1 School of Traditional Chinese Medicine, Hunan University of Chinese Medicine, 410208 Changsha, Hunan, China
2 Teaching and Research Section of Traditional Chinese Medicine Surgery, The First Hospital of Hunan University of Chinese Medicine, 410021 Changsha, Hunan, China
3 School of Pharmacy, Hunan University of Chinese Medicine, 410208 Changsha, Hunan, China
4 Department of Oncology, the Affiliated Hospital of Hunan Academy of Traditional Chinese Medicine, 410006 Changsha, Hunan, China
5 College of Integrated Traditional Chinese and Western Medicine, Hunan University of Chinese Medicine, 410208 Changsha, Hunan, China
6 Department of Oncology, The First Hospital of Hunan University of Chinese Medicine, 410021 Changsha, Hunan, China
Abstract
Objective: The use of immune
checkpoint inhibitors (ICIs) provides promising strategies for hepatocellular
carcinoma (HCC) treatment. This study aimed to explore impact and underlying
mechanism of the combination therapy of quercetin and anti-programmed cell death
1 (anti-PD-1) antibody on HCC. Methods: Orthotopically transplanted HCC
tumors in mice were treated with quercetin, anti-PD-1 antibody, or a combination
of both therapies. Histopathological changes and programmed cell death ligand 1
(PD-L1) expression were characterized by hematoxylin and eosin (H&E) and
immunohistochemistry (IHC) staining. The diversity and differences of gut
microbiota (GM) were evaluated through 16S rRNA sequencing. Levels of macrophage
immunity-related cytokines were quantified by enzyme-linked immunosorbent assay
(ELISA), quantitative real-time polymerase chain reaction (RT-qPCR), and Western
blot. Results: Combination therapy reduced necrosis,
fibrosis, and PD-L1 expression in liver tissues. Additionally, combination
therapy reduced GM imbalance and increased abundance of Firmicutes,
Actinobacteria, and Verrucomicrobiota at the phylum level as
well as Dubosiella and Akkermansia at the genus level.
Combination therapy improved macrophage immunity, raised the expressions of CD8a,
CD4, CD11b, interleukin (IL)-10, and interferon (IFN)-
Keywords
- quercetin
- anti-PD-1 antibody
- hepatocellular carcinoma
- gut microbiota
- macrophage immunity
Hepatocellular carcinoma (HCC) is a malignant tumor that arises in hepatocytes. HCC is the most common pathological type of primary liver cancer and a principal cause of cancer-related deaths globally [1]. Clinically, surgery is the most effective treatment for HCC and includes both hepatectomy and liver transplantation). However, most HCC patients frequently miss the opportunity to have surgery due to the poor rate of early disease diagnosis [2]. Therefore, research into HCC diagnosis and innovative treatments is a critical step to improving response to this form of cancer.
Transcriptome analysis has revealed a marked
local upregulation of both programmed cell death 1 (PD-1) and programmed cell death 1 ligand 1 (PD-L1) in
the tumor microenvironment (TME) of HCC. FoxP3-positive lymphocytic infiltration,
E-cadherin deficiency, epithelial-mesenchymal transition (EMT), and an extremely
poor histologic differentiation are also observed [3]. Immune checkpoint blockade
immunotherapy resulted in the activation of the nuclear factor kappa B
(NF-
Previous findings have indicated that PD-L1 and gut microbiota may serve as potential predictive biomarkers for immunotherapy responses in the case of advanced HCC [5, 6]. Recent studies have increasingly demonstrated that gut microbiota (GM) can affect the occurrence and progression of HCC through the gut-liver axis [7]. Cooperation between GM and immune checkpoint inhibitors (ICIs) has particularly improved the effectiveness of PD-1 blockade therapy for cancers [8]. As such, GM may play a critical role in the response of HCC patients who are treated with an anti-PD-1 immunotherapy regimen [9]. Research on the combination of ICIs and other drugs or therapies for the treatment of malignant tumors has been reported [10, 11]. Quercetin is characterized as an effective liver protector due to its antioxidant, anti-inflammatory, and anticancer activities [12]. Its therapeutic effects on HCC have been studied both in vitro and in vivo models [13]. Further, quercetin has been demonstrated as an alternative therapy for preventing HCC early in the process of tumorigenesis by regulating key TME components [14]. Prior research has shown that quercetin, functioning as a cancer chemopreventive agent, can attenuate the inhibition of PD-L1 on T lymphocytes by altering the PD-1/PD-L1 interaction [15]. However, the combination therapy of quercetin and anti-PD-1 immunotherapy on GM and TME in HCC requires further exploration.
