- Academic Editor
§LENA Consortium: Labeling of Enalapril from Neonates up to Adolescents Consortium
Background: Plasma renin activity (PRA) has gained relevance as
prognostic marker in adults with heart failure. The use of PRA as a clinically
meaningful parameter in children and children with heart failure requires a
thorough knowledge of the factors that influence PRA to correctly assess PRA
levels. We aim to evaluate the influence of age, heart failure and
angiotensin-converting enzyme inhibitor (ACEi) on PRA levels in children.
Methods: We conducted a systematic literature search to identify studies
on PRA levels in healthy children and in children with heart failure. In
addition, we analysed PRA data measured before (n = 35, aged 25 days–2.1 years),
4 hours after (n = 34) and within the first 8 days of enalapril treatment (n =
29) in children with heart failure from the European project Labeling of Enalapril from Neonates up to Adolescents (LENA). Results: Age has a profound
effect on PRA levels in healthy children, as PRA levels in the literature are up
to about 7 times higher in neonates than in older children. Children with heart
failure younger than 6 months showed 3–4 times higher PRA levels than healthy
peers in both the literature and the LENA studies. In the LENA studies, the ACEi
enalapril significantly increased median predose PRA by a factor of 4.5 in
children with heart failure after 4.7
Plasma renin activity (PRA), along with other markers such as N-terminal pro-B-type natriuretic peptide (NT-proBNP), is an important prognostic marker for adults with heart failure. This is based on the generally accepted view that neurohormonal activation of the sympathetic nervous system and the renin-angiotensin-aldosterone system (RAAS) profoundly contributes to the pathophysiology of heart failure [1]. Moreover, Aimo et al. [2] and Vergaro et al. [3] found that PRA independently predicted cardiovascular death in adults with heart failure and concluded that PRA could be used besides other markers for prognostic stratification of heart failure patients.
As in adults with heart failure, PRA and the other markers are often measured in children with heart failure because the neurohumoral activation of the sympathetic nervous system and the RAAS also contribute to the pathophysiology of paediatric heart failure [4], although the aetiology of heart failure in children is somewhat different from adults. So far, PRA has been measured in children with heart failure as a marker for RAAS activity [5] or to check whether clinical symptoms are related to PRA [6]. Regarding clinical symptoms, PRA showed a correlation with respiratory rate and an inverse correlation with weight gain in children with heart failure [6].
To correctly assess PRA levels in daily practice, factors influencing PRA levels have to be identified and subsequently standardised or considered. It is already well known from healthy children that position of blood draw must be standardised because upright position during blood draw can increase PRA [7]. In addition, sampling should be done at the same time of day because of the diurnal variation in PRA [8, 9]. For example, Dechaux et al. [8] recommend blood sampling at 7:00 AM in supine position. Since a low-sodium diet can increase PRA levels [10, 11], if present, a low-sodium diet should be considered when evaluating PRA.
Moreover, studies show that PRA decreases with increasing age [12, 13, 14]. Even though studies on the influence of age on PRA are available, they indicate a high variation in PRA levels. Because those studies have relatively small subject numbers, a systematic review for collecting as much as possible information, especially in young children, is mandatory to draw a precise picture on the magnitude of age dependency on PRA levels and furthermore to clearly separate PRA levels in healthy children from children with heart failure.
Heart failure medication can also influence PRA levels. It is known from adults with heart failure that diuretic [15] and angiotensin-converting enzyme inhibitor (ACEi) [16, 17] therapy increase PRA, whereas beta-blocker [17] and digoxin [18] therapy decrease PRA. For beta-blockers, the decrease in PRA was also shown in children with heart failure [19]. It can be assumed that treatment with ACEi affects PRA levels in children with heart failure in the same way as in adults with heart failure. ACEis inhibit the conversion of angiotensin I to angiotensin II by the angiotensin converting enzyme. As a result, the angiotensin II levels decrease and negative feedback of angiotensin II on the renin secretion is reduced. Therefore, the renin level and consequently the PRA increase [20]. Nevertheless, the findings from adults with heart failure have not yet been confirmed in children with heart failure. Furthermore, to our knowledge, there are no studies on the effect of enalapril on PRA in children with heart failure. However, information on the influence and especially the magnitude of the influence of ACEi on PRA is important for the proper evaluation of PRA in children with heart failure on ACEi treatment.
Here, we aim to evaluate the influence of age, heart failure and ACEi treatment on PRA levels in children. For that purpose, we investigated PRA levels in healthy children and children with heart failure on standard therapy (e.g., diuretics, digoxin, and beta blocker), with and without ACEi treatment. We performed a systematic literature review and analysed data from the European project Labeling of Enalapril from Neonates up to Adolescents (LENA) where children with heart failure were treated with enalapril.
A literature search was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [21] using MEDLINE database. Search terms were defined to identify literature on PRA in healthy children as well as in children with heart failure. In November 2021, the search term “(plasma renin activity) AND (Paediatric OR newborn OR infant OR toddler OR child) AND (Heart failure OR dilated cardiomyopathy OR congenital heart defect OR congenital heart disease)” was utilized to ascertain literature on PRA in children with heart failure. In January 2022, the search term “(plasma renin activity) AND (Paediatric OR newborn OR infant OR toddler OR child) AND (healthy OR “control group”)” was used to detect literature on PRA in healthy children. The following filters were set for both searches: Humans, English, German, Child: birth – 18 years.
The inclusion criteria were set as follows. Studies were included if they
provided PRA in healthy children or children with heart failure from birth to 18
years of age. In addition, for better comparability, PRA had to be reported in
the study as arithmetic mean
The exclusion criteria were set as follows. Studies were excluded if age, type of statistical parameters used, or health status were not accurately reported. In addition, studies in which PRA was measured in preterm infants, in fetal blood or in umbilical cord blood were excluded. Further exclusion criteria were if only stimulated PRA or only renin concentration was measured. As the influence of ACEi treatment was to be investigated, studies on children with heart failure were excluded if no information on therapeutic medication was provided. Moreover, studies on children with heart failure were excluded if only postoperative or intraoperative PRA was measured. Further details are provided in the PRISMA flow diagram (Fig. 1).
