Retraction of Frontiers in Bioscience-Landmark 2024, 29(4)
1 Department of Pharmaceutical Chemistry, Faculty of Pharmacy, The Islamia University of Bahawalpur, 63100 Punjab, Pakistan
2 Primary and Secondary Health Care Department, Government of Punjab, 63100 Punjab, Pakistan
3 Department of Medicinal Chemistry, College of Pharmacy, University of Minnesota, Minneapolis, MN 55455, USA
4 Department of Pharmacognosy, Faculty of Pharmacy, Gazi University, 06330 Ankara, Turkey
5 Department of Pharmaceutics, Faculty of Pharmacy, The Islamia University of Bahawalpur, 63100 Punjab, Pakistan
6 Instituto de Investigación y Postgrado, Facultad de Ciencias de la Salud, Universidad Central de Chile, 8330507 Santiago, Chile
7 Department of Organic Chemistry, Faculty of Pharmacy, University of Santiago de Compostela, 15782 Santiago de Compostela, Spain
Abstract
Background: A previously unstudied medicinal plant,
Leucophyllum frutescens (Berland.) I.M. Johnst. (Scrophulariaceae) was
investigated to evaluate its potential in preventing and treating
neurodegenerative diseases, including Alzheimer’s disease. Methods:
Methanolic leaf extract (MELE) and its fractions (HELE, CHLE, and BULE) were
evaluated for their polyphenolic content and antioxidant activity by five
different methods, including in vitro enzyme inhibition assays, which
are clinically linked to neurodegenerative diseases. The potentially active
n-butanol fraction (BULE) was further evaluated for its neuroprotective
effects using an albino rat animal model and phytoconstituents profiling using
Liquid chromatography with tandem mass spectrometry (LC–MS/MS), and in silico molecular docking by Maestro®
Schrödinger. Results: The n-butanol fraction (BULE) in the
hydroalcoholic leaf extract exhibited the highest total phenolic content (230.435
Keywords
- antioxidant activity
- enzyme inhibition assay
- liquid chromatography-mass spectrometry
- Leucophyllum frutescens
- Scrophulariaceae
- polyphenolic content
Dementia is a chronic and progressive neurodegenerative disease that impacts
more than 55 million older people worldwide [1]. The most frequent form of
dementia in older people is Alzheimer’s disease (AD), with age, genetics, and
traumatic brain damage all being contributory elements to its onset, which
ultimately, leads to mortality through the loss of cognitive function [2]. In the
US, AD accounts for 60–70% of the decline in cognitive function in the elderly
and is the seventh largest cause of death [3]. In Pakistan, the WHO reported in
2020 that dementia and AD were responsible for 1.3% of all mortality worldwide
[4]. The buildup of various-sized hyperphosphorylated beta-amyloid and tau
proteins in neural fibers is a hallmark of the histopathology of Alzheimer’s
disease [5]. Unfortunately, the development of safe and effective
disease-modifying drugs to treat the cognitive problems experienced by dementia
patients has started to lag. However, a recently approved monoclonal antibody
drug, lecanemab-irmb (Leqembi®), may delay cognitive
decline, and thus, represent a change in the history of AD therapy. Over the past
two decades, tacrine, a centrally acting acetylcholinesterase inhibitor with
indirect cholinergic agonist action, was withdrawn from the market due to
hepatotoxicity, thereby leaving cholinesterase inhibitors (e.g., donepezil,
galantamine, and rivastigmine) and N-methyl-D-aspartate (NMDA) antagonists (e.g., memantine HCl) as the
only medications approved for the treatment of AD [5]. Studies on phytochemicals
that inhibited acetylcholine esterase and butrylcholine esterase showed that they
produced higher levels of acetylcholine in the synaptic cleft, which promoted
neurotransmission and resulted in increased cognitive responses [6]. A study
where 150 lignans were used as inhibitors of various target enzymes, known to be
involved in several oxidative pathways, concluded that 139 of the lignans
inhibited two or more of the studied enzymes, including JNK-3, PTPIB, NOX1, etc.
[7]. Polyphenolic compounds have previously been used to treat various
neurological disorders, such as Parkinson’s and Alzheimer’s disease. Lignans,
which are found in a variety of plant sources, have been studied for their
