- Academic Editor
†These authors contributed equally.
Background and Aims: Previous studies have confirmed the
anti-inflammation effect of bone marrow mesenchymal stem cell-derived exosomes
(BMSC-Exo). We aimed to investigate the therapeutic effect of BMSC-Exo on
diabetic kidney disease (DKD), as well as the underlying mechanisms.
Methods: SD rats were induced by streptozotocin combined with a high-fat
diet to establish a diabetes disease model. BMSCs-Exo were injected via tail
veins at a weekly dose of 100 µg for 12 weeks. Pathological changes
in the rat kidneys were evaluated using HE, Masson, and Periodic Acid-Schiff and
immunohistochemical staining. TUNEL staining and western blot were used to
evaluate the expression levels of apoptosis-related proteins in the rat kidney
cells. The TNF-
Diabetic kidney disease (DKD) is one of the most common and serious
complications of diabetes and is the leading cause of end-stage renal disease.
Long-term hyperglycemia, hypertension, and inflammation are the main etiologies
that lead to the development of DKD [1]. The pathological manifestations of DKD
involve increased glomerular mesangial matrices and thickening of the basement
membrane and glomerular sclerosis, along with proteinuria, decreased glomerular
filtration rates, and other functional abnormalities [2]. The pathogenesis of DKD
is very complex and is still not fully understood, and treatments are not fully
optimized either [3]. The pathogenesis of DKD usually results from hemodynamic
dyshomeostasis, metabolic disorders, and abnormal hormone synthesis [4]. The
renin-angiotensin-aldosterone system, formation of advanced glycation end
products, activation of transforming growth factor
In classical DKD patients, standard therapy still focuses on blood glucose and blood pressure control, targeting to halt the DKD progression and regression of albuminuria. These drugs mainly include inhibitors of dipeptidyl peptidase 4 (DDP-4) and sodium-dependent glucose transporters 2 (SGLT2), and classic hypoglycemic drugs such as metformin and renin aniotension aldosterone system (RAAS) antagonists. However, the above strategies have been shown only to slow down the progression but cannot completely prevent DKD from progressing to end-stage renal failure or reduce mortality [5, 7]. Thus, there is an urgent need for new therapeutic approaches to improve renal function in DKD patients, delay disease progression, and reduce mortality.
Numerous studies have shown that stem cell transplantation can successfully prevent the progression of DKD [8]. The therapeutic effect of bone marrow mesenchymal stem cells (BMSCs) on DKD has been validated [9, 10, 11]. and investigated in clinical trials [12]. The therapeutic potential of stem cells rests on their paracrine function and is particularly related to exosomes [13]. Exosomes are nanoscale lipid bilayer extracellular vesicles that are actively released by mammalian cells into intercellular substances and circulation and are thus involved in various physiological and pathological processes in the body [14]. Previous work has shown that bone marrow mesenchymal stem cells derived exosomes (BMSC-Exo) and the microRNAs they contain can alleviate DKD, but the mechanism has not been fully elucidated [15, 16].
BMSC-Exo has also been shown to have anti-inflammatory and anti-apoptotic effects [14]. Thus, we speculate that BMSC-Exo can alleviate DKD by inhibiting inflammation and stressing anti-apoptosis pathways. Our study may provide new evidence for the treatment of DKD.
We selected 2-week-old male SD rats, isolated their femurs and tibias, washed
the bone marrow cavities with a complete medium, pipetted the marrow into a cell
suspension, and separated mononuclear cells using density gradient centrifugation
with Ficoll-Paque PREMIUM 1.084 (Cytiva, Boston, MA, USA). Cells from the tibia and femur
of one rat were inoculated into two T25 cell culture flasks. The isolated and
purified cells were grown in DMEM/F-12 medium (Gibco, Grand Island, CA, USA) containing 20%
fetal bovine serum (Gibco, CA, USA) and 1% penicillin-streptomycin solution
(Gibco, CA, USA) in a humidified cell culture incubator at 37 °C with
5% CO
In addition to the morphological observations under the light microscope, the P3 generation cells were induced to differentiate into adipogenic, osteogenic, and chondrogenic lines, and were subsequently characterized by oil red O staining, alizarin red staining, and allicin blue staining, respectively. The expression of cell surface markers CD29 (positive), CD44 (positive), and CD34 (negative) was detected by flow cytometry (Supplementary Fig. 1).