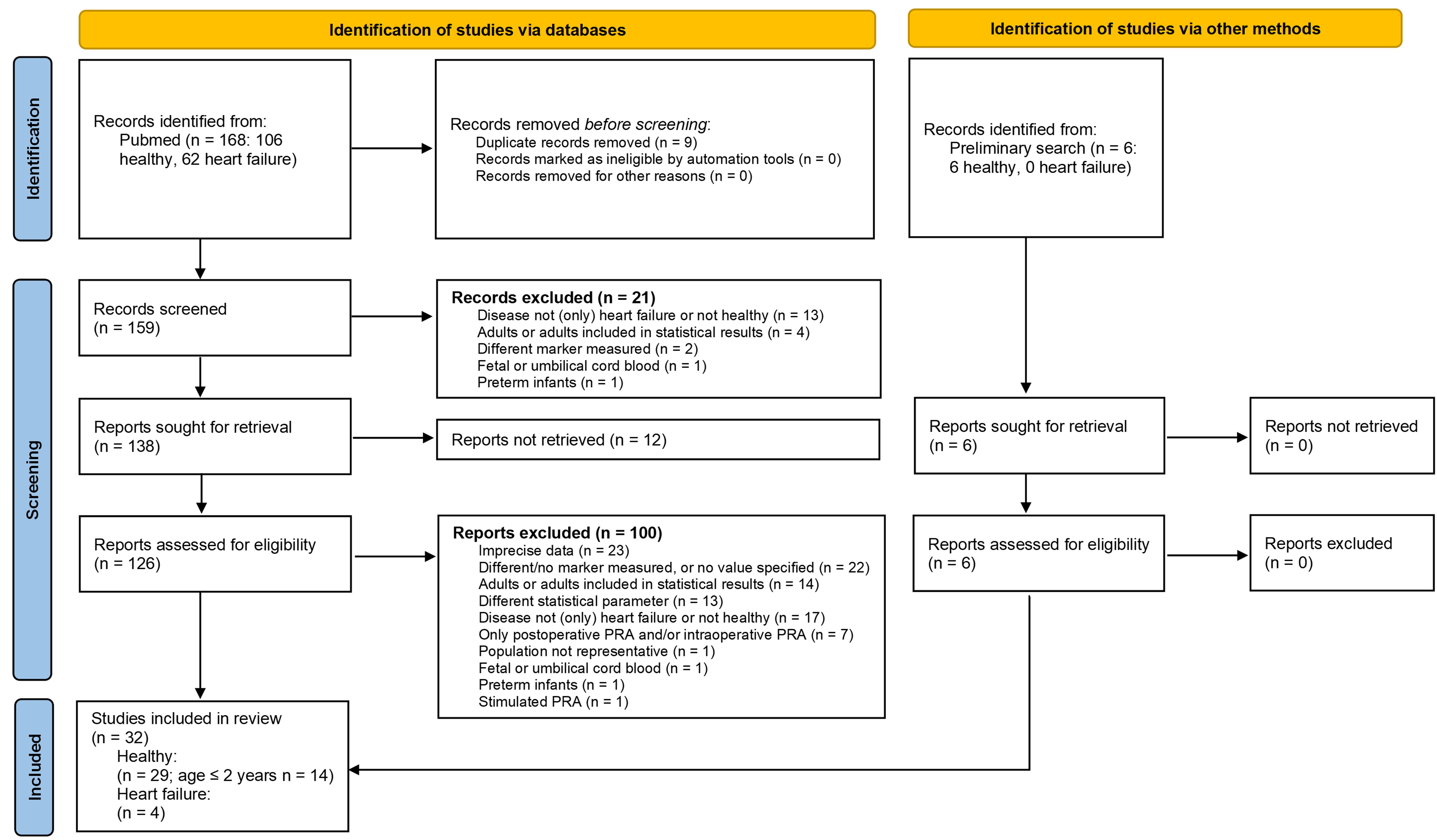 Fig. 1.
Fig. 1.Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of the conducted literature search.
In the first step of study selection, the titles and abstracts of all records identified with the above search terms were screened. Records that did not meet the inclusion criteria or fulfilled an exclusion criterion were excluded at this stage. Subsequently, the full text of the remaining records was sought. Reports for which neither a printed nor a digital version of the full text was available could not be considered further. In the next step, all available full texts were screened. Reports that did not meet the inclusion criteria or fulfilled an exclusion criterion were excluded. Finally, all studies that were not excluded and met the inclusion criteria were included in the review.
To obtain an overview of the RAAS in children, a non-systemic literature search for all RAAS parameters was conducted as a preliminary search before the systemic literature search. Additional publications found in the preliminary search that met all inclusion criteria were also included.
After inclusion of all suitable publications, it was evaluated how many publications contain information about PRA in young healthy children aged up to two years.
In addition to the literature review, data from the European LENA project
(EudraCT 2015-002335-17; EudraCT 2015-002396-18) were analysed [22]. In the LENA
project, enalapril in the form of orodispersible minitablets (ODMT) was tested in
102 children with heart failure due to congenital heart disease (CHD) or dilated
cardiomyopathy (DCM). Of 102 subjects, 35 subjects were ACEi naïve and were
included in our analysis. The dosing regimen of the LENA studies was established
using a physiologically based pharmacokinetic (PBPK) simulation. It includes age-
and weight-dependent doses which were predicted to result to similar enalapril
and enalaprilat exposures as observed in adults for a start dose of 2.5 mg and a
maintenance dose of 20 mg [23]. Daily doses
In addition to heart failure therapy, other drugs were also administered during the observation period that are not expected to influence PRA. Other concomitant medications were antiplatelet drugs, ampicillin/sulbactam, cephalosporins, chloral hydrate, folic acid, heparins, ibuprofen, iron supplement, levothyroxine, meropenem, methylprednisolone, morphine, palivizumab, paracetamol, polyethylene glycol, potassium, prednisone, ranitidine, red cell concentrate and vitamin D3.
As part of the pharmacokinetic/pharmacodynamic (PK/PD) studies, blood samples had been collected and analysed for PRA levels before, 4 hours after and within the first 8 days of enalapril treatment. Blood was collected in a cooled ethylenediamine tetraacetic acid (EDTA) tube, carefully mixed and immediately centrifugated under cooled conditions (0–4 °C). After centrifugation, the supernatant was transferred into a cryo tube and was stored at –80 °C until analysis. The sample was taken in supine position and, if possible, when the children were quiet. It was advised to collect the blood sample before 10:00 AM. If this time could not be kept, the sample collection should always take place around the same time to minimise the influence of the circadian rhythm as much as possible. Resting time and behaviour during sampling (relaxed, moving or crying) were noted. PRA was determined by using an validated in-house customised enzyme-linked immunosorbent assay (ELISA) [24]. Further information on the study procedure can be found in the study protocol [22].
To investigate the influence of ACEi treatment, only the data of the 35 subjects who were ACEi naïve at the onset of the LENA studies were examined. Out of 35, 32 subjects had heart failure due to CHD and three due to DCM. The age of the 35 ACEi naïve subjects ranged from 25 days to 2.1 years.
