Background: Coronary artery disease is a leading public health problem.
However, the mechanisms underlying mitochondrial damage remain unclear. The
present study verified and explored the novel mechanisms underlying ischemic
injury based on a metabolomic analysis. Methods: Mouse models of acute
myocardial infarction were established, and serum samples were collected for
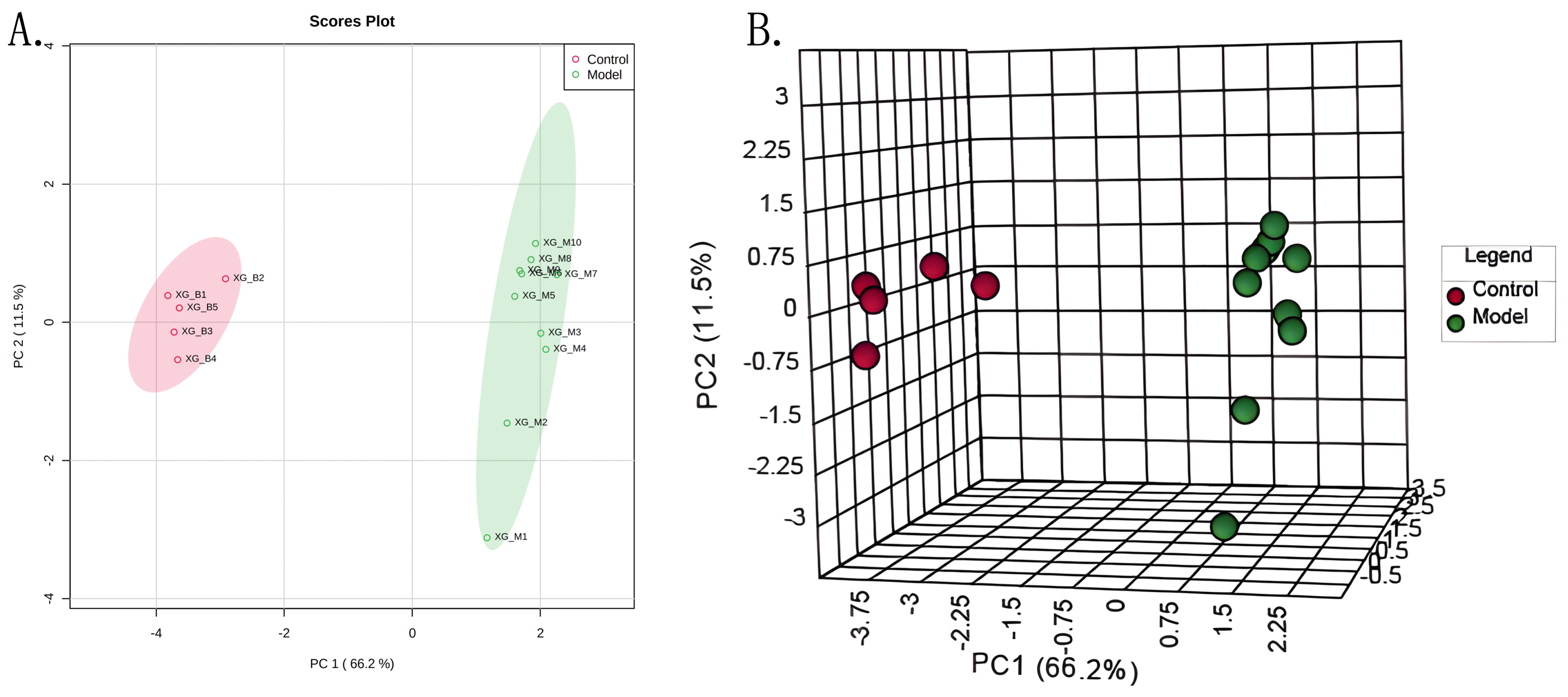
targeted liquid chromatography with tandem mass spectrometry analysis. Based on
metabolomic analyses, the N-methyl-d-aspartic acid receptor
(NMDAR)–related calcium transporting signaling pathway was selected. Primary
cardiomyocyte cultures were used, and N-methyl-d-aspartic acid
(NMDA) was used as an agonist to confirm the role of NMDAR in ischemic injury. In
addition, Bax, Bcl-2, mitochondrial calcium, potential, and mitochondrial
reactive oxygen species accumulation were used to explore the role of NMDAR in
mitochondrial damage–induced apoptosis. Results: Glutamate-related
metabolism was significantly altered following in acute myocardial infarction.
NMDA induces apoptosis under hypoxic conditions NMDAR was translocated to the
mitochondrial-related membrane after activation, and its mitochondrial expression
was significantly increased (p