1 Department of Obstetrics and Gynecology, The First Affiliated Hospital of Yangtze University, 434023 Jingzhou, Hubei, China
2 Department of Radiotherapy, Huabei Petroleum Administration Bureau General Hospital, 062550 Renqiu, Hebei, China
3 Department of Obstetrics and Gynecology, Hubei Clinical Medicine Research Center for Individualized Cancer Diagnosis and Therapy, The First Affiliated Hospital of Yangtze University, 434023 Jingzhou, Hubei, China
4 Department of Obstetrics and Gynecology, Hubei Clinical Medicine Research Center for Individualized Cancer Diagnosis and Therapy, The First Affiliated Hospital of Yangtze University, 434023 Jingzhou, Hubei, China
5 Department of Physiology, The First Affiliated Hospital of Yangtze University, 434023 Jingzhou, Hubei, China
†These authors contributed equally.
Abstract
As a spherical protein that acts as a repository for intracellular iron, Ferritin is the most important iron storage form and is known to influence tumor immunity. Unbound ferritin is composed of 24 subunits, made up of ferritin light chain (FTL) and ferritin heavy chain (FTH). Ferritin can be automatically put together to form hollow nanocages that measure 12 nm around the outside and 8 nm around the inside. Cancer causes the second-most deaths worldwide, effective elimination of tumor cells while protecting normal cells is the foundation of modern tumor therapy. To this end, the innate tumor-targeting activity of human FTH1, first identified ten years ago, is highly appealing. Unmodified human FTH1 binds to its receptor, transferrin receptor 1 (TfR1), which is frequently overexpressed in cancer cells. FTH1-TfR1 binding permits improved drug efficacy by promoting ferritin-mediated targeted delivery. In addition, FTH is also associated with the prognosis of multiple typies of cancer. The level of FTH1 is significantly and positively correlated with the infiltration of tumor-associated macrophages. FTH1 also plays an important role in regulating the tumor immunity of solid cancer. As such, FTH1 has been extensively applied in the targeted delivery of anticancer drugs, diagnostic molecules (e.g., radioisotopes and fluorophones), and inorganic nanoparticles (NPs) to tumors.This article reviews the role of FTH in cancer and its potential as a therapeutic target.
Keywords
- cancer
- ferritin
- FTH
- tumor
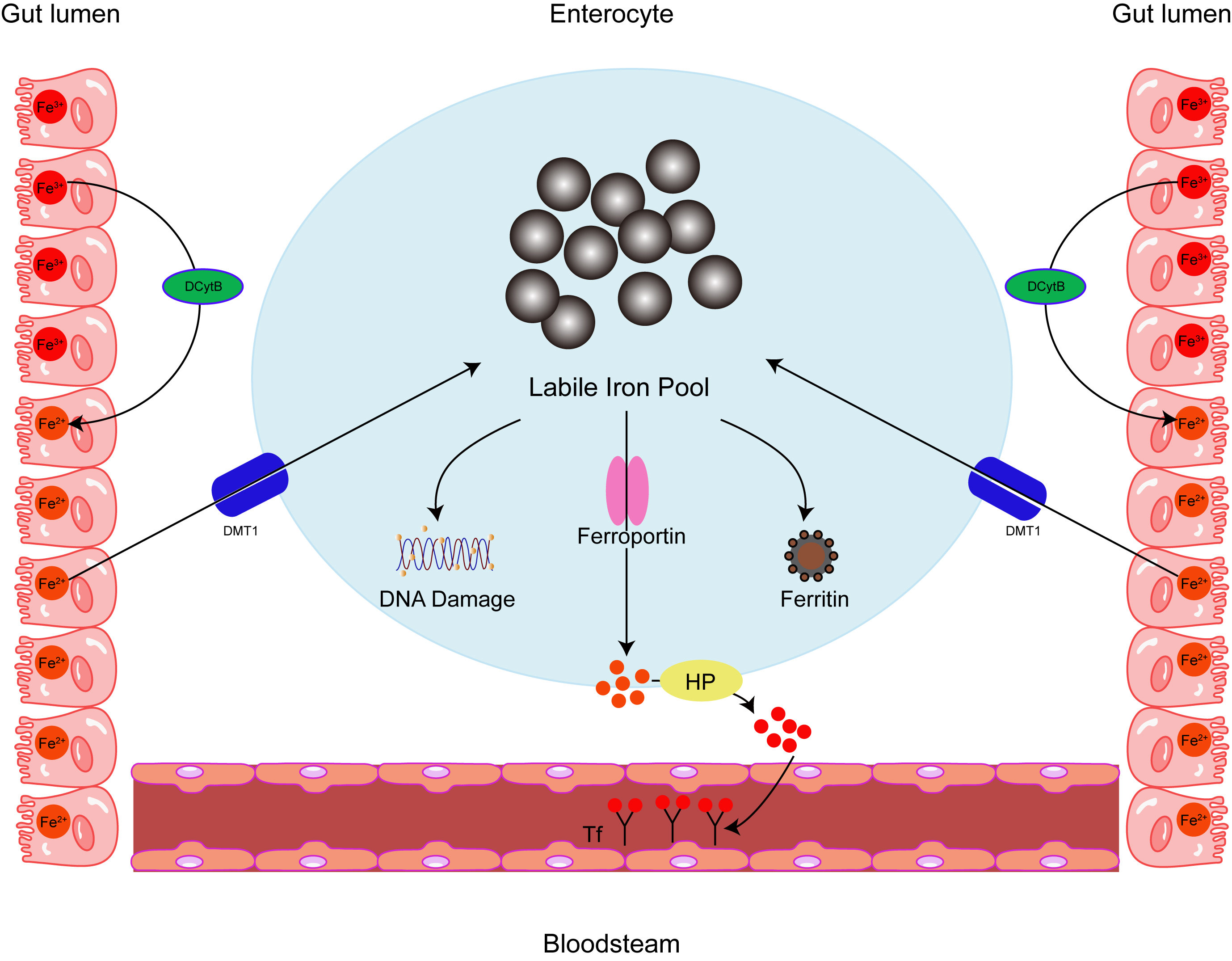
The history of ferritin begins just before the second World War, when it was isolated from horse spleen [1]. Human ferritin consists of two different subunits: light chain (L-ferritin) and heavy chain (H-ferritin), which are 19 kDa and 21 kDa in size, respectively, encoded by genes (ferritin heavy chain (FTH) and ferritin light chain (FTL)) on chromosomes 11q and 19Q. With approximately 55% sequence homology, the two subunits are structurally similar, comprising four parallel and antiparallel helices (A-D), and a shorter E-helix at a 60-degree angle to the others [2]. Although ferritin is primarily considered a cytoplasmic protein, it also localizes to the nucleus, mitochondria, and lysosomes, a small fraction of ferritin is found in the serum. As the major iron-storing protein, ferritin contains about 4500–5000 iron atoms and is found in most living cells in animals, plants, bacteria and algae [3, 4]. Ferritin is critically involved in many physiological and disease-related processes, including the regulation of the tumor microenvironment and immune metabolism [5]. In particular, ferritin is involved in ferroptosis [6], a mode of programmed cell death. Ferroptosis is peculiar compared to the other classes of cell death; for example, apoptosis, autophagy, and pyroptosis [7, 8], because it is brought about by iron build-up and known for the accompanying increase in lipid peroxidation. Ferritin’s role in ferroptosis is relevant for diseases such as cancer and may include a fundamental role in tumor immunity. Therefore, therapeutic approaches that target iron and ferritin homeostasis may be beneficial in cancer, particularly in conjunction with hapten therapy to amplify the anti-tumor response. Ferritin also protects DNA and proteins from iron-induced damage [9]. By shielding cells from the toxic properties of free iron, ferritin is effectively employed in several different cell activities, including immune regulation (Fig. 1).