As part of the clinical assessment during the studies, the modified Ross score
[25] was determined by the investigator. Diaphoresis, tachypnoe, breathing,
respiratory rate, heart rate and hepatomegaly are assessed, and zero to two
points are assigned to each depending on the severity. The maximum achievable
score is 12. To analyse the impact of heart failure severity on PRA, the children
were divided in asymptomatic children (modified Ross score
For the evaluation of the PRA level, the healthy children from the literature were divided in four groups after visual inspection of the data. In the visual inspection, age ranges in which the extent and variability of PRA was similar were defined as one age group. Based on mean age or centerpoint of age range of the study group, results reporting mean PRA were summarized into four groups of age ranges: Neonates up to 30 days of age, infants from 1–24 months, children from 2–10 years, and children and adolescents older than 10 years. For each age group, the weighted mean of the reported mean PRA was calculated as an overall approximation. The number of PRA measurements was used for weighing. The weighted mean of the four age groups was compared by calculating the percentage change between the groups.
As PRA and age were not normally distributed at all time points in the LENA
studies, we reported the median and where appropriate the range for our analyses.
For comparison with the literature data, we calculated mean and SD of age and PRA
to have better comparability with the literature data, which were all available
as mean
Since the conditions regarding normal distribution for the application of the parametric tests for dependent and independent samples were not fulfilled, nonparametric tests were performed.
To analyse the effect of ACEi on PRA, PRA levels before, after 4 hours of enalapril treatment and within the first 8 days of enalapril treatment were compared. For this purpose, the Friedman test for more than two paired samples was conducted. After that, the Wilcoxon test for paired samples was conducted to compare the PRA before and after 4 hours of enalapril treatment as well as the PRA before and within the first 8 days of enalapril treatment. For both the Friedman test and the Wilcoxon test for paired samples, data from those children who had a complete data set of three PRA measurements were used (n = 29).
The PRA of the asymptomatic children and children with symptomatic heart failure
was compared before and within the first 8 days of enalapril treatment using the
Wilcoxon test for unpaired samples. Similarly, the age of the asymptomatic
children and the children with symptomatic heart failure was compared using the
Wilcoxon test for unpaired samples. p values
In the literature search, a total of 168 records were identified, 62 for the children with heart failure and 106 for healthy children. Of the 168 records, nine records were identified as duplicates. Additionally, six records on PRA in healthy children were identified through a preliminary search. After screening the titles and abstracts, 21 records were excluded. A total of 12 reports were not available in print or digital form, and 100 reports were excluded after screening the full text. Finally, 32 studies fulfilled the criteria and were included in the review. One study contained information on PRA in both healthy children and children with heart failure. Thus, a total of 29 studies on PRA in healthy children and four studies on PRA in children with heart failure were identified. Further details on the literature search and exclusion criteria are provided in the PRISMA flow diagram (Fig. 1).
The literature search yielded 29 publications on PRA in healthy children (Fig. 1). The plasma renin activities reported in these 29 publications were from a
total of 1482 healthy children. A total of 14 of the 29 publications provided
values of PRA from healthy children younger than or equal to two years of age.
Overall, 344 of the 1482 healthy children were younger than or equal to two years
old. Visual inspection of the literature data revealed roughly four groups:
Neonates up to 30 days of age, infants from 1–24 months, children from 2–10
years, and children and adolescents older than 10 years. The results of PRA level
evaluation in healthy subjects showed a decrease of PRA level over age (Fig. 2,
Table 1, Ref. [5, 7, 8, 12, 13, 14, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49]). The overall approximation of mean PRA levels
for the four age ranges were 15.4, 11.8, 3.5, and 2.2 ng/mL/h. Compared to PRA
levels of neonates up to 30 days of age, this means a drop of 23% in infants
1–24 months, 77% in children 2–10 years and 85% in children and adolescents
older than 10 years. Comparing neonates with older children, PRA is up to 7 times
higher in neonates than in older children. The percentage decrease in PRA between
the different age groups is highest between infants aged 1 to 24 months and
children aged 2 to 10 years. The highest mean PRA was determined by Vincent
et al. [27] for 16 children between 6 and 30 days of age at 29.8
 Fig. 2.
Fig. 2.Age-related change of plasma renin activity (PRA) in healthy
children from birth to 18 years of age. PRA is expressed as mean
| Age | Sex | PRA (ng/mL/h) | Reference | |||||||||||
| Mean | SD | Centerpoint of range | Min | Max | Dimension | n | Mean | SD | Min | Max | n | Sampling procedure | ||