neuroprotective effects in H
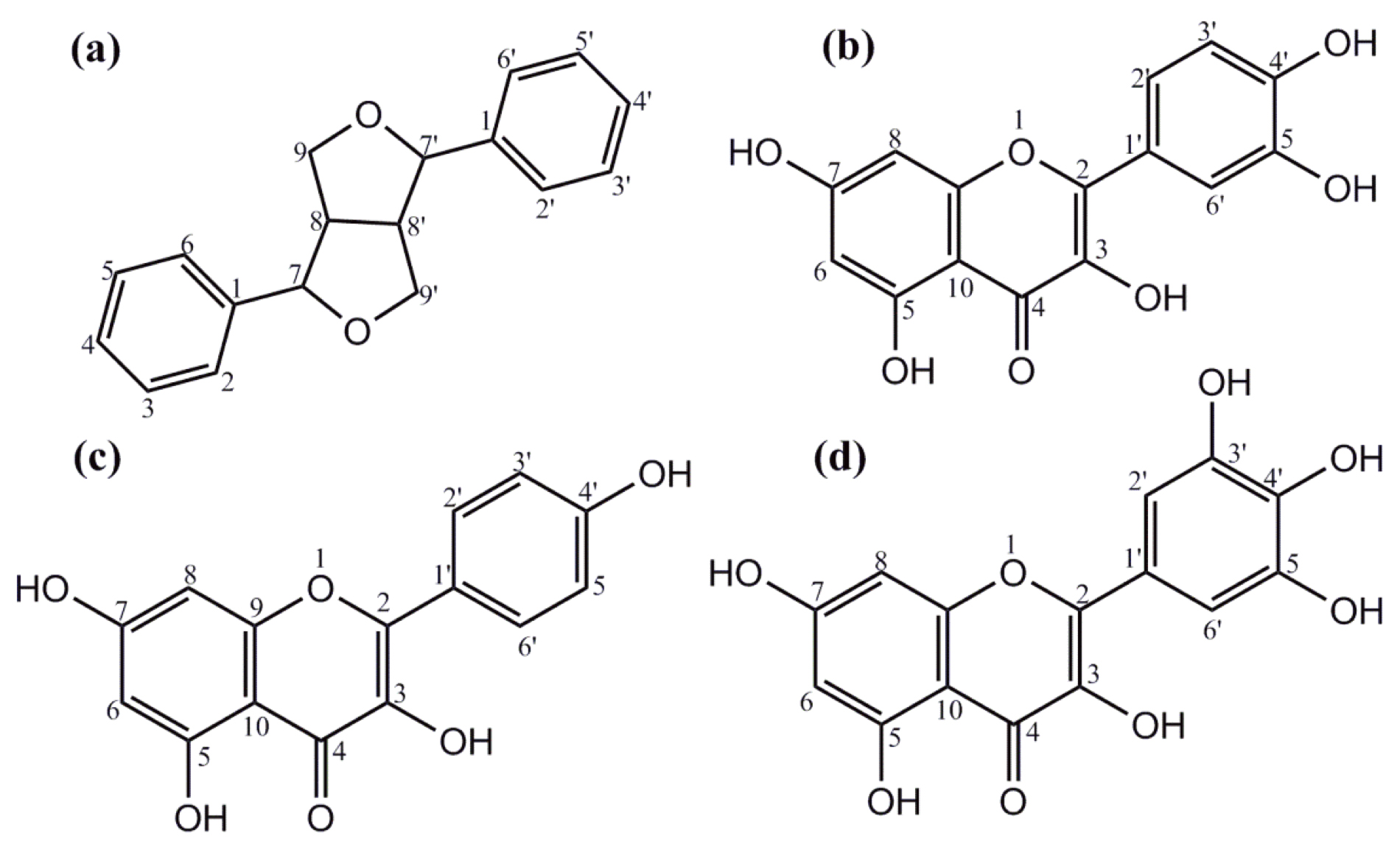 Fig. 1.
Fig. 1.Structures of phenolics and flavonoids. (a) The furanofuran lignan backbone and general structure are found in many plant-derived phenolics. Structures of some flavonoids found in L. frutescens leaf extracts: (b) quercetin, (c) kaempferol, and (d) myricetin.
Numerous studies [10] have demonstrated that people with diabetes mellitus have
an increased risk of developing AD, although the underlying biological
mechanism(s) linking the two diseases is unclear. Diabetes mellitus is
characterized by high blood glucose levels (hyperglycemia), characterized by the
destruction of pancreatic
There are various strategies being considered for directly treating or mitigating Alzheimer’s disease. Currently, a common strategy is the inhibition of acetylcholinesterase (AChE) and butyrylcholinesterase [5]. Native Americans have used the leaf decoction of Leucophyllum frutescens (Berland.) I.M. Johnst., known as Cenizo, for its mild sedative effects in addition to treating lung congestion, bronchitis, and chills associated with the common cold [13]. The aim of this study was to evaluate the potential of this plant against AD. Thus, a hydroalcoholic leaf extract (MELE) and its three different solvent fractions (HELE, CHLE, and BULE) were obtained and evaluated as potential sources of neuroprotective agents by examining their polyphenolic content, antioxidant potential, ability to inhibit enzymes clinically linked to neurodegeneration, and neuroprotection in an animal model.
This study was approved by the Advanced Studies and Research Board of the Islamia University of Bahawalpur, via letter No. 673/AS&RB, dated December 16, 2019. The plant was purchased from a local nursery for authentication by a taxonomist at the Herbarium Department of Botany, Faculty of Life Sciences, The Islamia University of Bahawalpur (Ref no. 60/botany; dated September 25, 2018).
Ammonium acetate, ferric chloride, methanol, absolute ethanol, n-hexane, chloroform, n-butanol, dimethyl sulfoxide, deionized water, and hydrogen peroxide were purchased from Merck KGaA (Darmstadt, Germany). All other chemicals, including acetylcholinesterase from human erythrocytes (C0663), butyrylcholinesterase from equine serum (C0157), alpha-amylase from human saliva (A1031; type XIII-A), 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2-azinobis (3-ethylbenothiazoline), 6-sulfonic acid, 2,4,6-tris (2-pyridyl)-s-triazine (TPTZ), and neocuproine were purchased from Sigma-Aldrich, St. Louis, MO, USA.
The 200 intact L. frutescens were purchased from a nearby nursery in
Bahawalpur, Pakistan (29°23
The qualitative analysis of secondary metabolites was performed according to the
methods described in Trease and Evan’s Pharmacognosy [14]. Total phenolic content
was determined colorimetrically using Folin and Ciocalteu’s phenol reagent [15].
The total phenolic content values were calculated using the straight-line
equation, Y = 0.0105x + 0.0702, in which Y is the absorbance of the sample and x
is the amount of total phenolic content in µg∙mL
Antioxidant activity was measured using five different methods. Total
antioxidant activity (TAA) was determined using the phosphomolybdenum method [15]
and the results were expressed as mg ascorbic acid Eq∙gm
Acetylcholinesterase and butyrylcholinesterase enzyme inhibition assay was
performed according to a modified version of the Ellman method [5]. The sample
solution of the MELE, HELE, CFLE, and BULE was diluted to prepare solutions of
different concentrations, including 150, 15.0, 1.5, 0.15, and 0.05
µg∙mL
Inhibition (%) = [1-(
where A_sample is the absorbance of the sample and A_control is the absorbance of the solution without the sample.
The ability of the leaf extract samples to inhibit alpha-amylase from human saliva (A1031, type XIII-A) was determined using the 96-microplate spectrophotometric method by Magaji et al. [17]. Acarbose was used as a positive control. The percent inhibition was determined using the following formula:
Inhibition (%) = [1-(
where A_sample is the absorbance of the sample and A_control is the absorbance of the solution without the sample.