 Fig. 1.
Fig. 1.Intracellular iron homeostasis. Ferritin acts as an iron
oxidase and iron is internalized and sequestered in the ferritin mineral core,
converting Fe
Ferritin production is subject to a tight translational
control made famous through undergraduate biochemistry lectures, as it is the
typical example provided for functional elements in the 5
Multiple autoimmune diseases are guilty of harboring increased ferritin levels, which are believed to be caused by cytokine-stimulated ferritin synthesis [12, 13]. Although many patients with rheumatoid arthritis have normal serum ferritin concentrations, ferritin levels may be elevated in synovial fluid and synovial cells [14]. High ferritin concentrations have also been documented in the urine of patients with nephritis associated with systemic lupus erythematosus [15]. Another study found that ferritin levels were elevated in thyroiditis patients and decreased after treatment with anti-inflammatory drugs [16]. Beyond the scope of autoimmune disease, elevated serum ferritin has also been observed following bacterial and viral infections, including Epstein-Barr virus (EBV), Human immunodeficiency virus (HIV), and tuberculosis [17].
In cancer cells, FTH1 is reminiscent of Janus, the two-faced god, because increased FTH1 may be both beneficial and detrimental for cancer growth under different circumstances. Biamonte et al. [18] found that increased FTH1 resulted in upregulation of P53 expression and decreased proliferation in non-small-cell lung carcinoma cells. However, work by Salatino et al. [19] in ovarian cancer cells showed that FTH1 is essential for the normal functioning of the antioxidant system, implying that FTH1 blockage may enhance cisplatin-induced cytotoxicity. In addition, Liu et al. [20] showed that high concentrations of cytoplasmic FTH1 implied good disease prognosis. However, elevated serum ferritin (SF) is an unfortunate sign for patient prognosis in the context of hematological malignancies, as high SF is associated with poor overall and progression-free survival. SF expression rose significantly with disease progression or recurrence. SF can be used