| na | na | 0.5 | 0 | 1 | days | 10 | m/f | 8.8 | 8.9 |
0.6 | 30 | 10 | Supine (2–3 h) in the morning (between 8:00 and 10:00 AM) | [29] |
| 1 | na | na | 1 | 1 | days | 20 | m/f | 19.04 | 9.48 | na | na | 20 | Sober (4 h) and supine (2 h) in the morning | [30] |
| na | na | 1.5 | 1 | 2 | days | 10 | m | 24.8 | 26.6 |
3.7 | 96 | 10 | Supine (for at least 2 h) in the early morning | [31] |
| na | na | 4.0 | 2 | 6 | days | 15 | m/f | 24.7 | 11 |
na | na | 15 | Recumbent position in the morning (between 9:00 and 11:00 AM) | [32] |
| 4 | na | na | 4 | 4 | days | 20 | m/f | 17.33 | 8.69 | na | na | 20 | Sober (4 h) and supine (2 h) in the morning | [30] |
| na | na | 4.5 | 3 | 6 | days | 15 | m/f | 11.6 | 10.5 |
1.4 | 40 | 15 | Supine (2–3 h) in the morning (between 8:00 and 10:00 AM) | [29] |
| 7 | na | na | 7 | 7 | days | 12 | m | 13.41 | 11.8 |
na | na | 12 | Sober (2 h) and supine (1–3 h) in the morning (9:00 AM) | [33] |
| na | na | 8.0 | 7 | 9 | days | 9 | m | 5.8 | 4.5 |
1.1 | 13.8 | 9 | Supine (for at least 2 h) in the early morning | [31] |
| na | na | 12.0 | 4 | 20 | days | 7 | m/f | 3.6 | 0.9 | na | na | 15 | Sober (2 h) | [34] |
| na | na | 15.6 | 0.667 | 30.5 | days | 17 | m/f | 25 | 20.6 |
1.5 | 70 | 17 | Supine (2 h) | [7] |
| na | na | 17.0 | 12 | 22 | days | 10 | m/f | 8.73 | 3 |
na | na | 10 | Recumbent position in the morning (between 9:00 and 11:00 AM) | [32] |
| na | na | 18.0 | 6 | 30 | days | 16 | m/f | 29.8 |
28.6 |
na | na | 16 | Recumbent (1 h) and sober (2 h) in the morning (before 10:00 AM) | [27] |
| na | na | 28.5 | 10 | 47 | days | 5 | m/f | 19.6 | 10.5 | 5.2 | 31 | 5 | Sober (2–3 h) and supine between 9:00 and 10:00 AM | [5] |
| 30 | na | na | 30 | 30 | days | 25 | m/f | 4.2 | 2.8 | na | na | 25 | na | [35] |
| na | na | 31.5 | 21 | 42 | days | 8 | m/f | 2.3 | 1.7 |
0.2 | 5.2 | 8 | Supine (2–3 h) in the morning (between 8:00 and 10:00 AM) | [29] |
| na | na | 45.5 | 28 | 63 | days | 9 | m | 8.1 | 3.2 |
3.5 | 12.4 | 6 | Supine (for at least 2 h) in the early morning | [31] |
| na | na | 2.0 | 1 | 3 | months | 25 | m/f | 22.4 |
13.7 |
na | na | 20 | Recumbent (1 h) and sober (2 h) in the morning (before 10:00 AM) | [27] |
| na | na | 4.5 | 3 | 6 | months | 14 | m/f | 20 |
8.5 |
na | na | 14 | Recumbent (1 h) and sober (2 h) in the morning (before 10:00 AM) | [27] |
| 5.9 | na | na | 1 | 12 | months | 20 | m/f | 3.85 | 2.1 |
na | na | 20 | Supine in the afternoon | [28] |
| na | na | 6.5 | 1 | 12 | months | 11 | m/f | 18 | 13.3 |
0.6 | 40 | 11 | Supine (2 h) | [7] |
| na | na | 7.5 | 3 | 12 | months | 18 | m/f | 6.27 | 4.1 |
na | na | 18 | Supine (10 h) in the morning | [13] |
| 8.8 |
2.2 |
na | na | na | months | 8 | m/f | 7.8 | 0.7 | na | na | 8 | Sober and supine | [36] |
| na | na | 9.0 | 6 | 12 | months | 15 | m/f | 20 |
14.9 |
na | na | 15 | Recumbent (1 h) and sober (2 h) in the morning (before 10:00 AM) | [27] |
| na | na | 0.5 | 0.016 | 1 | years | 13 | m/f | 3.3 |
3.2 |
na | na | 13 | Recumbent (3 h) and sober in the morning (between 8:00 and 9:00 AM) | [37] |
| na | na | 1.5 | 1 | 2 | years | 20 | m/f | 16.3 |
9.1 |
na | na | 20 | Recumbent (1 h) and sober (2 h) in the morning (before 10:00 AM) | [27] |
| na | na | 1.5 | 0.083 | 3 | years | 14 | m/f | 4.6 | 6 |
na | na | 14 | Sitting except infants (supine) | [12] |
| na | na | 2.5 | 1 | 4 | years | 16 | m/f | 4.47 | 3.1 |
na | na | 16 | Supine (10 h) in the morning | [13] |
| na | na | 2.5 | 1 | 4 | years | 8 | m/f | 3.5 |
3.1 |
na | na | 8 | Recumbent (3 h) and sober in the morning (between 8:00 and 9:00 AM) | [37] |
| na | na | 3.0 | 1 | 5 | years | 10 | m/f | 8 | 4.7 |
0.8 | 16.4 | 10 | Supine (2 h) | [7] |
| na | na | 3.5 | 2 | 5 | years | 15 | m/f | 6.6 |
3.3 |
na | na | 15 | Recumbent (1 h) and sober (2 h) in the morning (before 10:00 AM) | [27] |
| na | na | 4.5 | 3 | 6 | years | 17 | m/f | 2.5 | 2.1 |
na | na | 17 | Sitting | [12] |
| na | na | 5.0 | 4 | 6 | years | 36 | m/f | 3.42 | 2.02 | na | na | 36 | Sober and supine in the morning (between 6:00 and 7:00 AM) | [14] |
| na | na | 6.0 | 5 | 7 | years | 9 | m/f | 5.7 |
2.3 |
na | na | 9 | Recumbent (1 h) and sober (2 h) in the morning (before 10:00 AM) | [27] |
| na | na | 6.0 | 4 | 8 | years | 11 | m/f | 1.9 |
1 |
na | na | 11 | Recumbent (3 h) and sober in the morning (between 8:00 and 9:00 AM) | [37] |
| na | na | 6.1 | 0.167 | 12 | years | 63 | m/f | 1.6 | 1.6 |
0.33 | 6.4 | 63 | Sober and supine in the morning (between 9:00 and 11:00 AM) | [38] |
| 6.3 | 2.5 | na | na | na | years | 10 | m/f | 2.04 | 1.13 | na | na | 10 | na | [39] |
| na | na | 6.5 | 4 | 9 | years | 18 | m/f | 2.33 | 1.2 |
na | na | 18 | Supine (10 h) in the morning | [13] |
| 6.7 | 4.2 | na | 1 | 15 | years | 50 | m/f | 1.17 | 0.92 | 0.3 | 2.25 | 50 | Supine (30 min) | [40] |
| na | na | 7.5 | 6 | 9 | years | 24 | m/f | 1.4 | 1.5 |
na | na | 24 | Sitting | [12] |
| 7.5 | 2.1 | na | 6 | 9 | years | 2 | m | 2.1 |
1.9 |
na | na | 2 | Supine (1 h) | [41] |
| na | na | 8.0 | 7 | 9 | years | 10 | m/f | 5.3 |
4 |
na | na | 9 | Recumbent (1 h) and sober (2 h) in the morning (before 10:00 AM) | [27] |
| na | na | 8.0 | 7 | 9 | years | 38 | m/f | 3.03 | 1.43 | na | na | 38 | Sober and supine in the morning (between 6:00 and 7:00 AM) | [14] |
| 9.5 |
2.6 |
na | 7 | 15 | years | 8 | m/f | 2.68 | 1.6 |
0.3 | 5.1 | 8 | Sober and supine in the morning (7:00 AM) | [8] |
| na | na | 10.0 | 8 | 12 | years | 21 | m/f | 1.4 |
1 |