IC
The butanol fraction of hydroalcoholic leaf extract (BULE) was investigated
using reverse-phase liquid chromatography with mass spectral analysis (LC–MS/MS)
in an LTQ XL Linear Ion Trap Mass Spectrophotometer (Thermo Scientific, Waltham, MA, USA),
equipped with an electrospray ionization (ESI) source to qualitatively analyze
secondary metabolite modalities. The BULE was dissolved in methanol, filtered
through 0.22 µm, and injected into a direct syringe pump at a flow
rate of 8 µL∙min
In silico molecular docking of 11 selected molecules tentatively
identified by LC–MS/MS analysis of BULE was performed by using Maestro
The neuroprotective effects of BULE were assessed in vivo by performing a study using the animal model previously described in the literature [19].
A total of 30 female albino rats, aged 8–10 weeks, were procured from the
animal house of the Department of Pharmacology, Islamia University of Bahawalpur,
Pakistan. These animals were maintained in a specific pathogen-free environment
with a controlled temperature of 25
The animals were divided into five groups “A, B, C, D, E, and F”. Group A served as the normal control. AD was induced in groups B, C, D, E, and F by orally administering aluminum chloride (100 mg/kg/day) for 42 days. Group B served as the negative control (AD induced with no treatment). Groups C, D, and E (AD induced with treatment) were orally administered an aqueous suspension of BULE at doses of 100 mg/kg/day, 200 mg/kg/day, and 400 mg/kg/day, respectively. Group F was orally administered rivastigmine (0.3 mg/kg/day) and served as the positive control. The treatment was performed for a month after the development of AD in all the groups except A (normal control group). The induction of AD was assessed using the Morris water test [19].
The control and treated rats were sacrificed by cervical dislocation, and their brains were secured and the hippocampus quickly dissected, which was homogenized in 10% w/v ice-cold mixture of 50 mM Tris buffer (Sigma-Aldrich, St. Louis, MO, USA) pH 7.4 and 300 mM sucrose solution using a Potter–Elvehjem homogenizer. The homogenate was centrifuged at 3000 rpm for 10 minutes, and the supernatant was frozen at –80 °C until it was analyzed for acetylcholinesterase levels and butyrylcholinesterase activities.
Each experiment was performed in triplicate, and values were expressed as the
mean
Polyphenols are plant components that include chlorogenic acids, tannins, hydrolyzable tannins, flavonoids, and lignans, which are found mostly as glycosides in undisturbed tissues but may be released as biologically active aglycones by glycosidase during predation or extraction. Polyphenols are potentially useful as nutraceuticals and perform a variety of pharmacological effects on the body after consumption, based on their antibacterial, antiviral, antiparasitic, antidiabetic, anticancer, and antioxidant properties [20].
Phytochemical analysis of L. frutescens hydroalcoholic leaf extract and
its fractions confirmed the presence of alkaloids, phenols, flavonoids, saponins,
and glycosides. BULE exhibited the highest total phenolic content, while HELE