as a prognostic factor for patients with newly diagnosed or recurrent multiple myeloma. For patients with acute myeloid leukemia, increased ferritin at diagnosis may predict tumor burden [21]. Moreover, FTH may be a diagnostic biomarker for prostate cancer [22], renal FTH has been identified as a biomarker of renal cell carcinoma (RCC), and elevated FTH is predictive of poor prognosis in RCC [23]. Inhibition of overexpressed FTH by FTH small-interfering RNA sensitized malignant mesothelioma cells to apoptosis, suggesting that FTH may contribute to the pathophysiology of malignant mesothelioma [24].
Cancer poses a substantial challenge for healthcare systems worldwide. Cancer refers to a class of diseases involving the neoplastic transformation of ordinary cells. This transformation involves a series of progressive steps, dictated by selective pressures of the local environment. Despite major advances in cancer treatment, morbidity and mortality are predicted to increase in the coming decades.
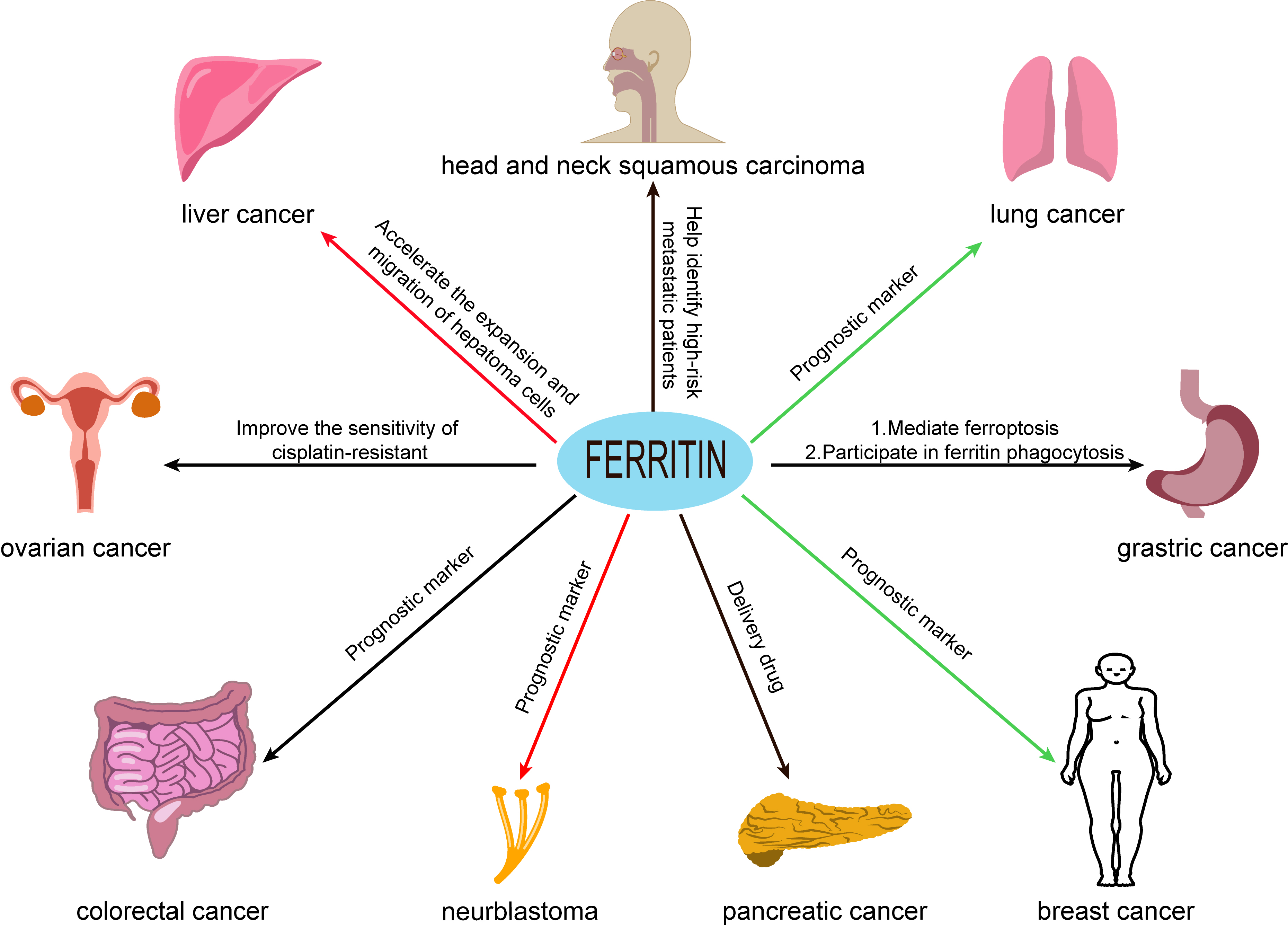
Ferritin may predict prognosis for some types of cancer as aberrant ferritin expression is strongly associated with many malignancies (Fig. 2), including breast cancer [25, 26], ovarian cancer [27], pancreatic cancer [28], colorectal cancer [29], liver cancer [30], lung cancer [31], diffuse large B-cell lymphoma [32], prostate cancer [33] and oral cancer [34]. Increased ferritin levels are often observed in cancer cells compared with healthy cells [19, 35]. Cancer stem cells (CSC) also display elevated ferritin compared to normal cells, together with dysregulated iron homeostasis and increased iron turnover [24, 26]. FTL and FTH1 are positively and significantly associated with the infiltration of tumor-associated macrophages and T-regulatory cells in many tumor types, thus opening up new therapeutic possibilities focused on the tumor microenvironment. These results suggest that FTL and FTH1 are key modulators of immunity against solid tumors (Table 1, Ref. [19, 39, 40, 44, 45, 49, 50, 51, 52, 59, 64, 65, 71, 78, 81, 87, 89, 92, 106, 107, 114]).
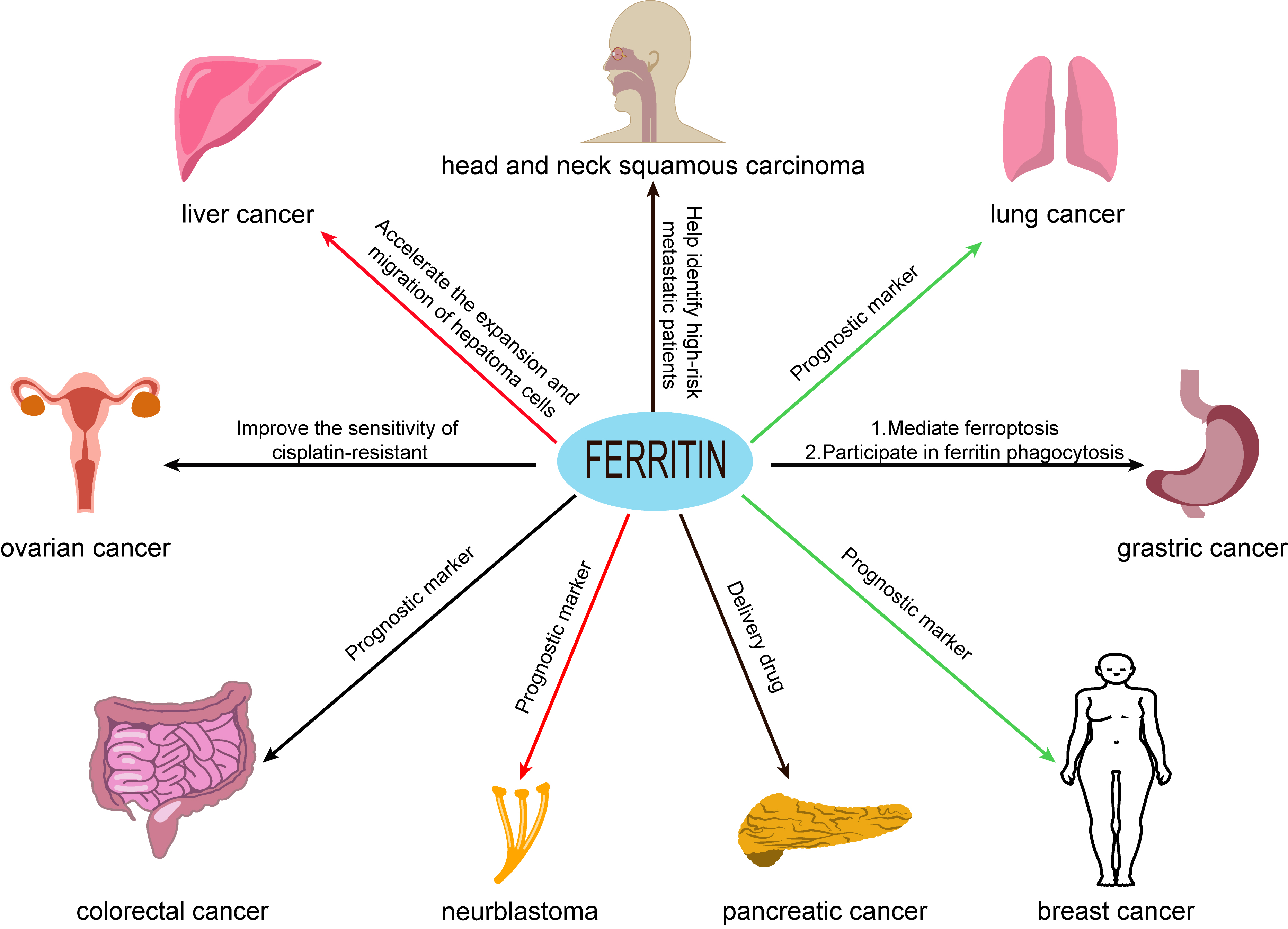 Fig. 2.