na | na | 21 | Recumbent (3 h) and sober in the morning (between 8:00 and 9:00 AM) | [37] |
| na | na | 10.0 | 4 | 16 | years | 50 | m/f | 9.2 |
7.5 |
na | na | 50 | Sitting in the morning (between 9:00 and 10:00 AM) | [42] |
| na | na | 10.5 | 5 | 16 | years | 19 | m/f | 3.5 | 3.1 |
0.6 | 11 | 19 | Supine (2 h) | [7] |
| na | na | 10.5 | 9 | 12 | years | 16 | m/f | 1.9 | 2 |
na | na | 16 | Sitting | [12] |
| na | na | 11.0 | 10 | 12 | years | 41 | m/f | 2.62 | 1.32 | na | na | 41 | Sober and supine in the morning (between 6:00 and 7:00 AM) | [14] |
| 11.2 | 4 | na | 3.1 | 16.7 | years | 32 | m/f | 0.4 | 0.2 | na | na | 32 | Sober (6 h) and supine (15 min) in the morning | [43] |
| na | na | 11.5 | 8 | 15 | years | 33 | m/f | 2.4 |
1.7 |
na | na | 33 | Supine (90 min) and sober in the morning | [44] |
| na | na | 12.0 | 9 | 15 | years | 17 | m/f | 2.07 | 2 |
na | na | 17 | Supine (10 h) in the morning | [13] |
| na | na | 12.0 | 9 | 15 | years | 11 | m/f | 2.3 |
0.8 |
na | na | 9 | Recumbent (1 h) and sober (2 h) in the morning (before 10:00 AM) | [27] |
| 12.3 | 2.5 | na | na | na | years | 24 | m/f | 2.5 | 1.3 |
na | na | 10 | Supine (20 min) and sober in the morning (between 9:00 and 10:00 AM) | [45] |
| 12.5 | na | na | 10 | 16 | years | 10 | m/f | 2.85 | 0.08 | na | na | 10 | Supine in the morning | [46] |
| 12.6 | 2.2 | na | na | na | years | 74 | m/f | 3.2 | 2 | na | na | 74 | Sitting (30 min) in the morning (7:00 AM) | [47] |
| 13.1 | na | na | 12 | 15 | years | 107 | m/f | 0.717 | 0.437 | na | na | 107 | Sober and sitting in the morning | [48] |
| 13.4 | 2 | na | 10 | 18 | years | 195 | m/f | 2.52 | 1.95 | 0.1 | 13.5 | 195 | Sitting | [49] |
| na | na | 13.5 | 12 | 15 | years | 16 | m/f | 1.8 | 1.2 |
na | na | 16 | Sitting | [12] |
| na | na | 14.0 | 13 | 15 | years | 41 | m/f | 2.07 | 1.14 | na | na | 41 | Sober and supine in the morning (between 6:00 and 7:00 AM) | [14] |
| 14 | 2.4 | na | na | na | years | 66 | m/f | 3.4 | 2.4 | na | na | 66 | Sitting (30 min) in the morning (7:00 AM) | [47] |
| na | na | 14.0 | 12 | 16 | years | 9 | m/f | 0.9 |
0.7 |
na | na | 9 | Recumbent (3 h) and sober in the morning (between 8:00 and 9:00 AM) | [37] |
| 14.2 | 2.2 | na | 12 | 17 | years | 4 | f | 2.6 |
1.2 |
na | na | 4 | Supine (1 h) | [41] |
| na | na | 16.5 | 15 | 18 | years | 10 | m/f | 1.8 | 1.3 |
na | na | 10 | Sitting | [12] |
Note:
In all studies, PRA was determined by radioimmunoassay (RIA). For age, the centerpoint of the range was only calculated if no mean value was available.
f, female; m, male; Max, maximum; Min, minimum; na, data not available; PRA, plasma renin activity; SD, standard deviation; SE, standard error.
For children with heart failure, the literature search revealed four studies with PRA data from a total of 58 children (Fig. 1). PRA levels in patients with heart failure also show a tendency to decrease with age (Fig. 3, Table 2). PRA levels in children with heart failure younger than 6 months were greater than in healthy peers. In patients with heart failure at this age, PRA levels were 3–4 times higher than in healthy subjects comparing equal age ranges (Tables 1,2).
 Fig. 3.
Fig. 3.PRA of healthy children and children
with heart failure. The PRA data from the healthy children are from the
literature (black, n = 344). The PRA data from the children with heart failure
are from the literature (blue, n = 58) and from the Labeling of Enalapril from Neonates up to Adolescents (LENA) studies (red, n = 35).
None of the children with heart failure had previously been treated with an
angiotensin-converting enzyme (ACE) inhibitor. PRA is expressed as mean
| Age | Sex | Indication | PRA (ng/mL/h) | Reference | ||||||||||||
| Mean | SD | Centerpoint of range | Min | Max | Dimension | n | Mean | SD | Min | Max | n | Sampling procedure | Analytics | |||
| 28 | na | na | 14 | 112 | days | 11 | m/f | severe congestive failure due to left-to-right shunts | 54 | na | 33 | 162 | 11 | na | na | [50] |
| 38 | na | na | 14 | 84 | days | 11 | m/f | CHD with left to right shunts (severe congestive heart failure) | 84 | 21 | 57 | 126 | 11 | Sober (2–3 h) and supine between 9:00 and 10:00 AM | RIA | [5] |
| 42 | na | na | 28 | 112 | days | 11 | m/f | severe congestive failure due to left-to-right shunts | 38 | na | 4 | 326 | 11 | na | na | [50] |
| na | na | 65 | 19 | 111 | days | 8 | m/f | CHD with left to right shunts (congestive heart failure) | 87.1 |
44.9 |
22 | 183 | 8 | Sober (2–3 h) and supine between 9:00 and 10:00 AM | RIA | [51] |
| 4 | 2 | na | na | na | months | 18 | m/f | CHD with left to right shunts | 35 | 40 | na | na | 7 | non-sedated infants | RIA | [6] |
| 6 | 2 | na | na | na | months | 30 | m/f | CHD with left to right shunts | 10 | 7 | na | na | 10 | non-sedated infants | RIA | [6] |
| 26.3 | 1.3 | na | 25 | 27 | days | 3 | m | CHD | 63.6 | 40.2 | 30.7 | 108.4 | 3 | Supine in the morning | ELISA | LENA studies |
| 55.1 | 14.3 | na | 35 | 85 | days | 12 | m/f | CHD | 38.1 | 37.2 | 6.4 | 101.2 | 12 | Supine in the morning | ELISA | LENA studies |
| 4.2 | 0.8 | na | 3.2 | 5.9 | months | 13 | m/f | CHD (n = 12) and DCM (n = 1) | 54.0 | 54.3 | 4.2 | 183.9 | 13 | Supine in the morning | ELISA | LENA studies |
| 11.1 | 6.8 | na | 6.9 | 25.1 | months | 7 | m/f | CHD (n = 5) and DCM (n = 2) | 11.2 | 6.0 | 3.4 | 19.7 | 7 | Supine in the morning | ELISA | LENA studies |
Note:
For age, the centerpoint of the range was only calculated if no mean value was available.
