids lack interaction with the microenvironment, such as inflammatory cells and hormones, which cannot completely simulate the internal environment. Fourth, there is a lack of vascular network in cardiac organoids, which mainly transports nutrients through diffusion, resulting in uneven distribution of nutrients in organoids and limiting the size of organoids.
Overall, despite the existing deficiencies and challenges in current cardiac organoid research, they hold significant potential for broad applications and irreplaceable research opportunities in the future. As technology advances and our understanding of organoids improves, these miniature tissue models have the capacity to revolutionize various aspects of cardiovascular research, drug discovery, disease modeling, and potentially contribute to personalized medicine approaches.
All the authors analyzed and discussed the literature, commenting on and approving the manuscript. YHW and GL performed the bibliographic research, drafted the manuscript, prepared the figures, and created the table. QOY, SZ, YZ, RZT, YPG, RZ and PZ all analyzed and discussed the literature, commented on and approved the manuscript. MIN edited the manuscript to ensure quality. All authors have participated sufficiently in the work to take public responsibility for appropriate portions of the content and agreed to be accountable for all aspects of the work in ensuring that questions related to its accuracy or integrity.
Not applicable.
Not applicable.
This research was funded by the National Natural Science Foundation of China (82271395 and 82001301), the Science and Technology Planning Project of Guangdong Province (2022B1212010010), the Guangdong Basic and Applied Basic Research Foundation(2023A1515030073); the Youth Talent Two-way Exchange Project of Guangdong and Macao (KD0120230024); and the Special Project of Dengfeng Program of Guangdong Provincial People’s Hospital (KY0120220133, DFJHBF202111, KJ012020630, DFJH201812 and KJ012019423) to GL and PZ.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
