- Academic Editor
†These authors contributed equally.
Background: The occurrence and development of chronic obstructive
pulmonary disease (COPD) are regulated by environmental and genetic factors. In
hypoxia, Erythropoietin (EPO) satisfies the body’s need for oxygen by
promoting the production of red blood cells. Hypoxia was proven to be a common
physiological condition in COPD progression and associated with many
complications. Some studies have found that EPO is involved in the
development of COPD. But the mechanism has not been fully proven.
Methods: We conducted a case-control study enrolled 1095 COPD patients
and 1144 healthy controls in Guangdong Province to evaluate the association
between EPO polymorphisms (rs1617640 A
Chronic obstructive pulmonary disease (COPD) is the most common pulmonary disease characterized by small airway lesions and chronic respiratory symptoms and continuous airflow restriction [1]. Its common pathological features include bronchiolitis and destruction of lung parenchyma (emphysema) [1]. COPD usually progresses slowly, often leading to the neglect of early treatment and prevention [2]. Thus, when the disease is brought to the patients’ attention, it has usually progressed to the third or fourth stage and may be accompanied by complications [3]. It is estimated that 4.5 million deaths yearly will be attributed to COPD by 2030 [4], and it is on track to become the third leading cause of death worldwide [5]. Due to the prolonged course and the high prevalence of the disease, COPD will also place a heavy burden on the global healthcare economy.
COPD is considered to be a process of chronic hypoxia and chronic systemic inflammation with complex pathogenesis. It is reported that environmental and genetic factors affect the risk of COPD jointly [6, 7]. It is well known that smoking is a recognized risk factor for COPD, still, this proportion in long-term smokers is only 10%–20%, and there is a considerable proportion of non-smoker people diagnosed with COPD, which indicates the importance of genetic factors in COPD [3]. With the development of genome-wide association analysis (GWAS), Pillai and colleagues first found that the single nucleotide polymorphisms (SNPs) of CHRNA3/CHRNA5/IREB2 located on chromosome 15q25 were associated with COPD significantly [8]. Since then, GWAS at home and abroad have reported lots of genes influencing susceptibility to COPD successively [9, 10, 11]. However, there is still largely unknown in the genetic etiology of COPD.
Erythropoietin (EPO) stimulated by Hypoxia is a recognized
hematopoietic cytokine that can control the oxygen-carrying capacity of blood and
enhance ventilatory function under hypoxic conditions [12, 13, 14]. EPO was
observed to play anti-inflammatory, anti-apoptosis, and anti-oxidant/fibrosis
roles in the lung by interacting with inflammation, apoptosis, oxidative stress,
or fibrosis-related pathways [15, 16, 17, 18, 19, 20]. Some studies indicated that the expression
of EPO was correlated with the severity of COPD positively, while
correlated with the pulmonary function index FEV
Therefore, we conducted a case-control study enrolled 1095 COPD patients and
1144 healthy controls in several hospitals in Guangdong to evaluate the
association between EPO polymorphisms (rs1617640 A
We had performed a case-control study among Han Chinese that included 1095 COPD
patients and 1144 healthy controls in several hospitals in Guangdong Province
(Songshan Lake Central Hospital of Dongguan, the Dongguan Binhaiwan Central
Hospital, and the Shenzhen Longhua District Central Hospital) during 2015 to
2019, to evaluate the association between EPO polymorphisms (rs1617640
A
872 participants, from a prevalence study in Zhuoni County of Gannan Tibetan Autonomous Prefecture of Gansu Province in 2019, was recruited to verify the effect of EPO polymorphisms on lung function.
All subjects from case-control study and prevalence study received the lung function test and the respiratory health questionnaire (including their demographic characteristics and environmental exposure factors, like education level and smoking status) for collecting their demographic characteristics and environmental exposure information [26]. We also obtained their 5 mL peripheral blood for genotyping after they signed informed consent.
This study was reviewed by the Ethics Committee of Guangzhou Medical University, Xi’an Jiaotong University and Gansu University of Chinese Medicine.