After the BMSCs grew to 50%–60% confluence, the original medium was removed,
washed three times with PBS (Gibco, Grand Island, CA, USA), and replaced with DMEM/F-12
containing 5% exosome-free FBS (OriCell, Wuhan, China). The cell culture
supernatant was collected after 48–72 h, and centrifuged at 500
To identify the extracted BMSC-Exo, transmission electron microscopy (TEM, Hitachi, Tokyo, Japan) was used to capture images of exosome morphology. Nanoparticle tracking technology (NTA, ParticleMetrix, Ludwigshafen, Germany) was applied to analyze the particle size distribution of exosomes, and western blotting was used to detect expressions of CD9 and CD81 on the exosomal surface.
5-week-old male SD rats (150–200 g) were purchased from HFK Bioscience Co, Ltd. (Beijing, China) and kept in the SPF animal laboratory while being fed a high-fat diet (HFD). After two weeks of acclimation, streptozotocin (STZ, 35 mg/kg/day, dissolved in 0.01 M citrate buffer, PH4.5) (Sigma, Saint. Louis, MO, USA) was intraperitoneally injected into the rats in the experimental group for three consecutive days, while the rats in the control group were injected with an equal volume of vehicle. To measure the postprandial blood glucose levels by a blood glucose meter (Roche, Basel, Switzerland), blood samples were collected each morning (8:00 AM) by acupuncturing the tail vein with a 28G needle. Rats with fasting glucose continuously above 11.1mM were considered to be successful diabetic models [17]. A total of 32 rats were divided into 4 groups and given different treatments up to 18 weeks. Excluding modeling failure and death, the remaining 20 rats were finally included in the follow-up experiments: the control group (NC group, n = 5), a diabetes group (DM group, n = 5), a metformin treatment group (Met group, n = 5), and an exosome treatment group (Exo group, n = 5). From the fourth week, the Met group was treated with 300 mg/kg/day of metformin by gavage, while the other three groups were given the same volume of pure water by gavage. The Exo group was given 100 µg (dissolved in 200 µL 0.9% sodium chloride solution) of exosomes through the tail vein every week, and the other three groups were given the same amount of normal saline at the same time points [17]. After 14 weeks of continuous administration, the rats were anesthetized with sodium pentobarbital (45 mg/kg body weight). Carotid blood was collected to assess blood glucose (GLU), urea nitrogen (BUN), and creatinine (Cr) levels. Each rat’s left kidney was fixed in a 4% paraformaldehyde solution overnight. The right kidney was kept in liquid nitrogen for qRT-PCR and Western blot detection. The rat grouping treatment strategy is shown in Fig. 1.
 Fig. 1.
Fig. 1.Preparation of rat diabetes model and group administration. HFD, high-fat diet; STZ, streptozotocin.
Blood samples obtained from rat carotid artery blood collection were allowed to stand at 37 °C to promote coagulation. After the blood was coagulated, it was centrifuged at 2500 rpm for 10 min, and the supernatant was aspirated. We used an Indiko automatic biochemical analyzer (Thermo Scientific, CA, USA) to measure GLU, BUN, and Cr concentration.
The kidneys fixed in 4% paraformaldehyde were clarified, dehydrated, embedded in paraffin, and cut into micron-thick sections. Sections were stained with hematoxylin-eosin (H&E) and Masson and Periodic Acid-Schiff (PAS) staining to assess the severity of diabetic kidney disease. Type IV collagen immunohistochemical staining was performed on the remaining sections. The sections were first incubated with Collagen IV polyclonal antibodies (1:200, Bioss, Beijing, China) at 4 °C overnight, washed with PBS for 5 min 3 times, and then incubated with biotin-labeled secondary antibodies for 1 hour at room temperature. The stained sections were scanned with an Aperio Scan Scope AT Turbo scanner (Leica, Wetzlar, Germany) to obtain images, and Image Scope software was used to analyze glomeruli morphology, renal tubules, and collagen deposition. For each kidney tissue, we selected three different slices and five fields of view in each slice. Twenty glomeruli were randomly selected from each kidney to assess the lesion.