exhibited the lowest (Table 1). The TPC and TFC contents of this study were
compared to a previous study conducted on the aerial parts of L.
frutescens, which revealed a significant difference in the polar fractions of
TPC and TFC, where the highest TPC was determined as 189.369
| Description | Total phenolic content | Total flavonoid content |
| (mg GA∙Eq∙gm |
(mg Qu∙Eq∙gm | |
| MELE | 148.243 |
210.164 |
| HELE | 3.533 |
113.645 |
| CHLE | 80.232 |
293.343 |
| BULE | 223.075 |
230.435 |
The LC–MS
| # | Retention time | ESI mode | Adduct (m/z) | Tentative identification | Class/subclass | Molecular formula | Observed mass | MS |
| 1 | 1.66 | Positive mode of ionization | [M+H] |
Theobromine | Alkaloid | C |
180.25 | 138, 110 [20, 21] |
| 2 | 1.86 | [M+H] |
Propylgalate | Galloyl ester | C |
211.50 | 196, 168, 140 [22] | |
| 3 | 3.67 | [M+H] |
Asarinin | Lignan | C |
355.50 | 337, 325, 135 [23] | |
| 4 | 4.29 | [M+H] |
Quercetin-3-beta-glucoside | Flavonoid glycoside | C |
463.33 | 337, 301, 215 [20] | |
| 5 | 4.63 | [M+H] |
Myricetin 3-acetylrhamnoside | C |
507.42 | 421, 381, 317, 287 [24] | ||
| 6 | 4.88 | [M+H] |
Isoquercitrin 6 |
Flavonoid derivative | C |
551.42 | 465, 303 [25] | |
| 7 | 5.49 | [M+H] |
Diosmetin-7-O-glucorinide-3 |
Flavonoid glycoside | C |
607.33 | 547, 531, 487, 473, 461 [26] | |
| 8 | 6.83 | [M+H] |
Pinoresinol diglucoside | Lignan glycoside | C |
683.50 | 665, 519 [27] | |
| 9 | 11.76 | Negative mode of ionization | [M-H] |
Eriodictyol | Flavonoid | C |
287.08 | 151, 135, 96 [28] |
| 10 | 11.93 | [M-H] |
Epigallocatechin | C |
305.17 | 275, 261, 125, 97, 54 [29] | ||
| 11 | 12.12 | [M-H-142] |
Methyl gallate derivative | Galloyl ester | [C |
325.25 | 18, 169 [30] | |
| 12 | 12.53 | [M-H-154] |
[C |
339.25 | 183, 170 [30] | |||
| 13 | 12.79 | [M] |
Eudesmin | Lignan | C |
386.08 | 369, 355, 342, 206, 165 [31] |
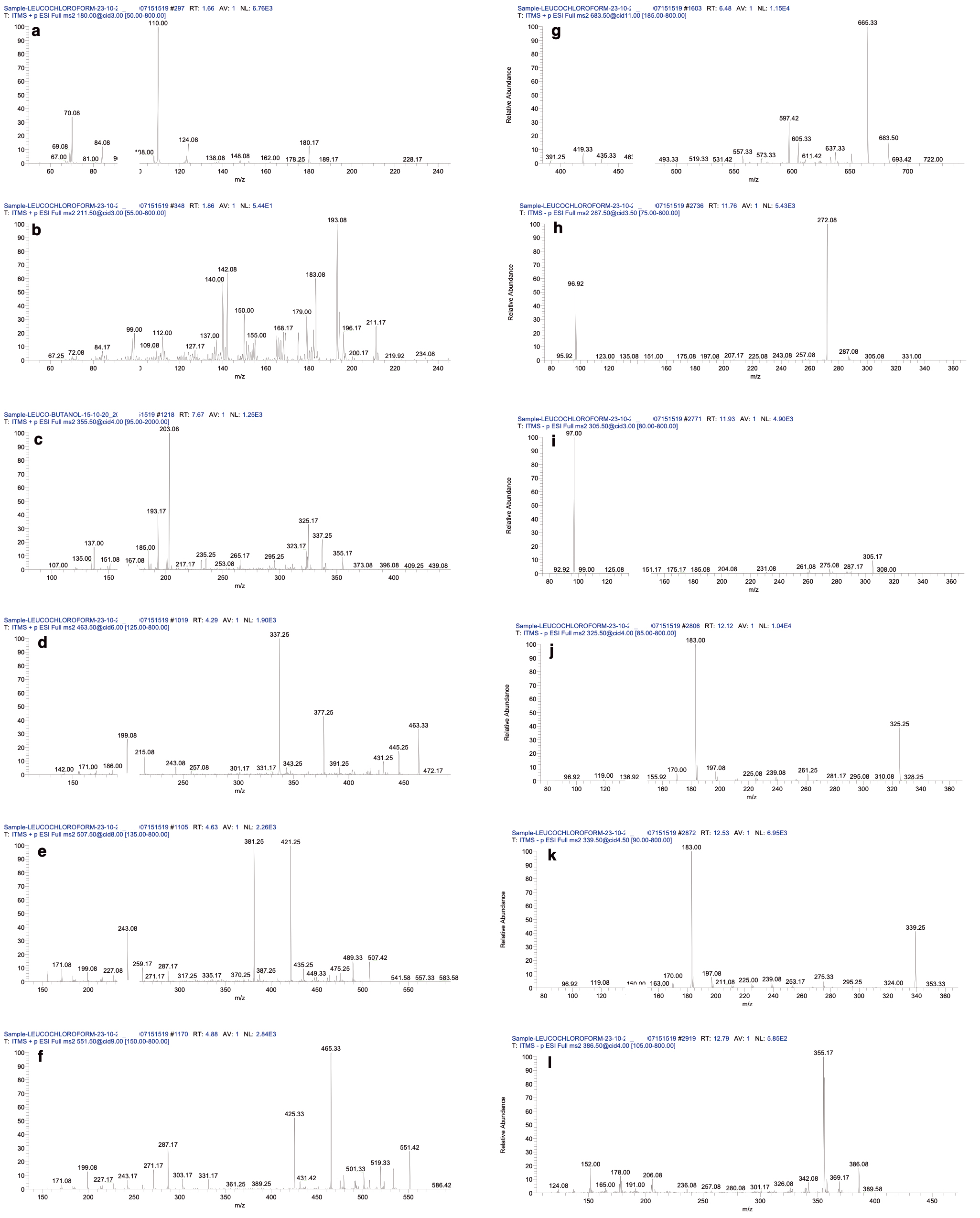 Fig. 2.
Fig. 2.The ms
Excessive accumulation of reactive oxygen species (ROS) is believed to play a
role in Alzheimer’s disease via the accumulation of beta-amyloid protein in the
neuronal tissues [32]. The radical scavenging potentials determined by DPPH and
TEAC were as follows: CHLE
| Description | TAA | DPPH | TEAC | CUPRAC | FRAP |
| mg AA∙Eq∙gm |
mg∙ trolx Eq∙gm |
mg∙ trolx Eq∙gm |
mg∙ trolx Eq∙gm |
mg∙ trolx Eq∙gm | |
| MELE | 152.603 |
171.336 |
170.866 |
356.343 |
335.232 |
| HELE | 166.625 |
84.247 |
70.570 |
249.121 |
190.232 |
| CHLE | 194.046 |
215.235 |
220.243 |
536.336 |
482.434 |
| BULE | 226.236 |
160.286 |
158.232 |
450.236 |
350.323 |
All values are expressed as mean
Acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) were inhibited by sample extracts and the positive controls, eserine and acarbose (Fig. 3 and Table 4). BULE exhibited significant inhibitory activity against AChE, whereas CHLE exhibited the highest inhibitory activity against BChE, which was consistent with them being the best starting sources for bioassay-guided purification of AChE and butyrylcholinestrase (BuChE) inhibitors. Phytochemical analysis and spectroscopic techniques indicated the presence of lignans and alkaloids in the leaves of L. frutescens.