According to the Global Initiative for Chronic obstructive pulmonary disease
2019 [27], we diagnosed COPD if participants experienced respiratory symptoms
like coughing, expectoration, dyspnea and wheezing in their daily lives, and the
ratio of forced expiratory volume in 1 second (FEV
Potential risk SNPs are screened through the dbSNP database
(http://www.ncbi.nlm.nih.gov/SNP) based on the following criterion: SNPs were
limited in the region between 2000bp upstream and downstream of EPO; the
minor allele frequencies (MAFs) were more than 0.05 in the Chinese population;
the linkage disequilibrium of the selected SNPs was low (LD, R
Using the TIANamp Genomic DNA Kit (Tiangen Biotech, Beijing) to extract DNA from the collected peripheral blood sample. TaqMan real-time polymerase chain reaction (PCR) was used for genotyping. Supplementary Table 4 shows the details of primers and probes. Furthermore, to ensure the reliability of the PCR reaction, we not only set up a negative control on each plate, and 10% random samples repeat tests were carried out.
The Chi-square test was used to assess the difference in demographic characteristics and the frequency distribution of genotypes between the case and control. After adjusting for age, sex, smoking, education, coal and biomass as fuels, logistic regression analysis was used to evaluate the relationship between EPO polymorphisms and susceptibility to COPD. Stratified analysis was then performed by age, sex, smoking, education level, coal and biomass as fuels. The consistency of ORs between the layers was tested by the Breslow-day test. The effect of genotypes on lung function was compared by the Kruskal-Wallis test.
The 10-year absolute risk was calculated by the method of Gail and Bruzzi [30, 31, 32, 33]. Based on a case-control study in Guangdong, the risk factors were screened by the backward stepwise logistic regression model. The relative risk and population-attributable risk were estimated by IRAP 2.2.0 (Bethesda, Maryland, USA).
IBM SPSS® Statistics 26.0 software (IBM Corp., Chicago, IL, USA) was used to analyze the data. The Odds ratio (OR) and 95% confidence interval (95% CI) were used as evaluation indexes. All analyses were two-sided with a significance level of 0.05.
Supplementary Table 1 shows the Demographic characteristics of the
case-control study in Guangdong with 1095 COPD patients and 1144 healthy
controls. The distribution of Education level, Smoking status, Coal as fuels, and
Biomass as fuels showed significant statistical differences between cases and
controls (all p
Supplementary Table 2 shows the Demographic characteristics of the
prevalence study in Gansu with 872 participants. There were 252 cases
We adjusted for sex, age, smoking status, education, coal as fuel and biomass as
fuel at a significance level of 0.05. As shown in Table 1, EPO rs1617640
C allele reduced COPD risk in southern Chinese significantly (AC vs. AA: adjusted
OR = 0.805, 95% CI = 0.669–0.969, p = 0.022; AC+CC vs. AA: adjusted OR
= 0.822, 95% CI = 0.689–0.980, p = 0.029). Unfortunately, we could not
observe the association between the other two SNPs (rs507392 A
| Models | Genotypes | Case (n = 1095) | Control (n = 1144) | Adjusted OR (95% CI) |
p |
AIC |
| n (%) | n (%) | |||||
| rs1617640 A |
AA | 725 (66.2) | 701 (61.3) | 1.000 (ref.) | ||
| AC | 317 (28.9) | 387 (33.8) | 0.805 (0.669–0.969) | 0.022 | ||
| Codominant | CC | 53 (4.8) | 56 (4.9) | 0.935 (0.628–1.392) | 0.742 | 3018.001 |
| Additive | p trend | 0.875 (0.757–1.012) | 0.073 | 3020.055 | ||