CHD, congenital heart disease; DCM, dilated cardiomyopathy; ELISA, enzyme-linked immunosorbent assay; f, female; LENA, Labeling of Enalapril from Neonates up to Adolescents; m, male; Max, maximum; Min, minimum; na, data not available; PRA, plasma renin activity; RIA, radioimmunoassay; SD, standard deviation.
Only the oldest children with heart failure, aged 6
The LENA studies also provided information on PRA in children with heart failure
without ACEi treatment. A total of 35 subjects in the LENA studies were not
pretreated with ACEi. Therefore, the PRA measured before the first enalapril dose
in these subjects can be compared with the literature data. The mean PRA of all
four age groups of the LENA subjects was comparable to the mean PRA in the
literature. Three of the four groups also had a mean PRA that was above the PRA
reported in healthy children. Analogous to the literature data, the oldest age
group (11.1
In contrast to the literature search, the LENA studies also provided information
on PRA in children with heart failure treated with ACEi. As mentioned earlier, 35
subjects (aged 25 days–2.1 years) had not received pretreatment with an ACEi.
Their predose PRA was compared with the PRA 4 hours after the first enalapril
dose and with the PRA within the first 8 days of enalapril treatment (Fig. 4).
The Friedman test showed a significant difference (p
 Fig. 4.
Fig. 4.PRA in children with heart
failure from the LENA studies at different time points of enalapril therapy.
PRA data were available from 35 subjects (aged 25 days–2.1 years, median age =
3.6 months) predose, from 34 subjects 4 h postdose, and from 29 subjects (aged 29
days–2.1 years, median age = 3.4 months) after 4.7
| Concomitant medication | Dosage at the start of the study | Duration of therapy before administration of enalapril | Change in dosage during observation period | Discontinuation of therapy during observation period | |||
| n | Median (Range) | Unit | Range | Duration |
n (%) | n (%) | |
| n (%) | |||||||
| Furosemide | 33 | 1.42 (0.27–3.20) | mg/kg/day | 1 day–4 months | 29 (87.9) | 2 (6.1) | 0 (0) |
| Spironolactone | 28 | 0.83 (0.27–1.88) | mg/kg/day | 3 days–5 months | 28 (100) | 0 (0) | 0 (0) |
| Digoxin | 3 | 11.06 (10.91–14.93) | µg/kg/day | 11–21 days | 3 (100) | 0 (0) | 0 (0) |
| Carvedilol | 1 | 0.55 | mg/kg/day | 19 days | 1 (100) | 0 (0) | 0 (0) |
| Milrinone | 1 | 0.30–0.45 |
µg/kg/min | 10 days | 1 (100) | 0 (0) | 1 (100) |
| No concomitant medication | 2 | - | - | - | - | - | - |
Note:
Out of the 35 LENA participants without ACEi pretreatment, eight had a Ross
score less than or equal to two and were therefore classified as asymptomatic.
Before the first enalapril dose, the median PRA of the children with asymptomatic
heart failure was 9.3 ng/mL/h (Fig. 5). In contrast, the LENA participants with
symptomatic heart failure (Ross score
 Fig. 5.
Fig. 5.PRA in asymptomatic children and
children with symptomatic heart failure from the LENA studies. The Wilcoxon test
for unpaired samples was conducted between the asymptomatic children with a Ross
score
The current literature review and the European LENA project on children with heart failure help to address the influence of age, heart failure and ACEi treatment on PRA levels in children more specifically. First, age does have a profound effect on PRA levels in healthy children, as in neonates the PRA is up to about 7 times higher than in older children. Secondly, children with heart failure younger than 6 months show 3–4 times higher PRA levels than healthy children of comparable age. Thirdly, the ACEi enalapril further increased PRA levels by a factor of 4.5 in children with heart failure.
Of the three influencing factors analysed, age has the greatest influence on PRA in childhood according to our analyses. The comparison of the results of the different studies in the systematic review confirms previous separate observations on the decrease of PRA with age [12, 13, 14] and furthermore provides information on the extent of the age-related decrease of PRA in healthy children.
Considering the whole childhood, our analyses show a strong decrease of PRA by up to 85%. Such a strong decrease with age in childhood is not extraordinary and is also known for example for NT-proBNP [52].
The reasons for the increased PRA in children and especially in neonates and infants are not known with certainty. It is known that renin release is promoted by a decrease in renal perfusion pressure [53]. Consequently, the increase in blood pressure with age in children [54], could be a possible explanation for the age-related decrease of PRA in childhood.
Neonates and infants up to two years of age not only have the highest PRA levels, but also the greatest variability between the different studies. Possible reasons for the variability of PRA between the different studies could be differences in sampling or sodium intake of the subjects as well as crying during sampling. It is unlikely that the variability is due to the influence of a different position during sampling [7] or a different time of sampling [8, 9] in the studies, as in most of the included studies with children under two years of age, sampling was performed in the morning in the supine position. Different sodium intake could be one reason for the variability between the studies, as other investigations show an inverse correlation between PRA and sodium intake [31, 55]. As sodium intake was rarely assessed in the included studies, differences in sodium intake between the studies could possibly explain parts of the variability between the different studies. In addition, crying during sampling increases PRA [56]. Neonates and infants are more likely to cry during blood collection than older children. A different proportion of crying subjects in the different studies could be another reason why the variability between the different studies is high for children under two years of age.
The second factor influencing PRA is heart failure, which appears associated with 3–4 times higher PRA levels in neonates and young infants compared to healthy peers. Children with heart failure younger than 6 months from both the literature and the LENA studies had higher PRA levels than healthy children of the same age.
The activation of the RAAS in children with heart failure is part of the pathophysiology [4]. Consistent with our results in children with heart failure younger than 6 months, Nijst et al. [57] found about 5 times higher PRA in adults with chronic heart failure on optimal medical therapy compared with healthy controls. Anand et al. [58] reported a 9.5 times higher PRA in adults with severe clinical congestive cardiac failure. The greater difference between patients and healthy subjects in the latter study could be due to the fact that the subjects of Anand et al. [58] were completely untreated patients, while LENA subjects had been pretreated with various heart failure medications other than ACEi.