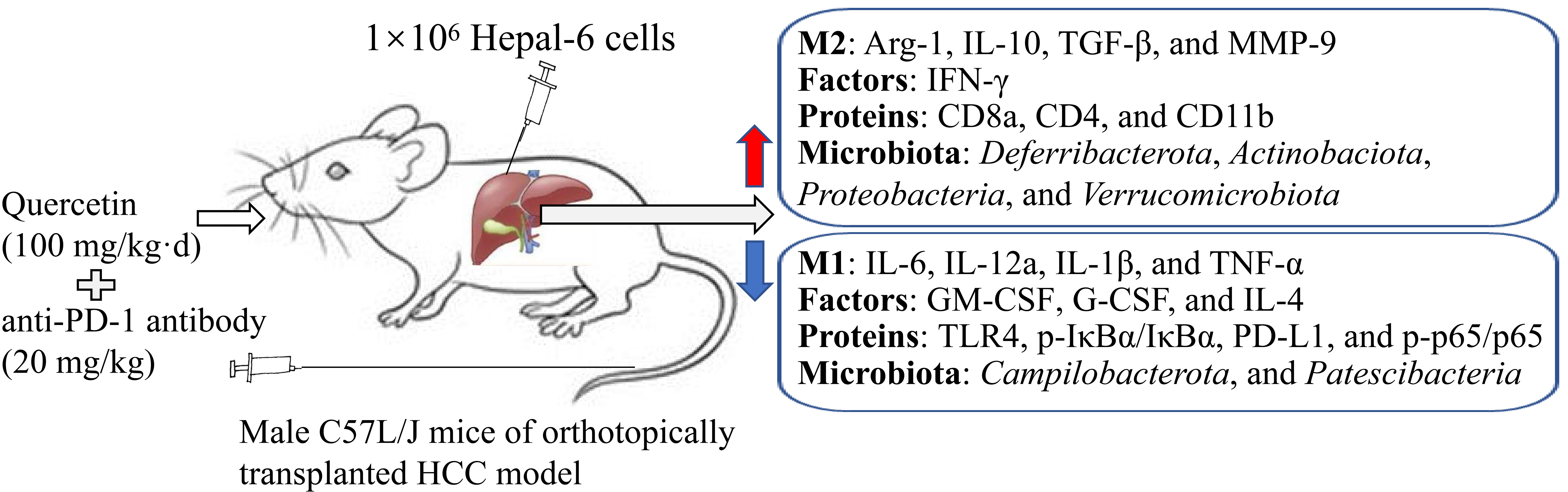
In this study, we used an orthotopically transplanted HCC mouse model that was treated with quercetin, anti-PD-1 antibody, or a combination therapy. We explored the impact of the combination therapy on GM and macrophage immunity in HCC mice as a means to provide new insights for the application of traditional Chinese medicine (TCM) and immunotherapy in HCC.
Male 8-week-old C57L/J mice were obtained from Hunan Slyke
Jingda Laboratory Animal Co., Ltd (Changsha, China). The construction of an
orthotopically transplanted HCC model was performed following one week of
adaption [16]. Briefly, 1
The experimental treatment groups were listed as follows: Control, Model,
Quercetin, anti-PD-1, and Quercetin+anti-PD-1, and each group contained 8 mice.
The corresponding intervention was administered to mice 14 days after cell
implantation. Mice in the Control and Model groups were gavaged with the same
dose of distilled water for 14 days. Mice in the Quercetin group were gavaged
daily with quercetin (100 mg/kg
The liver tissue slices were first dewaxed by placing them in xylene for 20 min followed by dehydration with an ethanol gradient (75–100%). After this, the slices were stained with hematoxylin (AWI0001a, Abiowell, Changsha, China) for 1–10 min and subsequently rinsed in phosphate buffer saline (PBS). Afterward, the slices were stained with eosin (AWI0029a, Abiowell) for 1–5 min, washed with distilled water, and then dehydrated with an ethanol gradient (95–100%). Finally, the slices were placed in xylene for 10 min for tissue transparency and sealed with neutral gum (AWI0238a, Abiowell) prior to observation.