| Dominant | AC+CC vs. AA | 370 (33.8) | 443 (38.7) | 0.822 (0.689–0.980) | 0.029 | 3018.506 |
| Recessive | CC vs. AA+AC | 53 (4.8) | 56 (4.9) | 1.004 (0.678–1.488) | 0.984 | 3023.279 |
| rs507392 A |
AA | 697 (63.7) | 682 (59.6) | 1.000 (ref.) | ||
| AG | 341 (31.1) | 398 (34.8) | 0.849 (0.707–1.020) | 0.080 | ||
| Codominant | GG | 57 (5.2) | 64 (5.6) | 0.877 (0.600–1.283) | 0.500 | 3020.052 |
| Additive | p trend | 0.890 (0.771–1.026) | 0.108 | 3020.688 | ||
| Dominant | AG+GG vs. AA | 398 (36.3) | 462 (40.2) | 0.853 (0.717–1.015) | 0.074 | 3020.078 |
| Recessive | GG vs. AA+AG | 57 (5.2) | 64 (5.6) | 0.928 (0.638–1.351) | 0.697 | 3023.128 |
| rs564449 G |
GG | 915 (83.6) | 957 (83.7) | 1.000 (ref.) | ||
| GT | 172 (15.7) | 172 (15.0) | 1.051 (0.832–1.330) | 0.670 | ||
| Codominant | TT | 8 (0.7) | 15 (1.3) | 0.557 (0.230–1.346) | 0.222 | 3021.279 |
| Additive | p trend | 0.976 (0.792–1.202) | 0.817 | 3023.226 | ||
| Dominant | GT+TT vs. GG | 180 (16.4) | 187 (16.3) | 1.013 (0.806–1.273) | 0.912 | 3023.267 |
| Recessive | TT vs. GG+GT | 8 (0.7) | 15 (1.3) | 0.552 (0.229–1.334) | 0.187 | 3021.460 |
OR, odds ratio; CI, confidence interval; AIC, Akaike Information Criterion.
The results of stratification and interaction analysis are shown in Table 2.
Compared with EPO rs1617640 AA genotypes, AC and CC genotypes remained
protective in the strata of non-smokers (OR = 0.782, 95% CI = 0.616–0.992),
avoiding coal and biofuels (OR = 0.812, 95% CI = 0.674–0.979; OR = 0.829, 95%
CI = 0.687–0.999). The homogeneity test showed no differences among the
sublayers (p
| Variables | Case (n = 1095) | Control (n = 1144) | AC+CC vs. AA | p |
p | |||||
| AA | AC | CC | AA | AC | CC | OR (95% CI) | ||||
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |||||
| Age | ||||||||||
| 288 (64.6) | 132 (29.6) | 26 (5.8) | 303 (59.2) | 187 (36.5) | 22 (4.3) | 0.763 (0.579–1.005) | 0.836 | 0.738 | ||
| 437 (67.3) | 185 (28.5) | 27 (4.2) | 398 (63.0) | 200 (31.6) | 34 (5.4) | 0.856 (0.673–1.090) | ||||
| Sex | ||||||||||
| Male | 456 (67.2) | 192 (28.3) | 31 (4.6) | 461 (62.9) | 229 (31.2) | 43 (5.9) | 0.851 (0.677–1.070) | 0.670 | 0.772 | |
| Female | 269 (64.7) | 125 (30.0) | 22 (5.3) | 240 (58.4) | 158 (38.4) | 13 (3.2) | 0.765 (0.571–1.025) | |||
| Education level | ||||||||||
| Primary school or below | 340 (66.9) | 145 (28.5) | 23 (4.5) | 272 (62.7) | 143 (32.9) | 19 (4.4) | 0.849 (0.644–1.118) | 0.982 | 0.846 | |
| Middle school or High school | 345 (65.2) | 157 (29.7) | 27 (5.1) | 388 (60.1) | 224 (34.7) | 34 (5.3) | 0.802 (0.629–1.021) | |||
| College, undergraduate or above | 40 (69.0) | 15 (25.9) | 3 (5.2) | 41 (64.1) | 20 (31.2) | 3 (4.7) | 0.707 (0.301–1.663) | |||
| Smoking status | ||||||||||
| No | 353 (64.5) | 168 (30.7) | 26 (4.8) | 402 (59.2) | 246 (36.2) | 31 (4.6) | 0.782 (0.616–0.992) | 0.710 | 0.688 | |
| Yes | 372 (67.9) | 149 (27.2) | 27 (4.9) | 299 (64.3) | 141 (30.3) | 25 (5.4) | 0.888 (0.677–1.165) | |||
| Coal as fuels | ||||||||||
| No | 629 (66.7) | 268 (28.4) | 46 (4.9) | 650 (61.7) | 352 (33.4) | 52 (4.9) | 0.812 (0.674–0.979) | 0.857 | 0.909 | |
| Yes | 96 (63.2) | 49 (32.2) | 7 (4.6) | 51 (56.7) | 35 (38.9) | 4 (4.4) | 0.788 (0.439–1.414) | |||
| Biomass as fuels | ||||||||||
| No | 596 (65.6) | 268 (29.5) | 44 (4.8) | 628 (61.1) | 350 (34.0) | 50 (4.9) | 0.829 (0.687–0.999) | 0.781 | 0.885 | |
| Yes | 129 (69.0) | 49 (26.2) | 9 (4.8) | 72 (62.9) | 37 (31.9) | 6 (5.2) | 0.721 (0.426–1.221) | |||
OR, odds ratio; CI, confidence interval.