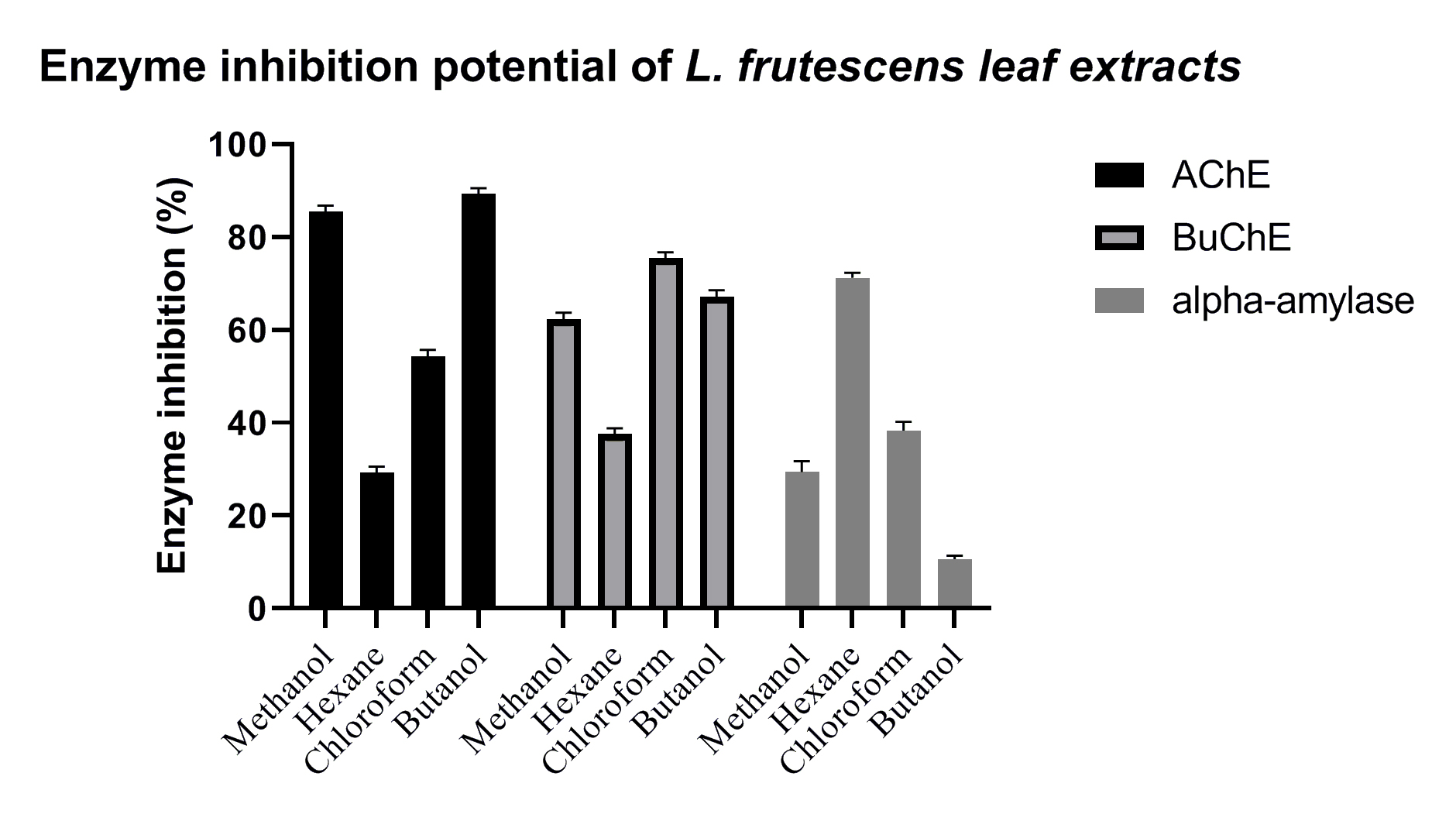 Fig. 3.
Fig. 3.Inhibition of enzyme activity by methanolic leaf extract (MELE), HELE, CHLE, and
BULE of L. frutescens leaves. Extracts were prepared with the solvents
indicated below the horizontal axis and examined for their ability to inhibit
human acetylcholinesterase (acetylcholinesterase (AChE), solid black bars), human butyrylcholinesterase
(butyrylcholinesrase (BuChE), gray bars with borders), and human salivary amylase (alpha-amylase, gray
bars with no borders). A significant difference (p
| Test materials | Acetylcholinesterase inhibition | Butyrylcholinesterase inhibition | ||||
| % inhibition | IC |
% inhibition | IC |
% inhibition | IC | |
| (0.5 mg∙mL |
(µg∙mL |
( 0.5 mg∙mL |
(µg∙mL |
(5 mg∙mL |
(mg∙mL | |
| MELE | 85.5 |
62.9 |
62.4 |
249.8 |
29.4 |
10.0 |
| HELE | 29.3 |
37.6 |
71.2 |
2.9 | ||
| CHLE | 54.3 |
428.6 |
75.4 |
129.7 |
38.3 |
6.7 |
| BULE | 89.3 |
12.1 |
67.2 |
156.3 |
10.9 |
|
| Eserine | 91.5 |
0.7 |
ND | 2.3 |
ND | ND |
| Acarbose | ND | ND | ND | ND | 98.9 |
0.1 |
Abbreviations: IC
Inhibition of glucose production from starch is one of several mechanisms used by effective antidiabetic drugs. By delaying starch digestion, alpha-amylase inhibitor drugs, such as acarbose, slow glucose production, and hence, absorption, resulting in reduced postprandial elevation of blood glucose. Type 3 diabetes mellitus (Type 3 DM) has been proposed to represent a major pathogenic mechanism of AD neurodegeneration. Type 3 DM corresponds to a state of chronic insulin resistance plus insulin deficiency, which is largely confined to the brain, yet, as in the case of non-alcoholic steatohepatitis (NASH), it can overlap with Type 2 DM. The study by De La Monte et al. [33] examined the postmortem brain tissue and found that AD may be associated with insulin signaling. Additionally, other studies linking diabetes with AD have focused on Type 2 diabetes as a cause of insulin resistance, oxidative stress, and cognitive impairment, although the aggregate of these effects still falls short of mimicking AD [34]. The HELE exhibited high alpha-amylase enzyme inhibition (see Table 3); this activity is potentially useful for decreasing blood sugar levels and ultimately decreasing oxidative stress, which is one of the factors involved in AD. The literature review revealed various classes of lignans, including those identified as present in BULE, effectively controlled plasma glucose levels in the diabetes-induced albino rat model [35]. The only alkaloid tentatively identified in BULE was theobromine. Theobromine is also a prescription drug, which is used against bronchoconstriction due to its vasodilating effects. Since recent studies have indicated that high cholesterol levels can contribute to AD development, theobromine was evaluated for its effects on cognitive behavior changes in albino rats fed a lard-enriched diet. Interestingly, it was shown to improve cognitive functions due to the restoration of A1 receptors and beta-amyloid [36].
The AChE and BuChE in vitro enzyme inhibition assay revealed that BULE showed the highest potential against AChE among all tested samples. Therefore, the in vivo neuroprotective effect of BULE was investigated in female albino rats where hippocampal damage had previously been induced by oral administration of aluminum chloride at 100 mg/kg/day for 42 days. The neuroprotective effects of BULE were demonstrated in animal groups treated with BULE and the positive control (rivastigmine) by a reduction in the following degenerative changes: decreased cellular population, increased cellular degeneration signs, and hypertrophy due to an enlargement in the mononucleate cells (Fig. 4).