Total RNA was extracted from liver tissues using Trizol reagent (15596026,
Thermo, Waltham, MA, USA). Following this, mRNA was reverse transcribed into cDNA
using a reverse transcription kit (CW2569, CWBIO, Beijing, China), followed by quantitative real-time polymerase chain reaction (RT-qPCR). The primers were as follows:
Liver tissues from different groups of mice were suspended in radio-immunoprecipitation assay (RIPA) lysate (AWB0136,
Abiowell) to obtain total protein extracts. After separation by
sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE), proteins
were transferred onto the nitrocellulose (NC) membranes. After blocking with 5%
skimmed milk (AWB0004, Abiowell) for 1.5 h, the NC membranes
were incubated with primary antibodies at 4 °C overnight separately.
Primary antibodies were as followed: CD8a (1:1000, ab33786, Abcam, Cambridge,
UK), CD4 (1:1000, 19068-1-AP, Proteintech, Chicago, IL, USA), CD11b (1:1000,
ab133357, Abcam), glyceraldehyde-3-phosphate dehydrogenase (GAPDH,
1:5000, 10494-1-AP, Proteintech), toll-like receptor 4 (TLR4,
1:1000, 19811-1-AP, Proteintech), inhibitor of nuclear factor
For the detection of IL-6 (KE10007, Proteintech), IL-10
(KE10008, Proteintech), interferon-
The liver slices were dewaxed by soaking in xylene for 20 min. Subsequently, dehydration was carried out using an ethanol gradient (75–100%). The slices were then boiled in citrate buffer (0.01 M, pH 6.0) (AWI0206a, Abiowell) for antigen retrieval. 1% periodic acid was employed to inactivate the endogenous enzymes. The slices were incubated with antibodies of PD-L1 (1:200, ab238697, Abcam) overnight at 4 °C, and then incubated with 100 µL of HRP-anti-Rabbit-IgG for 30 min at 37 °C. Following these steps, 100 µL of diaminobenzidine (DAB) was added to slices and incubated for 5 min. The slices were counterstained with hematoxylin for 5 min, rinsed with distilled water, and returned to blue in PBS. Dehydration was carried out with an ethanol gradient (60–100%), 5 min for each level. Finally, the slices were clarified in xylene for 10 min and sealed with neutral gum for observation.
DNA was extracted from fecal samples using a TIANamp Stool DNA Kit (#DP328-02,
Tiangen, Beijing, China). PCR amplification and library construction were
conducted using Phusion enzyme (K1031, APExBIO, Houston, TX,
USA) and the V3-V4 region primers (341F 5′-CCTACGGGNGGCWGCAG-3′ and 805R
5′-GACTACHVGGGTATCTAATCC-3′) of the 16S rRNA gene. An Illumina
NovaSeq6000 instrument was used for paired-end (PE250) sequencing to collect raw
data. Qiime 2 (2020.2) was used to conduct data quality control, calculate the
GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, CA, USA) was applied for
data analysis. All experimental data were expressed as mean
To assess the impact of combination therapy on the progression of HCC, mice injected with the fourth generation of Hepal-6 cells were treated with quercetin both with and without coordinate injection of anti-PD-1 antibody. The liver tissues in the Model group exhibited obvious tumors, confirming the successful construction of the orthotopically transplanted HCC model. Tumors in the liver tissues of mice were reduced when mice were treated with either quercetin or anti-PD-1 antibody alone. Combination therapy more effectively inhibited the growth of HCC than either monotreatment (Fig. 1A,B). Hematoxylin and eosin (H&E) staining of the liver tissues taken from different groups displayed that the liver cells of mice in the Control group were uniformly sized, neatly arranged, and without inflammatory infiltration. In the Model group, the volume of liver cells increased, nuclear staining deepened, and cell necrosis and fibrosis were observed. However, upon receiving combination therapy, the appearance of hepatocellular lesions was improved, and cell necrosis and inflammatory infiltration were significantly reduced (Fig. 1C). Western blot exhibited that the expressions of CD8a, CD4, and CD11b in liver tissues of the Model group were markedly downregulated compared to Control. Contrarily, combination therapy resulted in markedly increased expression of CD8a, CD4, and CD11b in liver tissues (Fig. 1D). Taken together, these findings indicated that combination therapy inhibited the progression of HCC.