The effect of rs1617640 A
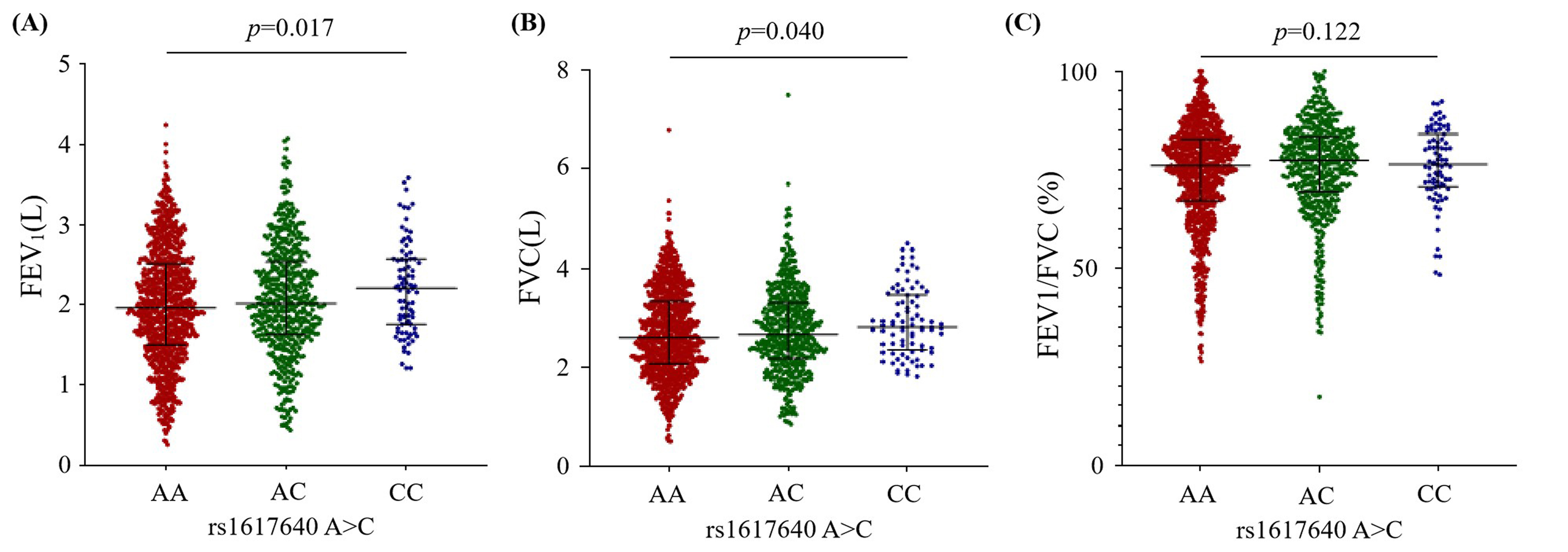 Fig. 1.