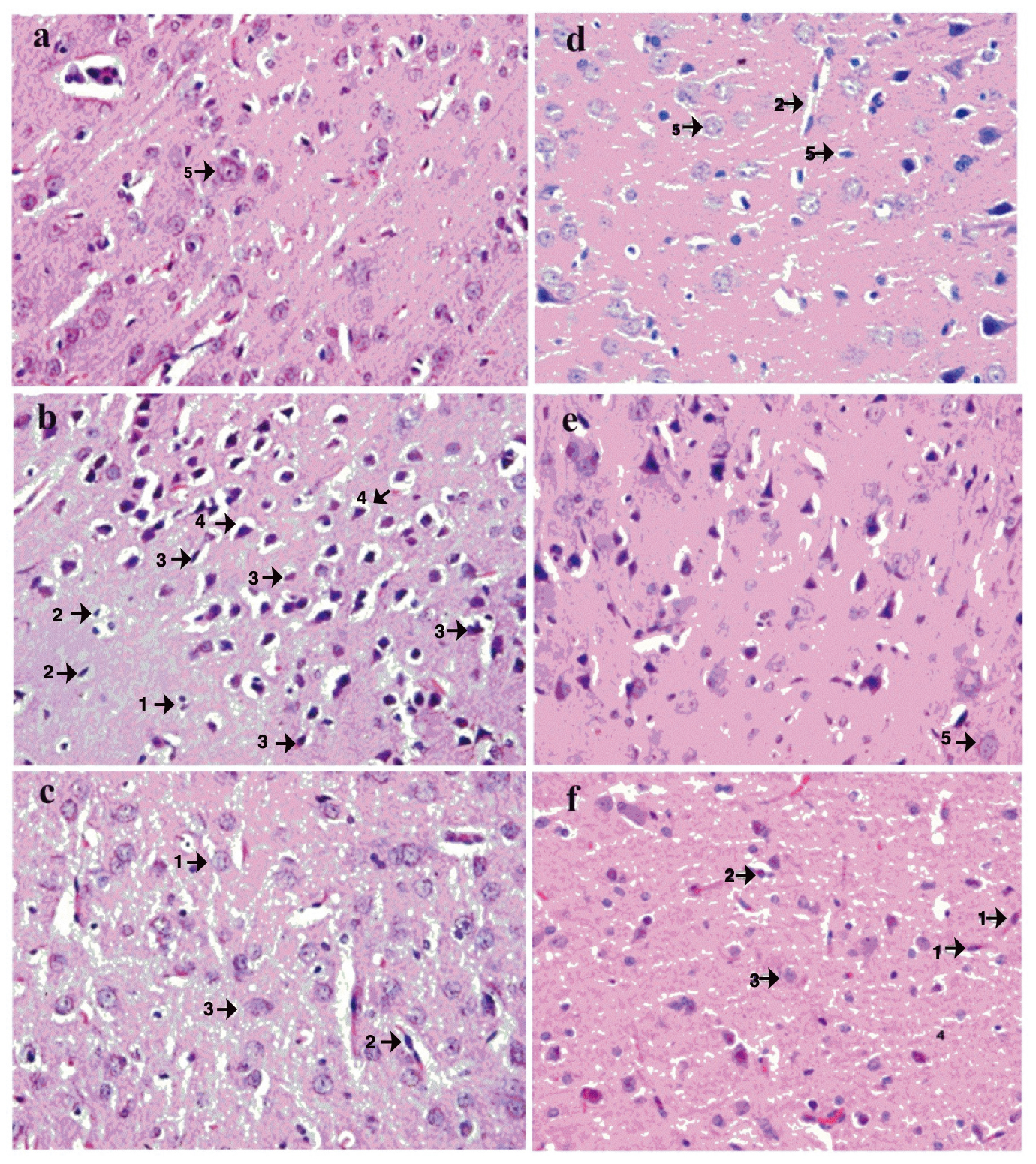 Fig. 4.
Fig. 4.Effects of BULE L. frutescens leaf extract on histopathology in representative photomicrographs of postmortem brain sections of rats administered aluminum chloride-induced (50 mg/kg/day) for 30 days. Photomicrographs are from the following treatment groups: (a) normal control receiving no aluminum chloride; (b) negative control receiving aluminum chloride but no BULE; (c) aluminum chloride-treated rats orally receiving BULE daily at 100 mg/kg/day; (d) aluminum chloride-treated rats orally receiving BULE daily at 200 mg/kg/day; (e) aluminum chloride-treated rats orally receiving BULE daily at 400 mg/kg/day; (f) positive control aluminum chloride treated rats orally receiving rivastigmine daily at 0.4 mg/kg/day. Arrow 1 shows degeneration of glial cells; arrow 2 shows degeneration of pyramidal cells; arrow 3 shows vacuolation of glial cells; arrow 4 shows vacuolation of pyramidal cells; arrow 5 shows normal large vesicular nuclei. The magnification is 40× (objective lense SP40/0.65 160mm).