Paraffin sections of left rat kidneys were stained with a TUNEL staining kit (Roche, Basel, Switzerland) to identify apoptotic cells in kidney tissue via fluorescein-dUTP labeling of DNA fragmented strands. Images were acquired using an Eclipse NI microscope (Nikon, Tokyo, Japan).
Total RNA was extracted from right kidney tissue frozen in liquid nitrogen using TRIzol (Thermo Fisher Scientific, MA, USA). The Revertaid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, MA, USA) was used for reverse transcription, and cDNA amplification was carried out using an SYBR Green Master Mix Kit (Takara, Otsu, Japan). Relative mRNA levels were calculated using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal standard. All primers were synthesized by Sangon Biotech and are listed in Table 1.
| Primer sequences | Probes | |
| TNF- |
Forward primer | 5′-AAACACACGAGACGCTGAAG-3′ |
| Reverse primer | 5′-ATCCAGTGAGTTCCGAAAGC-3′ | |
| GAPDH | Forward primer | 5′-CAACTCCCTCAAGATTGTCAGCAA-3′ |
| Reverse primer | 5′-GGCATGGACTGTGGTCATGA-3′ | |
The right kidney tissue was homogenized with RIPA and centrifuged to obtain
total tissue protein. The protein molecules were separated with 12.5% SDS PAGE
gel electrophoresis, and the protein was transferred to a 0.22 µm PVDF
membrane by a wet transfer method. A rapid blocking solution (Servicebio, Wuhan,
China) was used for blocking at room temperature for 1 hour. PVDF membrane and
rabbit-derived primary antibodies against against Bax (1:1000, Cell Signaling Technology, Boston, MA, USA),
Caspase 9 (1:1000, CST, MA, USA), Caspase 3 (1:1000, CST, MA, USA),
NF-
Data are presented as means
TEM images revealed that BMSC-Exo had a typical saucer-like structure (Fig. 2a). NTA showed that the average particle size of the particles in the
sample was 136.5
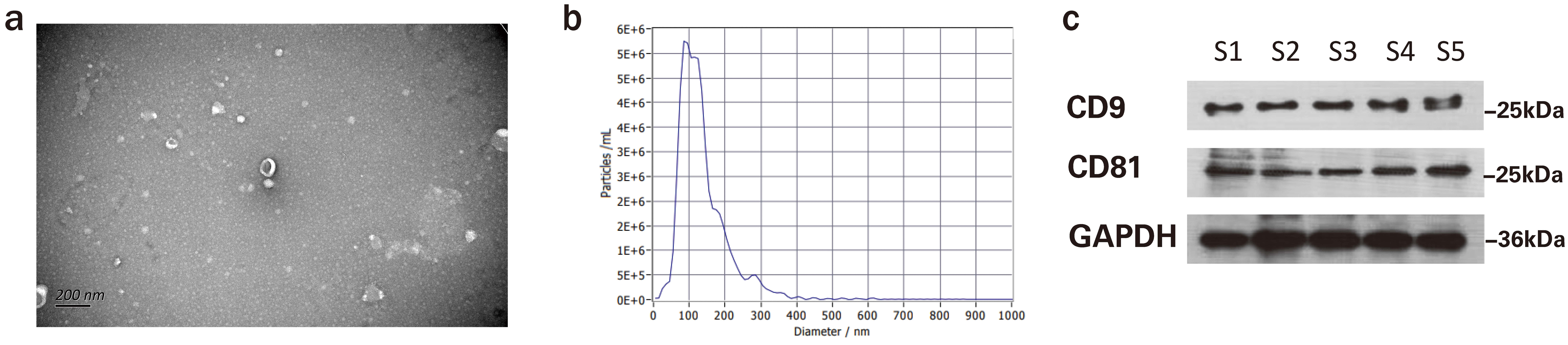 Fig. 2.