Fig. 1.The effect of the EPO rs1617640 A
 Fig. 2.
Fig. 2.The effect of the EPO rs1617640 A
We calculated the incidence of COPD from a clinical cohort constructed by electronic medical records from 2016 to 2020 in several hospitals in Guangdong Province (see Supplementary Table 3). The incidence of the 40-50 age group was 1436.73/100,000 person-years in males and 697.82/100,000 person-years in females. In the 50-60 age group was 2972.24/100000 person-years in males and 1321.07/100000 person-years in females. In the 60-70 years group was 5795.21/100,000 person-years in males and 2260.12/100,000 person-years in females.
A backward stepwise logistic regression model was fitted (Table 3), which
finally retained four factors, including education, smoking status, coal as
fuels, and rs1617640 A
| Factors | RR (95% CI) | ||
| Male | |||
| Education level | |||
| College, undergraduate or above | 1.000 (ref.) | ||
| Middle school or High school | 1.218 (0.766–1.937) | ||
| Primary school or below | 1.793 (1.111–2.894) | ||
| Smoking status | |||
| No | 1.000 (ref.) | ||
| Yes | 1.973 (1.559–2.495) | ||
| Coal as fuels | |||
| No | 1.000 (ref.) | ||
| Yes | 2.058 (1.436–2.951) | ||
| rs1617640 (Dominant) | |||
| AC+CC | 1.000 (ref.) | ||
| AA | 1.163 (0.925–1.463) | ||
| Population–attributable risk | 0.449 (0.258–0.641) | ||
| Female | |||
| Smoking status | |||
| No | 1.000 (ref.) | ||
| Yes | 1.809 (1.039–3.150) | ||
| Biomass as fuels | |||
| No | 1.000 (ref.) | ||
| Yes | 2.219 (1.440–3.418) | ||
| rs1617640 (Dominant) | |||
| AC+CC | 1.000 (ref.) | ||
| AA | 1.317 (0.987–1.758) | ||
| Population–attributable risk | 0.262 (0.128–0.396) | ||
RR, relative risk.
Combined with the retention factors, tables were developed to show the 10-year
absolute risk of COPD for men (Table 4) and women (Table 5) at different
individual relative risks [32, 33]. For example, a 45-year-old woman wants to
know her risk of developing COPD in 10 years. It is known that the woman with the
rs1617640 AA genotype does not smoke, and has a history of biomass as fuels.
According to Table 3, the individual relative risk of this woman is 2.922 (r =
RR
| Initial age (years) | Individual relative risk | ||||||||
| 0.00 | 1.00 | 2.00 | 3.00 | 4.00 | 5.00 | 6.00 | 7.00 | 8.00 | |
| 40–49 | 0.000 | 0.075 | 0.145 | 0.209 | 0.269 | 0.324 | 0.375 | 0.421 | 0.465 |
| 50–59 | 0.000 | 0.147 | 0.272 | 0.378 | 0.468 | 0.545 | 0.611 | 0.666 | 0.714 |
| 60–69 | 0.000 | 0.258 | 0.447 | 0.586 | 0.687 | 0.762 | 0.818 | 0.858 | 0.889 |
| Initial age (years) | Individual relative risk | ||||||
| 0.00 | 1.00 | 2.00 | 3.00 | 4.00 | 5.00 | 6.00 | |
| 40–49 | 0.000 | 0.050 | 0.097 | 0.142 | 0.185 | 0.226 | 0.265 |
| 50–59 | 0.000 | 0.092 | 0.175 | 0.251 | 0.319 | 0.382 | 0.438 |
| 60–69 | 0.000 | 0.150 | 0.276 | 0.384 | 0.475 | 0.552 | 0.618 |
To reveal the role of EPO polymorphisms on COPD, we investigated the
relationship between three SNPs of EPO (rs1617640 A
EPO, a pleiotropic factor, can affect the occurrence and progression of many diseases [34, 35]. EPO was reported that expressed in the lung and responded to Hypoxia by promoting erythropoiesis and neovascularization [13, 36, 37, 38]. In addition, in acute lung injury, EPO could activate the proliferation of pulmonary endothelial cells and reduce the infiltration of inflammatory cells, which plays a key role in controlling pulmonary ventilation. Some studies have shown that the inflammatory response and hypoxemia caused by COPD change the expression of EPO. For example, COPD patients have up-regulated EPO expression due to increased inflammatory factors and decreased erythrocytes in the body [39, 40]. Thus, an increase in hemoglobin concentration by upregulation of EPO’s expression can correct hypoxemia seen in COPD patients commonly [22, 41, 42]. Existing researches indicate that EPO may participate in the development of COPD. However, no study has explored whether EPO polymorphisms modify the susceptibility to COPD.