The effects on the enzymes involved in neurodegeneration, which could be exerted
by a series of 11 L. frutescens leaf extract components that had been
identified by LC–MS/MS, were further characterized using computational chemistry
to dock the molecules into the active sites of human acetylcholinesterase (PDB
code: 4M0E), human butyrylcholinesterase (PDB code: 4BBZ), and human salivary
alpha-amylase (PDB code: 1NHY). The binding affinity, binding energy, and amino
acids interacting with the enzyme active sites were determined for each of the 11
components identified in BULE and are presented in Table 5. The interactions of
the three L. frutescens leaf extract components with the highest binding
affinities to the active sites of the three enzymes were modeled and the
interactions are shown in Fig. 5. The binding of the L. frutescens leaf
extract components to the active sites predominantly resulted from one or both of
the two types of interactions: hydrogen bonding and
| Compound | Butyrylcholinestrase | Acetylcholinesterase | Human pancreatic alpha-amylase | |||
| (PDB code: 4BBZ) | (PDB code: 4M0E) | (PDB code: 1HNY) | ||||
| Binding affinity (kcal/mol) | Interacting amino acid(s) and position number(s) | Binding affinity (kcal/mol) | Interacting amino acid(s) and position number(s) | Binding affinity (kcal/mol) | Interacting amino acid(s) and position number(s) | |
| Asarinin | –7.395 | Trp 231, Trp 430 | –3.632 | Thr 125 | –5.365 | Trp 59, Gln 63, His 201 |
| Aschantin | –6.691 | Ser 198, Trp 82 | –4.377 | Thr 125, Ala 458, | –4.904 | Gln 63, Thr 163, His 305 |
| Diosmetin-7-O-glucuronide-3 |
–9.268 | Glu 197, Ser 287, Asn 189, Ser 198, His 438, Phe 329 | –6.743 | Val 122, Ser 462, Gln 461, Val 459, Ala 458, Gln 539, Glu 542 | –7.247 | Asn 53, Try 58, Trp 59, Asp 197, Arg 195, Glu 233 |
| Epigallocatechin | –8.104 | Thr 120, Asp 70, His 438, Glu 197 | –5.469 | Thr 125, Gly 535 | –6.840 | Thr 163, Asp 197, Glu 233 |
| Eudesmin | –5.597 | Ser 198, Phe 329, His 438 | Thr 125, Ala 458 | –4.697 | Gln 63 | |
| Isoquercetin-6 |
–10.533 | Gly 115, Gly 116, Glu 197, Trp 82, Ala 328, Pro 285 | –5.339 | Ser 462, Ala 458, Gly 535, Leu 537 | –6.855 | Gln 63, Trp 59, Trp 58, Asp 356, Asp 300 |
| Myricetin-3-O-acetylrhamnoside | –9.844 | Asn 83, Glu 197, Ser 198, Ser 287, His 438 | –5.469 | Gln 539, Gly 535, Leu 537, Glu 542 | –6.796 | Trp 59, Gln 63, Asn 352, Asp 356 |
| Pinoresinol diglucoside | –9.071 | Glu 197, Tyr 332, Phe 329, His 438, Ser 287, Asn 289 | –6.575 | Val, 122, Thr 125, Tyr 456, Glu 542, Gln 460, Pro 534, Ser 462 | –7.888 | Trp 58, Asp 197, Glu 233, Gly 304, Gly 351, Asn 352 |
| Propyl gallate | –3.526 | Ser 198, Gly 116, Glu 197, His 438 | –3.911 | Gly 535, Gln 539 | 4.641 | Gln 63 |
| Sesamin | –7.955 | Trp 231, Trp 430. | –4.233 | - | 4.681 | Gln 63 |
| Eridictoyl | –8.927 | Gly 115, Glu 197, Ser 198 | –4.733 | - | –6.158 | Gln 63, Trp 59, Glu 233, Arg 195, Asp 197 |
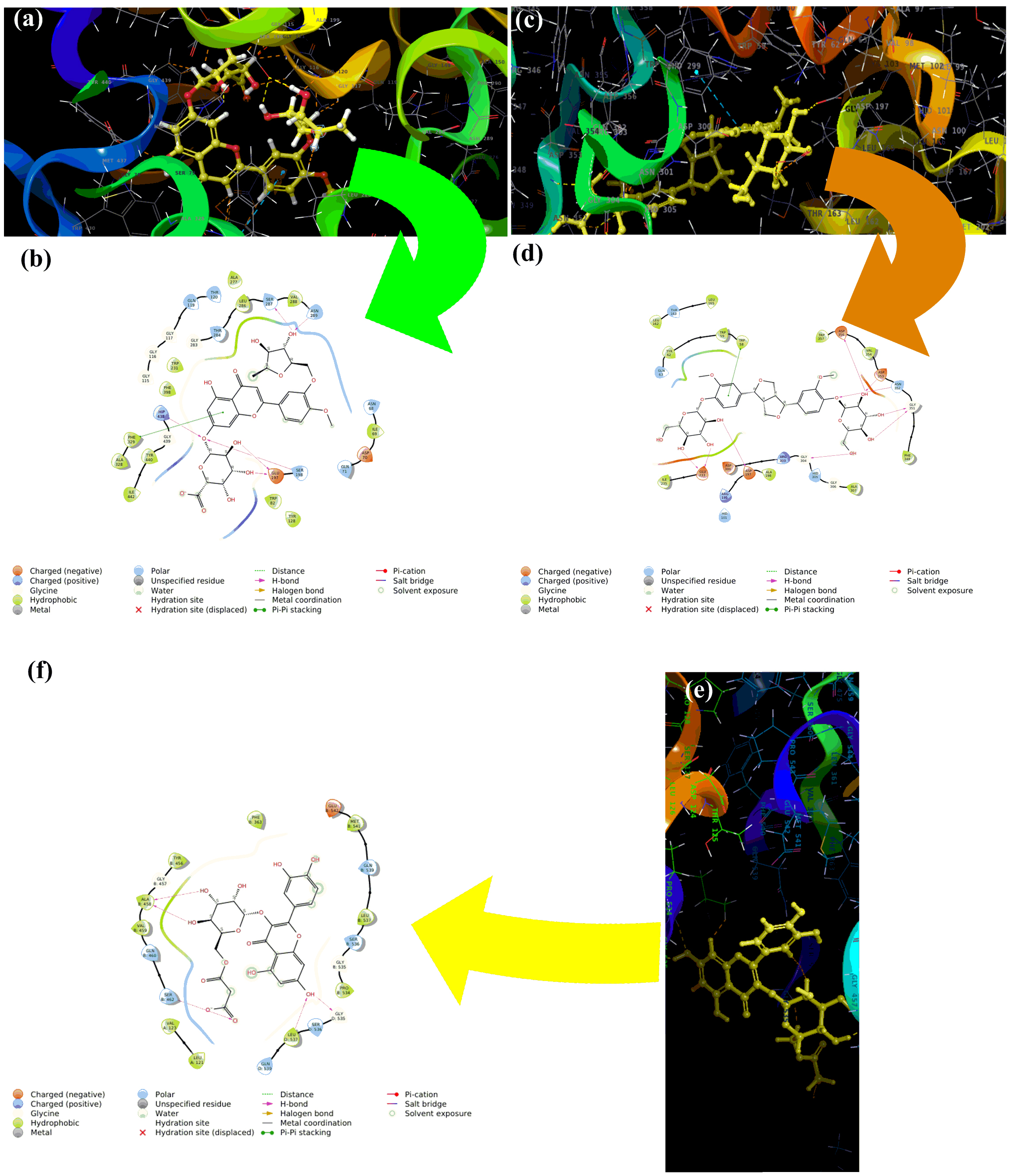 Fig. 5.