Fig. 2.Identification of BMSCs-Exo. (a) Transmission electron microscopy (TEM) shows that BMSCs-Exo have a “sauce-like” structure. (b) Analysis of BMSCs-Exo Particle Size Distribution by Nanoparticle Tracking Technology (NTA). (c) Exosome markers (D63, and CD81) are detected by Western Blot (S1-S5, 5 batches of samples).
GLU, BUN, and Cr were notably increased in the DM group compared with the NC,
Met, or Exo groups (p
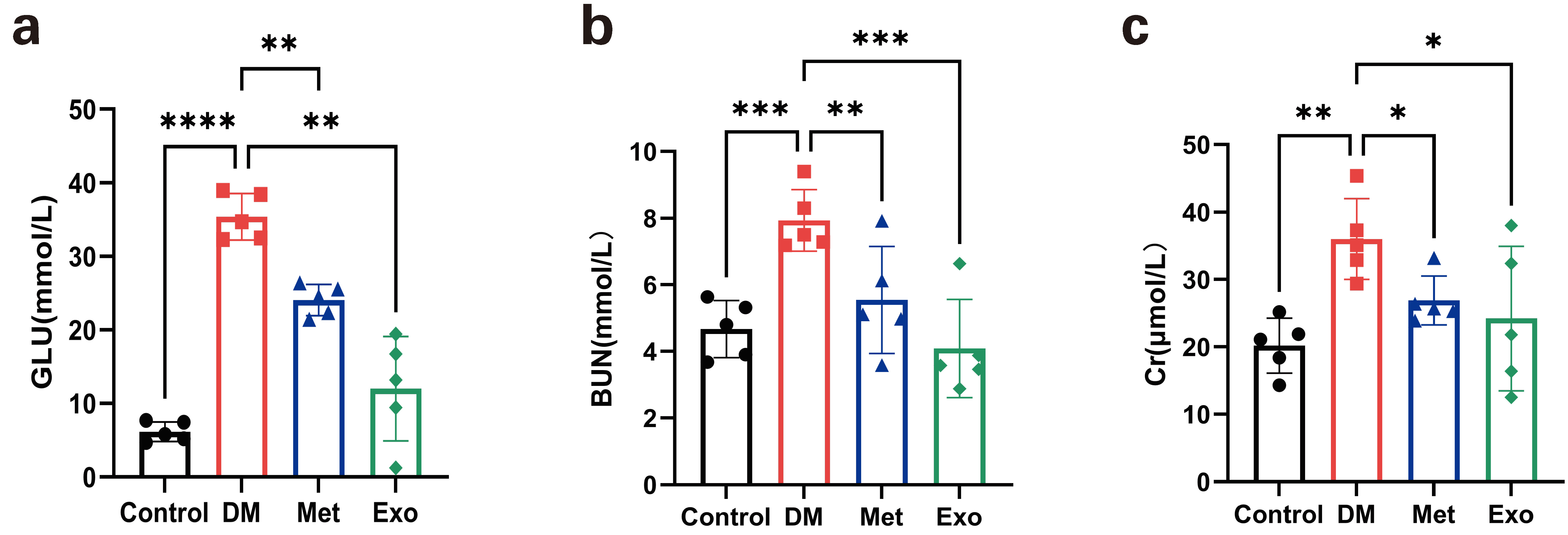 Fig. 3.