Fig. 2.The relationship between ferritin and cancer. Ferritin may be a prognostic factor for certain types of cancer, and ferritin expression levels are closely associated with many malignancies, including breast cancer, ovarian cancer, gastric cancer, pancreatic cancer, colorectal cancer, liver cancer, lung cancer, neuroblastoma, and head and neck squamous carcinoma. Note: The red arrow represents the promotion of FTH tumorigenesis. The green arrow represents the inhibition of FTH tumorigenesis. The black arrow indicates that the effect of FTH on tumor is unclear.
| Primary tumor | Ferritin expression | Functions | References |
| Colorectal cancer | UP | 1. Prognostic marker | [39, 40] |
| 2. Pro-tumorigenic effect | |||
| Head and neck squamous cell carcinoma | UP | Helps identify high-risk metastatic patients | [44, 45] |
| Breast cancer | Down | 1. Inhibits tumor growth | [19, 49, 50, 51, 52, 59] |
| 2. Prognostic marker | |||
| Lung cancer | Down | 1. Prognostic marker | [64, 65, 71] |
| 2. The up-regulation of FTH level in cells will lead to a significant decrease in cell proliferation rate | |||
| Liver cancer | Compared with normal samples, FTH was significantly up-regulated in primary liver cancer tissues and the expression of FTH in advanced cancer was significantly increased | 1. Accelerates the expansion and migration of hepatoma cells | [78] |
| 2. Pro-tumorigenic effect | |||
| Gastric cancer | FTH1 is up-regulated in 5-FU resistant cells and xenografts | 1. Mediates ferroptosis | [81, 87] |
| 2. Participate in ferritin phagocytosis | |||
| Pancreatic cancer | Ferritin is overexpressed in pancreatic cancer | Use natural or engineered FTH as a nano carrier system to deliver drugs to tumor blocks | [89, 92] |
| Neuroblastoma | The basic level of FTH in N2A cells was more than three times lower than that in neural stem cells | 1. High serum FTH level in patients is a marker of poor prognosis in high-risk neuroblastoma | [106, 107] |
| 2. The promotion of neuroblastoma growth and inhibition of cell death | |||
| Ovarian cancer | Compared with primary tumor, the expression of ferritin H chain in metastatic samples is higher | Improve the sensitivity of cisplatin-resistant | [114] |
Abbreviations: FTH, ferritin heavy chain; FTH1, ferritin heavy chain 1; 5-FU, 5-Fluorouracil; N2A, Nmouse neuroblastoma cell lines.
Colorectal cancer (CRC) is the second leading cause of cancer
death worldwide. The incidence of CRC has steadily climbed over the last few
decades, with nearly 2
Increased ferritin expression also serves to prevent ferroptosis. In p53-/- cells, ferritin was significantly increased following cisplatin administration. Treatment with oxaliplatin combined with CPT-11 and 5-FU combined with both oxaliplatin and CPT-11 increased ferritin levels to a greater degree than monotherapy, with the most significant findings including increases in IL-8 induced by oxaliplatin and cisplatin-incited increases in ferritin. Identification of previously unknown drug-specific mechanisms of effectiveness or toxicity may suggest new directions for targeting combination therapies to tumors [38]. Green tea epigallocatechin-3-gallate (EGCG) has also displayed, therapeutic effect efficacy in colorectal cancer cells (CRC). EGCG up-regulated the protein-level expression of transferrin receptor (TFR) and down-regulated FTH, demonstrating that it has iron-chelating effects in CRC. At the same time, molecular docking studies showed that EGCG could bind to glutamic acid 64 and lysine 71 through strong hydrogen bonding and strong binding affinity to ferritin (–7.3 kcal/mol), with hydrophobic interactions between the L-asparagine 74 and lysine 71 hydrophobic pockets. EGCG may impede ferritin activity via their strong interaction, consistent with the downregulation of FTH that has been noted in vitro. In vitro data also show that the molecular docking of TFR and EGCG cannot be regulated [41]. Thus, EGCG has iron-chelating properties in colorectal cancer, which shows potential for further development in CRC treatment.
Ranked sixth among the most widespread cancers around the world [42], head and neck squamous cell carcinoma (HNSCC) accounts for ~600,000 new cases reported annually, of which China accounts for a significant proportion. The ferroptosis inhibitor FTH1, which is also a prognostic factor, was associated with the infiltration of M2 macrophages in HNSCC, suggesting that induction of ferroptosis directly affects the infiltration of M2 macrophages. Therefore, targeting iron immunomodulation may enhance immunotherapeutic activity [43]. Chemiluminescence immunoassy showed the iron content and expression of the heavy chain (FTH) and light chain (FTL) of ferritin in tumor tissues were higher than those of normal tissues [44]. The study also showed that the expression level of FTH in HNSCC with transfer was higher than that in HNSCC without transfer. The Geo database further validated these results and reported a correlation between the FTH expression level and the prognosis of HNSCC patients. While the evidence suggests that ferritin is not an appropriate biomarker for the early diagnosis of HNSCC, ferritin expression levels are associated with cervical metastasis of HNSCC [45]. Hence, the correlation between ferritin and HNSCC is likely to be valuable for identifying patients at high risk of metastasis. Overall, ferritin is a strong candidate biomarker for identifying cervical metastasis of HNSCC.
Breast Cancer (BCA) is one of the most prevalent cancers worldwide, and the most common malignant tumor in women. In 2015, there were an estimated 272,400 newly diagnosed cases of breast cancer and nearly 70,700 breast cancer deaths in China. Treatment options include the usual suspects (surgery, radiotherapy, chemotherapy) as well as hormonal treatments. In the process of formulating the ideal treatment plan, several clinical and pathological features need to be evaluated in addition to histological subtypes. This is no mean feat, and BCA remains a clinical puzzle in terms of prognostic assessment and treatment selection. The targeted inhibition of iron metabolism is a double-edged sword that cuts both ways in the regulation of carcinogenesis. Research suggests that increased FTH1 expression is associated with enhanced resistance to chemotherapeutic molecules [46, 47, 48], but other studies indicate that FTH1 can act as an important tumor suppressor in BCA. Moreover, BCA cells have been found to have decreased FTH1 [49, 50, 51] while higher FTH1 expression is considered to denote favorable prognosis in triple-negative breast cancer [19, 52]. Furthermore, BCA tissues that are abundant in FTH1 are commonly highly enriched for interferons that are produced by CD8+ T cells, but not CD4+ T cells, implying FTH1 has a regulatory role on the activity of the adaptive immune system within the tumor microenvironment [19]. Enhanced expression of ferritin heavy chain 1 (FTH1) inhibited the c-MYC expression in breast cancer (BCA) cells, correlated with the import of iron as well as its storage and output, along with decreased BCA cell growth [53]. Meanwhile, knockdown of FTH1 enhanced cell growth and mammary gland sphere formation, while promoting greater c-MYC expression and elevated chemotherapy resistance. A central role of c-MYC is suggested by the correlation between the increased c-MYC expression induced by FTH1 silencing and the enhanced growth and migration of BCA cells [54]. Conversely, overexpression of FTH1 inhibited BCA cell growth and migration, associated with decreased c-MYC expression. Moreover, decreasing c-MYC increases the sensitivity of BCA cells to chemotherapy drugs [55]. The effects of FTH1 are most likely c-MYC-dependent, as silencing c-MYC recapitulated the effect of FTH1 overexpression. These findings indicate that FTH1 has significant tumor suppression activity in BCA cells and inhibits tumor growth by preventing the expression of key oncogenes such as c-MYC [56].