Fig. 5.Molecular modeling of the three L. frutescens
leaf extract components that bound with the highest affinity to the active sites
of the three enzymes clinically linked to neurodegenerative diseases. Diosmetin-7-O-glucuronide-3
Herbal medications are generally complex mixtures of substances that usually have different mechanisms of action. Thus, they have the potential to use multiple mechanisms simultaneously to treat disease conditions that respond to combination therapy. In the case of attempting to use L. frutescens leaf extracts for neuroprotection, the three identified component phytochemicals that bound with the highest affinity to the active sites of the three mediator enzymes had very limited structural similarity (Fig. 6). These three components all have aromatic, polyphenolic core structures with peripheral glycoside moieties. The two that bind to acetylcholinesterase are flavonoid glycosides with saccharide-like free hydroxyl groups in the 3-region of the flavonoid core.
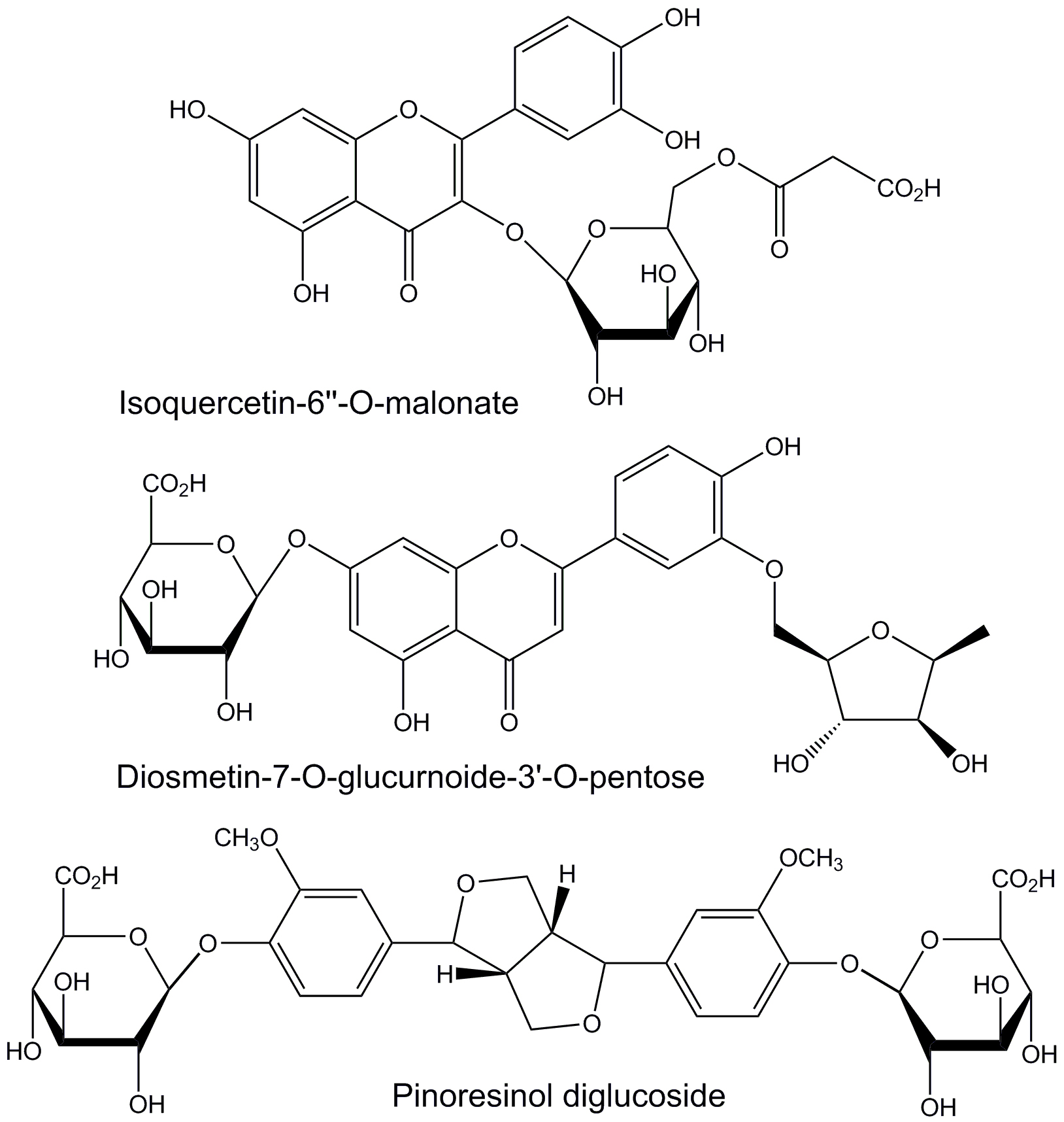 Fig. 6.
Fig. 6.
Structures of L. frutescens leaf extract
components that bind with the greatest affinity to the active sites of human
butyrylcholinesterase (isoquercetin-6
Polar solvent extracts of L. frutescens leaves containing polyphenols
with significant antioxidant activities as measured by five different methods.
BULE, the n-butanol extract, which exhibited high biological activity
levels, contained many phytochemicals, 14 of which could be identified by
LC–MS
Datasets used and/or analyzed for this study are available from the corresponding author upon appropriate request.
Conceptualization: IA, EKA, and WTS; methodology: IA, SA, HR, and NS; validation: IA and NS; formal analysis: IA, NS, and MN; investigation: IA, BA, and WTS; data curation: IA, EKA, BA and ES-S; writing-original draft preparation: IA, EKA and ES-S; writing-review and editing: IA, EKA, and ES-S. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work to take public responsibility for appropriate portions of the content and agreed to be accountable for all aspects of the work in ensuring that questions related to its accuracy or integrity.
This study was approved by the Advanced Studies & Research Board of the Islamia University of Bahawalpur via letter No. 673/AS&RB dated December 16, 2019. This study was approved by the Research and Ethics Committee of the Faculty of Pharmacy, Islamia University of Bahawalpur Procedures.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest. EKA had served as one of the Guest editors of this journal. We declare that EKA had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Luigi De Masi.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.