Fig. 3.BMSCs-Exo reduces blood glucose and improves renal function in
diabetic rats. (a) Rats grouping and dosing schedule. (b,c) Comparison of GLU,
BUN, and Cr in the NC group, DM group, Met group, and Exo group. *p
To evaluate the pathological changes of kidney damage in diabetic rats, we performed PAS staining and Masson staining in addition to conventional H&E staining and detected the synthesis and secretion of collagen IV (the most important matrix collagen in diabetic kidney disease) with immunohistochemical techniques. PAS staining showed that, compared with the NC group, the glomerular basement membrane of the DM group was homogeneously thickened, the mesangial matrix was increased, and there were no Kimmelstiel-Wilson (K-W) nodules with obvious arteriolar hyalinization. The basement membrane in the renal tubular region was markedly thickened (Fig. 4a). Masson staining showed that the glomerular basement membrane, mesangial matrix, surrounding tubules, and surrounding blood vessels were stained blue in the DM group, indicating that there was obvious collagen deposition in the kidney (a manifestation of renal fibrosis; Fig. 4b). Immunohistochemistry for collagen IV showed that collagen IV in the DM group was slightly distributed in the glomerular mesangial matrix, but was mainly in the tubular cells and interstitium and that the staining was more obvious than it was in the NC group (Fig. 4c). The above-mentioned manifestations were significantly alleviated after the administration of BMSCs-Exo and metformin. Compared with the Met group, the renal pathological manifestations of the BMSCs-Exo group were lighter.
 Fig. 4.
Fig. 4.BMSC-Exo alleviated diabetic kidney disease in the Exo group.
(a) PAS staining of rat renal tissues (200
To evaluate the renal cell apoptosis in diabetic rats, we performed TUNEL
staining on the pathological sections of rat kidneys. The results showed that the
DM group had more severe apoptosis than the NC group. Administration of Met
significantly reduced apoptosis, and fewer apoptotic cells were detected with Exo
than with Met (Fig. 5a). The pro-apoptosis protein Bax was detected by western
blot, and the results corresponded with TUNEL staining. The relative expression