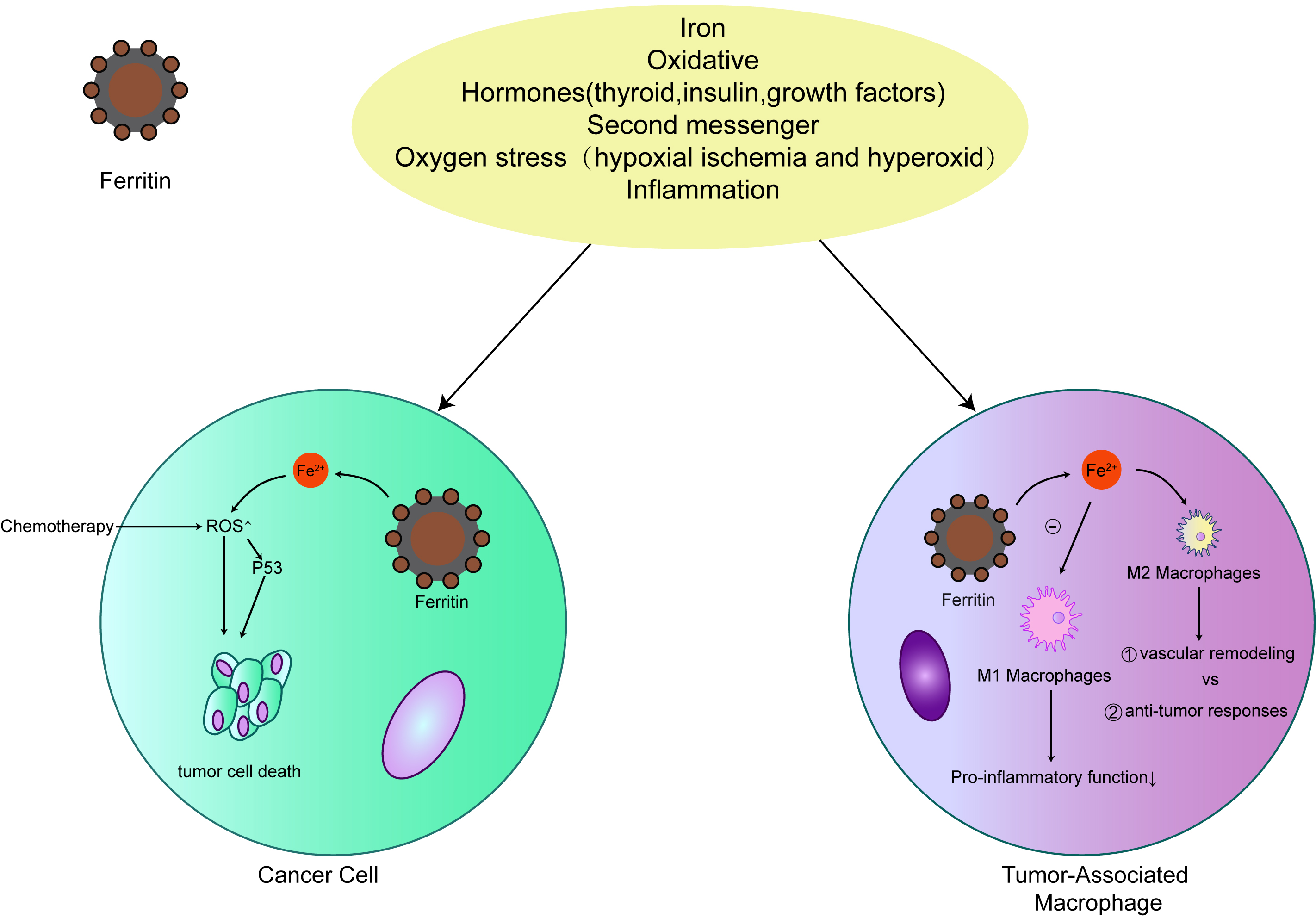
Early studies have shown that the role of iron in tumorigenesis is that iron accumulates in matrix in iron-induced tumors, rather than malignant cells [57]. The involvement of matrix indicates that iron-dependent tumorigenesis may be mediated by non-malignant tumors, such as macrophages, which have high iron storage capacity. Extracellular ferritin secreted by macrophages can stimulate the occurrence of tumors at various levels. More and more evidences show that ferritin plays a multifunctional role in human biology, such as angiogenesis [58], increasing the proliferation of cancer cells [59], transporting iron [60, 61], and inhibiting lymphocyte reaction [62, 63]. Thus, the secretion of ferritin may play an important role in promoting and maintaining tumor. In addition, ferritin may play a role in preventing macrophages from initiating an effective pro-inflammatory (M1) phenotype to maintain wound healing (M2) phenotype by isolating intracellular iron [40]. Macrophages rich in ferritin increase the infiltration of breast tumors, which may directly affect the occurrence of tumors through the production and secretion of ferritin (Fig. 3). Increased expression of FTH1 may indicate good prognosis and anti-cancer drug effect [59]. Thus, understanding the role of ferritin in BCA development may provide an opportunity to explore a novel area of therapeutic development.