We assume that the PRA in children with heart failure is influenced by the
severity of heart failure. The influence of the severity of heart failure on PRA
is supported by the fact that our subjects with symptomatic heart failure (Ross
score
Data from the literature and the LENA studies indicate that PRA levels, similar to healthy children, tend to decrease with age also in patients with heart failure. However, the amount of data available and the age range of children with heart failure were too small to interpret the influence of age in children with heart failure more precisely.
A surprising finding was that the oldest subjects with heart failure from the
literature and the LENA studies even had PRA levels in the range of PRA of
healthy children. We suggest two reasons why PRA is not increased in older
children with heart failure. The first reason could be that the older children
have milder heart failure, as patients with more severe clinical symptoms due to
CHD are usually operated earlier. The subjects in the oldest study group (aged 6
The third factor influencing PRA is the ACEi treatment, which increases PRA by 4.5-fold in children with heart failure. Thus, our results suggest that treatment with an ACEi may have an even greater influence on PRA than heart failure itself.
Previous studies on PRA after captopril administration in children with heart failure either did not find a significant increase in PRA [61, 62] or did not state whether the increase in PRA was significant [50, 51]. The studies that did not find a significant increase investigated only a small number of 8 respectively 12 subjects [61, 62]. In contrast, our analysis is based on a considerable higher number of 29 subjects.
In accordance with our results in children with heart failure, enalapril administration significantly increases PRA by approximately a factor of four in adults with heart failure [16]. Compared to adults with heart failure, the increase in PRA in children with heart failure was not yet significant 4 hours after administration, but only within the first 8 days of treatment. The delayed effect of enalapril in children with heart failure could have several reasons. One reason could be the higher starting dose used in the adult study compared to the starting dose in the LENA studies. In the study on enalapril in adults with heart failure a high dose between 10 and 40 mg was used as the first dose. The children in the LENA study received a dose comparable to a starting dose of 2.5 mg enalapril in adults. Another reason may be that the maximum enalaprilat concentration is reached later in children under one year of age. The pharmacokinetic analysis of the LENA studies revealed that the subjects younger than one year had a median time to reach maximum enalaprilat concentration of 6 hours [23]. Whereas in subjects older than one year, the median time to reach maximum enalaprilat concentration was 4 hours [23], comparable to that of adults [63]. Since 28 of the 29 subjects analysed were younger than one year, the time of PRA measurement in these children may have been too early to observe a significant increase in PRA. As a third reason, the PRA increase may be due not only to a direct negative feedback of angiotensin II on renin release but also to an increase in renin synthesis, as treatment with enalapril for several days causes an increase in renin mRNA in rats [64]. Finally, the number of subjects may have been too small to observe a significant increase in PRA after 4 hours in children with heart failure.
As concomitant medication could have an impact on PRA during the observation period, we investigated duration, changes and discontinuations of concomitant medication. Most of the concomitant medication was taken at least three days before the start of enalapril administration. It can therefore be assumed that the majority of subjects and their PRA had stabilized on their therapy before enalapril was administered. Since furosemide [15] and milrinone [60] increase PRA, reducing the dosage of furosemide and discontinuing milrinone therapy in one subject may at most have attenuated the observed increase in PRA due to enalapril. As the increase in furosemide dose in one subject only compensates for the reduced bioavailability due to the switch from intravenous to peroral administration, this change is not expected to have any effect. For the above reasons, we consider an impact of concomitant medication on PRA during the observation period as unlikely.
Of importance is the fact that the significant difference in PRA between asymptomatic children and children with symptomatic heart failure disappeared under enalapril therapy. The reason for this could be that, according to our results, the influence of ACEi treatment appears to be greater than the influence of heart failure itself. However, the comparison between asymptomatic children and children with symptomatic heart failure is based on a small number of eight asymptomatic patients, and the improvement in heart failure with therapy may have attenuated the increase in PRA differently in the two groups.
The existing studies on PRA after captopril administration in children with heart failure had to be excluded from the systematic review due to missing information on PRA values [61], contradictory information on the unit [62] or imprecise or missing information on age [50, 51]. Therefore, to our knowledge, this is the first report of PRA after ACEi administration in children with heart failure with exact age information and the first report of PRA after enalapril in children with heart failure ever.
The systematic review may not include all publications on PRA in healthy children and children with heart failure due to limitations of the method. Only publications listed in the MEDLINE database in English or German were considered. Publications in English and German for the literature search on PRA in healthy children and in children with heart failure, however, encompass the majority of all publications under the search terms used, with 92% and 90% respectively. Publications in which parts of the search terms are not mentioned in the title or abstract may have been overlooked. For healthy children, six additional publications from the preliminary search were included. In three of these cases were only the title and not the abstract available in the MEDLINE database. In the other three cases, other keywords were used for healthy children, for example, they were named normal children. Due to the nature of the literature data, it was not possible to analyse the literature data beyond descriptive statistics. Our relatively strict inclusion criteria led to the exclusion of some studies. Excluding studies with inaccurate age information reduced the data set, but as these analyses show, accurate age information is necessary to compare PRA values. Specifying the allowable statistical parameters in the inclusion criteria resulted in a smaller data set but improved the comparability of the data. Despite these limitations, the systematic review contains a large amount of data on PRA from almost 1500 healthy children and almost 60 children with heart failure.
Our age classification for the evaluation of the PRA level of the healthy children from the literature is based only on the visual inspection of the data. Both the age group of children from 2–10 years and the age group of children and adolescents over 10 years encompass a relatively wide age range. However, the age classification chosen is comparable to the age classification of the European Medicines Agency and the US Food and Drug Administration, where the age groups are for children from 2–11 years and for adolescents from 12–18 years.
The analysis of PRA in children with heart failure from the LENA studies is faced with limitations. One limitation is that the number of subjects that could be analysed for the effect of enalapril on PRA was limited because only a part of the subjects was without ACEi pretreatment. However, almost all ACEi naïve children were under one year old. Considering this very young age, a large number of very young subjects with heart failure were studied. Furthermore, PRA was analysed in children with heart failure of different aetiology. However, due to the small number of three ACE naïve children with DCM, an analysis regarding a possible influence of the aetiology of heart failure was not possible. Data from the LENA studies cannot provide information on the prognostic value of PRA in children with heart failure. The number of 35 ACE naïve subjects was unfortunately too small to perform a multiple regression analysis. Thus, an overlap of the influences of age, heart failure and ACEi treatment is possible. However, when comparing PRA between healthy children and children with heart failure, the LENA subjects were divided into four age groups to compare them with healthy peers and to keep the influence of age as low as possible. When comparing PRA between children with asymptomatic and symptomatic heart failure, there was no significant age difference between the two groups. The influence of enalapril on PRA was investigated in an observation period with a maximum of eight days. Therefore, we consider the influence of age to be negligible in the selected observation period. Since PRA decreases with age, age could at most have attenuated the observed effect of enalapril on PRA. As the Ross score improved in some of the subjects during the observation period, the improvement in heart failure may have attenuated the observed increase in PRA due to enalapril. However, as only two subjects had a change in Ross score large enough to change the heart failure classification, we assume that the effect of the improvement in heart failure on PRA will be rather small here. Moreover, we analysed the effect of enalapril on PRA in children with heart failure only in the first days of therapy. Further analysis of the studies data is planned to determine whether PRA remains elevated with prolonged ACEi therapy.