We finally retained three SNPs (rs1617640 A
In this study, we first discovered that the variation of EPO rs1617640
A
Our clinical cohort found that the incidence of COPD and the mortality without COPD differed between men and women, both indicators were higher in males than in females in all age strata (see Supplementary Table 3). This is consistent with the findings of Garcia Rodriguez in the incidence of COPD [51]. Hence, we decided to fit a 10-year absolute risk model for COPD in southern Chinese by sex. We found the factors retained in the models of males and females were slightly different (see Table 3), which may be related to the reduction of sample size after stratification. At the same time, the differences in the social division of labor and lifestyles between men and women may also lead to differences in risk factors [52, 53]. In the model for the female, the population attributable risk was 0.262 (0.128–0.396), indicating that additional risk factors need to be considered to predict the risk of COPD accurately [54].
There are some limitations in this study. First, this was a case-control study, and there was a certain degree of recall bias when obtaining environmental exposure information. Secondly, the participants in this study were mainly Han Chinese, which may restrict the extrapolation of conclusions to other populations. Moreover, this study clarified the association between EPO polymorphisms and COPD using epidemiological methods, which needed further verification by cellular and molecular experiments. Finally, this study did not conduct whole genome sequencing and analysis, it’s a potential limitation of the study.
In conclusion, this study reported for the first time that the EPO
rs1617640 A
COPD, chronic obstructive pulmonary disease; EPO, Erythropoietin; GWAS,
genome-wide association analysis; SNPs, the single nucleotide polymorphisms;
FEV
The data that support the findings of this study are available from the corresponding author upon reasonable request.
YW and ZL conceived and designed the study with support from XW and JL as well as wrote the manuscript; XZ conducted the experiment; YW, ZL, AL and XZ preformed the statistical analysis of the results; YP, CM, CC, CX, DH, YD, and XZ participated in sample collection for the case-control study and prevalence study, as well as the electronic medical record entry. XW and JL were responsible for the design and quality control of the whole research and the guidance of the methodology. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work to take public responsibility for appropriate portions of the content and agreed to be accountable for all aspects of the work in ensuring that questions related to its accuracy or integrity.
This study was performed under the Declaration of Helsinki and approbated by the institutional review boards of Xi’an Jiaotong University Health Science Center (approval: XJTU 2016-411) and Guangzhou Medical University (approval: GZMC2007-07-0676).
In addition, we explained the purpose of the study to all participants at the time of the study and obtained their signed informed consent.
Thanks to the Institute of Public Health, Guangzhou Medical University. Thanks to the researchers of this laboratory for their guidance on experimental technology.
This study was supported by the following grants: National Key Research and Development Project of China 2017YFC0907202 (Xinhua Wang); National Natural Science Foundation of China 81872694, 82173609 (Jiachun Lu); Local Innovative and Research Teams Project of Guangdong Pearl River Talents Program 2017BT01S155 (Jiachun Lu); Guangdong Medical Science and Technology Research Fund Project A2020324 (Cuiyi Chen); National Natural Science Foundation of China (81460123) and Guangxi Natural Foundation (2018GXNSFAA281187) (Yibin Deng); National Natural Science Foundation of China (82260889) (Xuhui Zhang); the Science and Technology Project of Guangzhou (No.202103000073).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