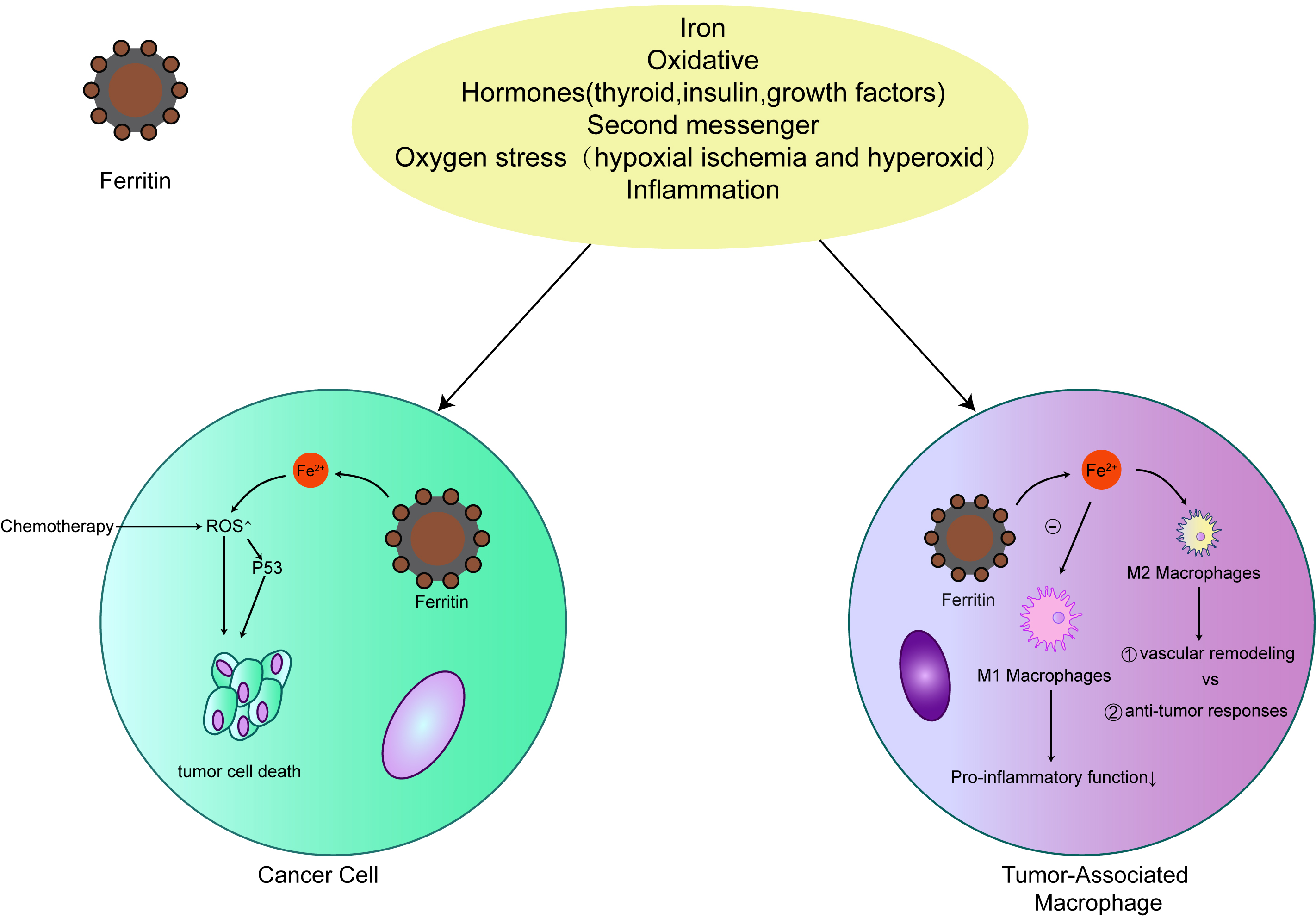 Fig. 3.
Fig. 3.The relationship between ferritin and cancer. Ferritin expression levels are closely associated with many malignancies. Ferritin protects cancer cells from the production of iron-induced reactive oxygen species (ROS) to increase their resistance to chemotherapy. In tumor-related macrophages, ferritin promotes the M2 process of primary disease of macrophages.
Lung cancer is responsible for the most cancer-related deaths worldwide, according to data published by the World Health Organization and global cancer statistics. Despite some progress in the comprehensive treatment of advanced lung cancer, current therapies are limited by problems such as serious adverse reactions related to dose-limiting toxicity and off-target effects, as well as the development of drug resistance. The most common lung cancer is non-small cell lung carcinoma (NSCLC). Accounting for 80–85% of all lung cancer cases, NSCLC is responsible for high morbidity and mortality worldwide. With a 5-year survival rate of only around 10%, early diagnosis is critical for maximizing treatment options and improving prognosis of lung cancer. The analysis of Expressed sequence tag (EST) database showed that the expression of FTH gene in lung tumors was lower than that in normal tissues [64]. NSCLC has elevated ferritin levels in serum and bronchoalveolar fluid [65, 66]. Research suggests that high levels of serum ferritin are associated with poor prognosis [65]. Recent research into potential strategies for early diagnosis has identified potential tumor markers. A study showed that curcumenol induced cell death and inhibited the proliferation of lung cancer cells. Long-chain non-coding RNA H19 (lncRNA H19) was found to be significantly decreased in lung cancer cells treated with curcumin versus controls. Consistent with this, lncRNA H19 overexpression abrogated the anticancer effects of curcumin, whereas knockdown of lncRNA H19 promoted curcumin treatment-induced ferroptosis. Mechanistically, lncRNA H19 was found to exhibit competitive binding to miR-19b-3p, thereby enhancing the transcriptional activity of its endogenous target, FTH1. The data suggest that curcumin exerts its anti-tumor efficacy on lung cancer by inducing ferroptosis, and that the lncRNA H19/Mir -19 B-3p/FTH1 axis plays an important role in curcumin-induced ferroptosis [67]. The study confirmed the close relationship between p53 activity and FTH expression [68, 69]. P53 increases the expression of FTH at the translation level by regulating IRP1-IRE translation regulation system, while overexpression of tumor suppressor factors negatively regulates FTH transcription [70]. In contrast, FTH physically binds p53 and stabilizes protein levels under oxidative stress. Recent studies have demonstrated that FTH can enhance p53 expression by downregulating miR-125b homeostasis in A549, H460, SW1573 and LXF-289 non-small cell lung cancer cell lines. Regardless of their redox related activities [18], the up-regulation of FTH level in cells will lead to an increase in p53 protein expression in non-small cell lung cancer (NSCLC) and a significant decrease in cell proliferation rate [71]. Therefore, FTH have the potential to improve the early diagnosis of lung cancer, in order to enhancing the accuracy of lung cancer treatment.
Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer, accounting for 75–90% of total cases. As the third leading cause of cancer-related death worldwide, HCC is both common and deadly, with primary liver cancer deaths projected to reach 1 million by 2030. Prognosis is typically poor, with a 5-year overall survival rate of less than 20% [72, 73]. HCC is associated with diseases involving liver inflammation, as this process leads to dysplasia of hepatocytes, making them prone to liver cancer [74]. Therefore, the recent increase in HCC incidence is not unexpected, given the rise in nonalcoholic fatty liver disease, alcoholic cirrhosis, and chronic hepatitis. The high mortality and morbidity associated with HCC is largely due to the late stage of disease, and early accurate diagnosis of HCC is a key challenge. Recently, it has been found that the degradation of FTH by autophagic degradation of ferritin can cause ferroptosis [75, 76]. FTH expression is strongly correlated with tumor grade, tumor stage and prognosis of HCC. Importantly, protein interaction studies elucidate that FTH participates in the maintenance of iron homeostasis and lysosome-dependent degradation [77]. The study found that compared with normal samples, FTH was significantly up-regulated in primary liver cancer tissues and the expression of FTH in advanced cancer was significantly increased [78]. Overexpression of FTH accelerates the expansion and migration of hepatoma cells and makes them resistant to iron poisoning but does not prevent cell death as a result of cytotoxic molecules such as oxaliplatin, irinotecan and doxorubicin. In FTH-recombinant cells, the build-up of peroxide and mitochondrial reactive oxygen species (ROS) was decreased, damage to mitochondrial respiratory function was lessened, and mitochondrial homeostasis was restored [78]. Notably, with greater PCNA staining and decreased lipid peroxide production, FTH expression also enhanced tumorigenic potential in vivo. FTH is involved in the occurrence and development of hepatocellular carcinoma as it regulates iron metabolism and sustains mitochondrial homeostasis. Concordantly, FTH overexpression is thought to exacerbate the malignancy of HCC cells; therefore, FTH may be a new prognostic tool and promising therapeutic target.
In terms of prevalence, gastric cancer (GC) ranks second in China and fourth in the world compared to other malignant tumors. Although postoperative radiotherapy, chemotherapy, and targeted therapy improve survival, the 5-year survival rate for GC is only 36%. In 90% of cases, gastric cancer has the usual appearance of adenocarcinoma [79]. The main treatment of gastric cancer is chemotherapy, but the drug resistance of 5-FU limits its clinical application [80]. Studies have shown that inhibiting STAT3 induced ferroptosis may provide a new strategy for the gastric cancer therapy and drug resistance improvement. FTH1 and STAT3 were up-regulated in 5-FU resistant cells and xenografts. Further studies have demonstrated that STAT3 mediates ferroptosis in gastric cancer by binding to and regulating the expression of common DNA response elements in SLC7A11, GPX4 and FTH1 gene promoters [81]. Similar to many diseases, ferroptosis also plays a key role in gastric cancer [82, 83, 84] and represents a potential therapeutic target. Ferroptosis is closely linked to ROS, which also feature in other important cell events such as apoptosis and autophagy; however, their mechanism of formation is different in each case. Mitochondria are the main source of ROS production due to electron escape from the electron transport chain [85]. ROS is also produced by the proteasomal and lysosomal degradation of ferritin [86]. Ferritin degradation by ferritinophagy has been shown to be mediated by nuclear receptor coactivator 4 (NCOA4), a part of the autophagosome that can interact with arginine residues on the surface of ferritin heavy chain 1 (FTH1) [87]. This causes iron to be released into an unstable iron pool, triggering the Fenton reaction and ROS production. Some iron chelators can induce phagocytosis, glutathione production, and ROS level [88]. Therefore, it is crucial to elucidate the molecular mechanisms underline ferroptosis and identify related therapeutic targets in the fight against gastric cancer.
Pancreatic cancer (PC) is the eighth most frequent source of cancer death. The only cure for PC is surgery, but few patients are diagnosed early enough for this to be a curative option. Therefore, PC has an incredibly low survival rate. The ability to detect biomarkers in patients with PC at an early stage is key to successful treatment. Surgery (resection) is first considered, followed by chemotherapy or radiotherapy. Unfortunately, there are inherent disadvantages with traditional treatment methods, such as a high frequency of adverse effects, the development of drug resistance, likelihood of recurrence, and the unavoidable risk of postoperative complications associated with invasive surgery. Therefore, alternative therapies with improved efficacy and reduced toxicity are needed to conquer these limitations. Ferritin is overexpressed in pancreatic cancer and can be used as a target for radiation transmission at tumor site [89]. Human heavy chain ferritin (FTH) can embed different types of drugs in the lumen and bind to the receptor CD71 in several solid tumors, thus highlighting the potential use of ferritin in tumor targeted therapy [90]. CD71 is identified as the receptor of human ferritin H chain (FTH) [91]. FTH binds to different receptor regions of its homologous ligand, while maintaining its receptor mediated endocytosis. Therefore, a new research route has emerged recently, which uses natural or engineered FTH as a nano carrier system to deliver drugs to tumor blocks [92]. Transfer it to human pancreatic tumor xenotransplantation in vivo, with good therapeutic effect [93, 94]. In recent years, light therapy, which comprises both photothermal therapy (PTT) and photodynamic therapy (PDT), has entered the scene as a potent cancer treatment technique as it is non-invasive and has low long-term mortality and complication rates, and greater selectivity [95, 96]. Combining PTT and PDT allows us to take advantage of a synergistic effect with fewer side effects than other treatments. Meanwhile, in situ oxygen production is most commonly undertaken to enhance ROS and improve the efficacy of PDT and PTT in cancer [97, 98]. The effects of phototherapy can be optimized by increasing ROS and suppressing the anti-oxidative stress defense system. However, although oxygen production enhances ROS, excess ROS has tumor-activating properties [99]. Nuclear factor erythroid 2-related factor (Nrf2) is a transcription factor that is activated following oxidative stress and modulates the expression of genes involved in redox homeostasis. Brusatol, a Nrf2 inhibitor, was added to a silicon dioxide nanonetwork to produce a self-synergistic tumor nano-platform. By inhibiting NRF2 and the genes upon which it acts, including heme oxygenase-1 (HO-1), glutathione peroxidase 4 (GPX4), and FTH [100, 101], Brusatol impairs the antioxidant and hyperthermic capabilities of tumors, thereby exerting good anti-tumor effects. More importantly, Brusatol inactivates GPX4 and FTH, thus promoting ferroptosis and greatly enhancing synergistic phototherapeutic effects. Therefore, phototherapy is a favorable new approach for the treatment of pancreatic cancer.