In summary, we have shown that age, heart failure and ACEi treatment have a notable influence on PRA. In children with heart failure, not only age but also ACEi treatment must be considered when assessing PRA as a clinically meaningful parameter, as ACEi treatment leads to a 4.5-fold increase of PRA that is not due to the disease state. In detail, it should be examined whether an ACEi is included in the medication and how long the ACEi treatment has already been given. In studies on PRA, subjects with and without ACEi should preferably be evaluated separately.
ACEi, angiotensin-converting enzyme inhibitor; CHD, congenital heart disease; DCM, dilated cardiomyopathy; ELISA, enzyme-linked immunosorbent assay; LENA, Labeling of Enalapril from Neonates up to Adolescents; NS, not significant; NT-proBNP, N-terminal pro-B-type natriuretic peptide; ODMT, orodispersible minitablets; PBPK, physiologically based pharmacokinetic; PK/PD, pharmacokinetic/pharmacodynamic; PRA, plasma renin activity; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RAAS, renin-angiotensin-aldosterone system; RIA, radioimmunoassay; SD, standard deviation; SE, standard error.
All data generated or analyzed during the systematic review are included in this published article. Datasets from the LENA project analyzed during the current study are not publicly available because a marketing authorization application has been submitted to the EMA.
Conception and design of the work were developed by MS, WC and SL. Data collection for the systematic review was performed by MS. MB, JMPJB, MD, CM, SNW and SL were involved in the acquisition of the LENA data. MS and WC analyzed the data, SL critically reviewed and discussed the analysis. MS drafted the manuscript. WC and SL critically reviewed the draft and the improved versions of the manuscript. MB, JMPJB, MD, CM and SNW critically checked the final manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The study protocols responding to specific national requirements were submitted to the responsible Independent Ethics Committees (IECs) in the participating countries for review and received approval. The address from the Ethics committee of the coordinating principle investigator’s IEC of Study EudraCT 2015-002335-17 (DCM patients) was: Secretariaat Medisch Ethische Toetsings Commissie Erasmus MC, Postbus 2040, 3000 CA Rotterdam, The Netherlands, NL Dossiers: date: 4 December 2015 No.: NL54914.078.15 and NL54738.078.15 and MEC-2015-634 and MEC-2015-635; Ethics committee UMC Utrecht, NL: date 17 February 2016 No.: Mvd/vb/16/004864, Mvd/vb/16/004964; Medical Research Council, Ethics Committee for Clinical Pharmacology, National Institute of Pharmacy and Nutrition, Budapest date 30 November 2015, No.: OGYI/36681-7/2015 and OGYI/36999-9/2015; Ethikkommission Medizinische Universität Wien; date 21 December 2015 No.: 1803/2015; Address of the coordinating principle investigator’s IEC of study EudraCT 2015-002396-18 (CHD patients) was: Ethics Committee of the University Children’s Hospital in Belgrade and the Institute of Mother and Child Health “Dr Vukan Čupić” Univerzitetska Dečja Klinika, Tirsova 10, 11129 Belgrade, Serbia. Dates 29 February 2016 and 5 April 2017; No.: 26/307 and 8/9; Ethikkommisison der Ärztekammer Hamburg, Germany 22 May 2017, PVN9495 and PVN5496. Informed consent was obtained from all subjects involved in the study.
We thank the LENA Consortium for having discussed the results of the primary objective throughout the LENA project from 2013 to 2019 at meetings. The complete LENA consortium is listed below. LENA Beneficiaries and Principal Investigators other than the authors: Jörg Breitkreutz, Germany. Ingrid Klingmann, Belgium. Florian Lagler, Austria. Jan de Hoon, Belgium. Anne Keatley Clarke, United Kingdom. Laszlo Ablonczy, Hungary. Thomas Mir, Germany. Vladislav Vukomanovic, Serbia. Milan Dukic, Serbia. Ida Jovanovic, Serbia. Advanced Scientists other than the authors: Björn B. Burckhardt, Karl Kleine, Angelika Moder, Emina Obarcanin, Peter Wagner, Jennifer Walsh, Anne van Hecken, Lucie Spatenkova. Scientists and young scientists: Mohsin Ali, Bojana Božić, Maja Bijelić, Ilja Burdman, Agnes Ciplea, Muhammed Faisal, Samieh Farahani, Martin Feickert, Tanja Gangnus, Milica Lazic, Nina Makowski, Fabian Süssenbach, Marijke van der Meulen, Saša Popović, Miro Parezanović, Nori Smeets, Vanessa Swoboda. Clinical investigators: Dragana Bojanin, Stefan Đorđević, Jasminka Dragić, Ann-Kathrin Holle, Bosiljka Jovičić, Jovan Košutić, Gordana Kozomara, Haidara, Majid, Jadranka Mitrović, Sanja Ninić, Miro Parezanovic, Vojislav Parezanovic, Andrija Pavlović, Sergej Prijić, Branislava Rebić, Igor Stefanović, Daniel Tordas, Irena Vulićević. Study nurses, Technicians, Social workers: Anke Bartel, Andjelka Čeko, Marissa Herborts, Annelies Hennink, Bosiljka Kosanović, Sanja Kostic, Ljiljana Isailović, Jasmina Maksimovic, Badies Manai, Nada Martinović, Gyöngyi Máté, Miloš Perišić, Jelena Reljić, Regina Pirker Marta Salamomovic, Claudia Schlesner, Jutta Tins, Eva Wissmann. We thank Holger Schwender, Institute of Mathematical Sciences, Department of Applied Statistics, Heinrich-Heine-University Düsseldorf, for advice on statistical analysis.
The research work was a part of project LENA (labeling of enalapril from neonates up to adolescents) which is funded by a European Union Seventh Framework Program (FP7/2007-2013) under the grant agreement no. 602295.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