of Bax in the rat kidneys in the DM group was significantly elevated compared
with those in the NC group (p
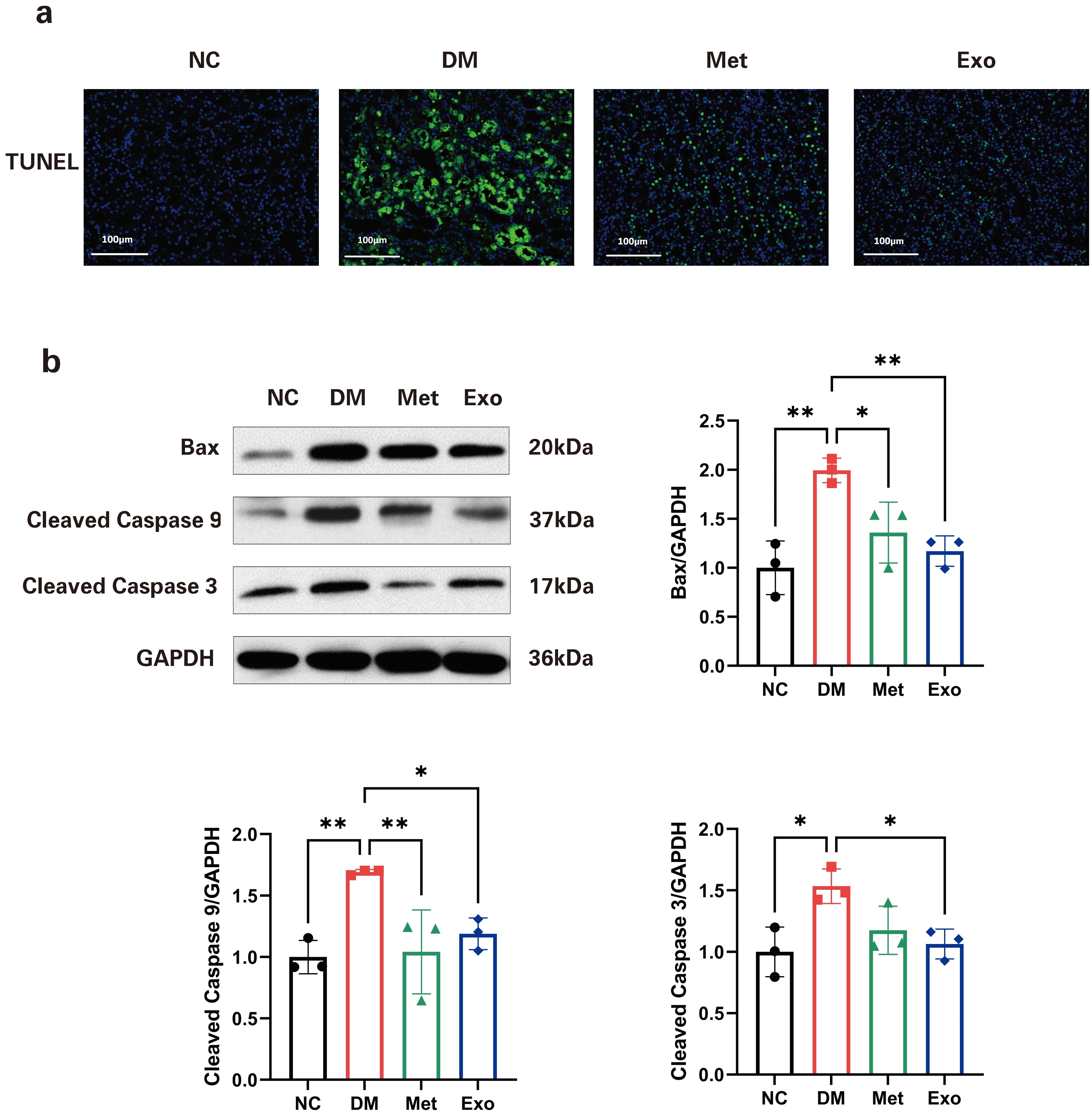 Fig. 5.
Fig. 5.BMSCs-Exo reduced apoptosis in renal tissue. (a) TUNEL staining
of rat renal tissues (200
To observe the renal inflammatory state of diabetic rats and the interventional
effects of Exo, we observed the distribution of inflammatory cells in kidney
tissue using H&E staining, detected the transcription level of pro-inflammatory
factor TNF-
 Fig. 6.
Fig. 6.BMSCs-Exo attenuates kidney inflammation in diabetic rats. (a)
H&E staining showed the rat kidney tissue structure and inflammatory cell
infiltration (400
This study found that exosomes derived from BMSCs reduced GLU, BUN, and Cr levels in diabetic rats, and also reduced glomerulosclerosis and collagen deposition in diabetic rats. Furthermore, we found that the inflammation and apoptosis in the kidney tissue of diabetic rats were significantly aggravated, and the BMSC-Exo could reduce the infiltration of inflammatory cells, the release of inflammatory factors, and apoptosis in the kidney tissue, and regulate apoptosis-related genes, apoptosis-related proteins, and inflammatory signaling pathways, thus improving renal function and alleviating renal damage in diabetic rats. This experiment provides laboratory evidence for the treatment of diabetic kidney disease BMSC-derived exosomes.
Obtaining reliable exosomes is the first step in this experiment. We tested the exosomes isolated from the supernatant of BMSCs in terms of three aspects: morphology, particle size analysis, and surface molecules. The characteristics are in line with Minimal Information for Studies of Extracellular Vesicles (MISEV) guidelines for the field, issued by the International Society for Extracellular Vesicles in 2018 (ISEV) [18]. This provides support for the reliability of the experimental results.
We observed that BMSC-Exo had a hypoglycemic effect that was not inferior to
metformin and also had a reversal effect on serum BUN and Cr, which reflect
glomerular function. Hyperglycemia is associated with the pathological
progression of diabetic kidney disease and can lead to increased glycation end
products (AGEs), which are the major causes of hyperglycemic kidney damage [19].