An elevated CSF-to-serum ferritin ratio is considered a marker of active tumorigenesis in patients with glioblastoma multiforme, while serum ferritin levels have been demonstrated to be useful for determining disease activity and treatment guidance of this devastating illness. Neuroblastoma is particularly common amongst children, manifesting as an extracranial solid tumor and responsible for around 15% of childhood cancer deaths. Although the 5-year survival rate is at a relatively optimistic 75%, the recurrence rate is 50–60%. Tumors in infants are generally less problematic and typically progress to spontaneous maturation or regression, but tumors in patients older than 18 months frequently progress rapidly and cause significant morbidity and mortality. Neuroblastoma is not limited in anatomical location, occuring in the neck, chest, abdomen, or even bone marrow. Chemotherapy is a common treatment option, with drugs such as cyclophosphamide, vincristine, doxorubicin, cisplatin, etoposide and temozolomide available for neuroblastoma treatment [102]. Unfortunately, as is often the case with chemotherapy, drug resistance and toxic side effects can be debilitating; for example, bone marrow failure is unfortunately common [103, 104] and can lead to death. At the time of diagnosis, a careful assessment of the patient and the specific tumor should be conducted to obtain considered risk stratification in order to optimally select treatment. The ferritin heavy chain, which effectively neutralizes iron by storing it in a soluble and non-toxic state, attenuates iron-mediated ROS [105] and may be implicated in the sensitivity of N2A cells to iron poisoning treatment. In N2A cells, basal FTH levels are more than three times lower than in neural stem cells, which may lead to high levels of toxic free iron, cause various modifications in DNA, and enhance lipid peroxidation [106]. However, the overexpression of FTH significantly decreased ROS levels and cell death in N2A cells. In addition, low levels of FTH result in the sensitivity of neuroblastoma to upper iron inducers. High serum FTH in patients with neuroblastoma is a marker of poor prognosis [107], which may be due to the promotion of neuroblastoma growth and inhibition of cell death by FTH. However, some FTH is nonetheless necessary for life, as embryonic loss of FTH is debilitating [108], possibly because FTH has more conserved and important functions, such as its ferro-oxidase activity, which protects cells from oxidative stress. FTH inhibits the activity of iron oxidase and, combined with an iron inducer, may be a beacon of hope for patients with neuroblastoma who have poor prognosis.
Ovarian cancer causes many deaths in women worldwide, and the most fatal of gynecological cancers. Because early clinical signs are not obvious, more than two-thirds of ovarian cancer cases are not diagnosed until the tumor has metastasized to the abdominal cavity or other organs [109], and the five-year survival rate for patients in advanced-stage ovarian cancer is poor. Despite significant progress towards curing the disease, huge challenges remain. Radiotherapy, chemotherapy, surgery and other adjuvant therapies are the mainstays of ovarian cancer treatment [110, 111, 112], but the treatment effect is suboptimal and the recurrence rate is high. About 90% of ovarian cancer deaths can be traced to chemoresistance [113]; therefore, there is an urgent need to address the issue of chemoresistance. Research suggests that showed higher expression of ferritin H-chain in metastatic samples compared to primary tumor [114]. Cisplatin is a widely used chemotherapeutic agent for ovarian cancer. However, cisplatin resistance occurs frequently when ovarian cancer cells enhance the activity of their antioxidant system [115]. Therefore, the final reactive oxygen species (ROS) concentration induced by cisplatin exposure is critical for the effectiveness of this pro-oxidative cancer therapy. Ferritin is an invaluable component of the antioxidant system because it is able to store iron in a non-toxic form. In the antioxidant enzyme family, the role of FTH is to sequester iron with bioavailability and catalytic passivation, thus preventing its build-up in the intracellular unstable pool (LIP) and its involvement in the Fenton reaction, which generates ROS. FTH may be the key protein associated with cisplatin-based chemotherapy resistance, and inhibition of FTH may be a potential strategy to improve the sensitivity of cisplatin-resistant ovarian cancer cells.
Since ferritin plays an important role in the development of cancer, it’s necessary to make applications of ferritin during anti-cancer therapies.
As mentioned above, ferritin is an important prognostic indicator in treatments of multiple types of cancer, other than predicting the effect of surgery [116] and chemo-therapy [117], increased serum ferritin level could be served as an independent risk factor after standard intensity-modulated nasopharyngeal carcinoma radiotherapy [118], Greenbaum et al. [119] found CAR-T related toxicities is correlated to high ferritin level, indicating a novel strategy to prevent therapy toxicity. Among most type of cancers, ferritin level upregulation indicated a poor prognostic, but overexpression of ferritin reduced the tumor cell growth in BCA and NSCLC, these conflictive results may be due to different experiment system and need to be verified in future.
Besides, it is interesting that ferritin could be synthesized into nanocarrier in tumor targeted therapies. Anti-tumor drugs can be transported via ferritin nanocage. It has been suggested that after being combined to superparamagnetic iron oxide, ferritin is suitable to improve the sensitivity of liver cancer MRI diagnosis [120]. Ferritin based carriers deliver drugs to CD71 highly expressed tumor, which significantly improved the chemo-therapy efficiency [121]. Moreover, photosensitizer materials conjugated ferritin showed a promising application prospect in photo-dymamic cancer therapy [122].
However, as we showed in last part, ferritin is typically upregulated after cancer chemotherapy or radiotherapy, and protected cancer cells through ferroptosis inhibition, thus ferritin targeted treating may be helpful for reducing the ferritin mediated resistance to chemotherapy or radiotherapy. Ferritin siRNA was administered to chemotherapeutic drug doxorubicin treated breast cancer MCF-7 cell, then the chemoresistance was reduced through ROS production and p21 expression [35]. Radiation sensitivity was also improved by ferritin siRNA in astrocytoma cell line [123]. Hayashima’s study showed cystine deprivation induced cell death via FTH1 degradation [124]. Although these studies indicated ferritin targeted treatment is a promising way to improve the effects of cancer therapy, these strategies need to be further demonstrated in animal models and clinic trails.
Serum ferritin is critical for iron homeostasis in the human body due to its central role in the storage and release of iron. To fully understand the role of ferritin in the regulation of iron balance and disease, future research should explore the links between the mechanism of action, localization, structure and composition of ferritin, its interaction with other molecules, as well as its effects on unstable iron levels.
Ferritin is also associated with anti-oxidative stress, immune regulation and angiogenesis functions. More and more studies have found that ferritin expression is closely related to colorectal cancer, HNSCC, breast cancer, lung cancer, prostate cancer, liver cancer, and other malignant tumors. In addition, ferritin is useful as a carrier for the delivery of anti-tumor drugs because of its unique structural advantages. At present, more clinical data are needed to draw firm conclusions on the role of ferritin in anti-cancer therapy.
CY performed for the study design, drafted and re-vised the manuscript. XS wrote the manuscript and interpreted data. FY contributed to the data collection and database organization. AZ and JL participated in the statistical analysis of the data. XW interpreted the results. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work to take public responsibility for appropriate portions of the content and agreed to be accountable for all aspects of the work in ensuring that questions related to its accuracy or integrity.
Not applicable.
Not applicable.
This research was funded by a major project of Jingzhou Science and Technology Plan (2022CA48), Exchange Fund of China International Medical Association (CIMF-F-H001-313), Hubei Province health and family planning scientific research project (WJ2019M085), Fund of Hubei Clinical Medicine Research Center for individualized cancer diagnosis and therapy.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.