In vivo, AGEs can activate the local renin-angiotensin aldosterone
system (RAAS) in kidney tissue, stimulate endothelial cells to release vasoactive
substances, and dilate afferent arterioles, resulting in increased glomerular
filtration pressures and an imbalance between the bulbs and tubes and
causing disturbance of renal vascular hemodynamics. This series of processes
eventually lead to a decrease in the glomerular filtration rate, which manifests
in renal dysfunction [20, 21]. BMSC-Exo improved renal function while lowering
blood sugar. This dual-therapeutic effect could be related to the above-mentioned
mechanisms. In terms of the hypoglycemic mechanism, several studies have shown
that BMSC-Exo can chemoattract pancreatic tissue through the pancreas and
duodenal homeobox1 pathway and promote the regeneration of pancreatic
At the tissue level, rats in the model group only showed early manifestations of diabetic kidney disease (for example, no typical K-W nodular structure), and some studies have shown that standard diabetic animal models induced with low-dose STZ can only reflect mild renal damage [26]. It is worth noting that we observed that BMSC-Exo had significant effects on pathological manifestations known to be related to diabetic nephropathies, such as glomerular basement membrane thickening, mesangial matrix expansion, tubular basement membrane thickening, inflammatory cell infiltration, and matrix collagen deposition. Nagaishi et al. [27] have observed that BMSC-Exo can alleviate the renal pathological damage caused by diabetes in HFD and STZ-induced mouse models of diabetic kidney disease, which is consistent with what we observed in STZ combined with HFD-induced diabetic rat models.
Furthermore, our TUNEL results showed that the apoptosis rate of renal cells in diabetic rats was significantly increased but that these effects were alleviated by BMSC-Exo. Previous studies have confirmed that diabetes leads to increased renal AGEs and local metabolic disorders, and induces glomerular podocyte apoptosis, resulting in proteinuria [28]. Diabetic kidney disease is also accompanied by apoptosis of proximal convoluted tubule epithelial cells, resulting in renal tubular atrophy and thus a lack of connection between the glomeruli and proximal convoluted tubules [29, 30, 31]. BMSC-Exo induces autophagy through the mTOR signaling pathway to alleviate fibrosis in diabetic kidneys. The inhibition of autophagy is an inducing factor for apoptosis, suggesting that the observed BMSC-Exo-mediated reduction of apoptosis in diabetic kidneys may be achieved by inducing autophagy [32]. In diabetic kidney disease, apoptosis may result from an imbalance in the interaction between the pro-apoptotic Bax and anti-apoptotic Bcl-2 family members [33]. We also observed that Bax protein increased in diabetic rats but decreased after BMSC-Exo treatment. Caspases also play a key role in apoptotic programs [34]. We also observed that BMSC-Exo regulated the activation of initiator caspase 9 and executioner caspase 3. Thus, BMSC-Exo may improve diabetic kidney disease by regulating several stages of renal cell apoptosis.
We also observed that BMSC-Exo attenuated the inflammatory state in diabetic
kidney disease kidneys. BMSC-Exo reduced inflammatory cell infiltration in renal
tissue. However, only TNF-
Our study did not verify or further explore the mechanism of action of BMSC-Exo in vitro. In addition, exosome contents contain proteins, lipids, and various nucleic acid molecules. Many of these key molecules need to be further screened to identify new molecular targets for the treatment of diabetic kidney disease. Besides, whether metformin and BMSC-Exo have a synergistic effect on the treatment of diabetes and related kidney damage deserves further exploration.
In summary, the proposed mechanism of BMSC-Exo in the treatment of DKD has been shown in the graphical abstract. Because DKD is caused by a combination of factors, the pathogenesis is complex. The hypoglycemic, anti-inflammatory, and anti-apoptotic pleiotropic effects of BMSCs-Exo may provide a potential new therapeutic approach for the treatment of diabetic kidney disease.
BMSCs-Exo ameliorates renal pathological damage and improve renal function in diabetic rats by reducing apoptosis and reducing inflammation.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
JT, CG and XS designed the research study. LL and YZ performed the research. XZ, XY, HZ, and XW were involved in the animal feeding, model construction, and sample collection processes. ZA analyzed the data. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
All animal experiments were approved by the Animal Experiment Center of Beijing Anzhen Hospital ethics committee at Capital Medical University (approval No. 2022152X), which complies with the guide for the care and use of laboratory animals (eighth edition).
We would like to thank the public experimental platform of the Beijing Institute of Heart Lung and Blood Vessel Diseases for providing technical support for this study.
This work was supported by the Beijing Municipal Science and Technology Project (Z161100000516139), National Nature Science Foundation (82270341 and 82100486), ‘qingmiao’ plan (QML20210603), the Capital Health Research and Development of Special (No. 2022-1-2061).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