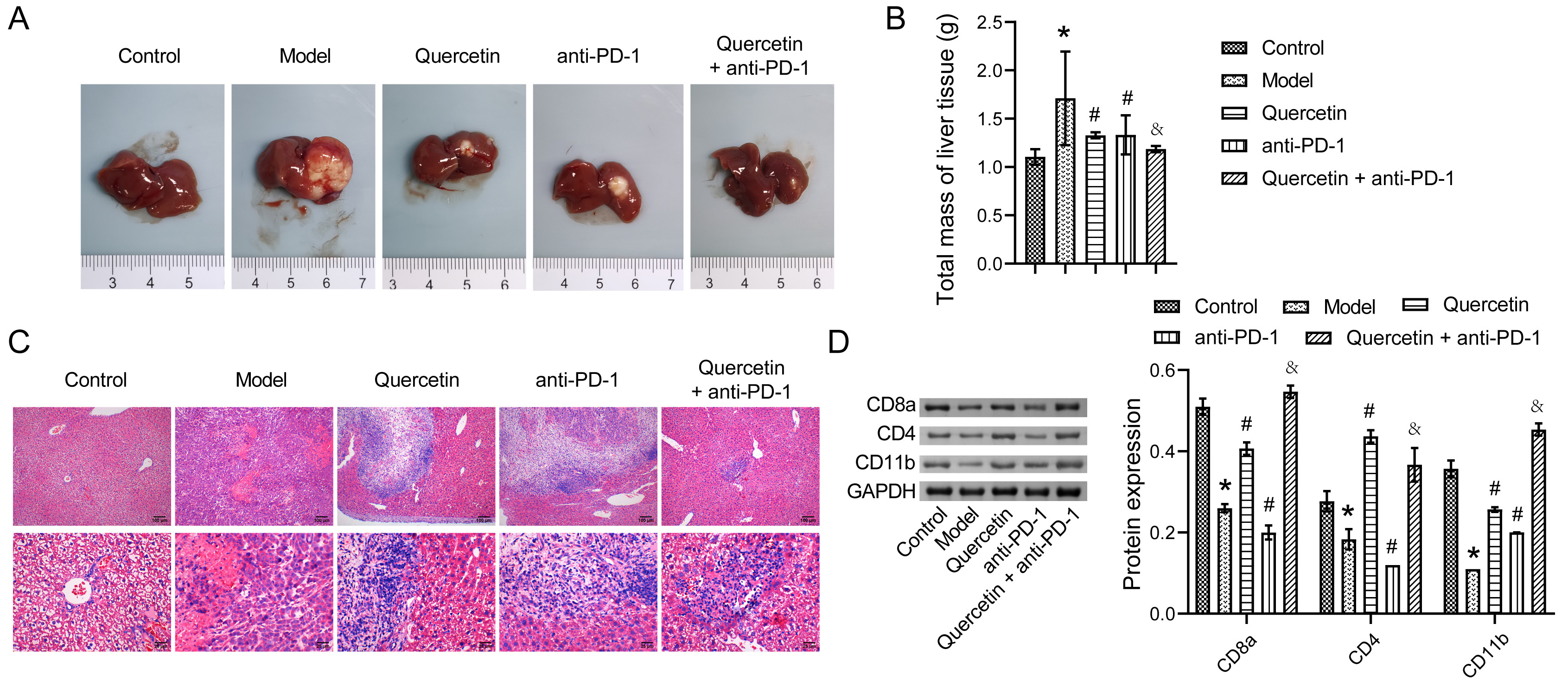 Fig. 1.
Fig. 1.The combination therapy inhibited the progression of
hepatocellular carcinoma (HCC). (A) The distribution of tumors in liver tissues; (B) The total mass of
liver tissues; (C) Hematoxylin and eosin (H&E) staining analysis of the pathological changes in liver
tissues; (D) Western blot analysis of the expressions of CD8a, CD4, and CD11b in
liver tissues. * p
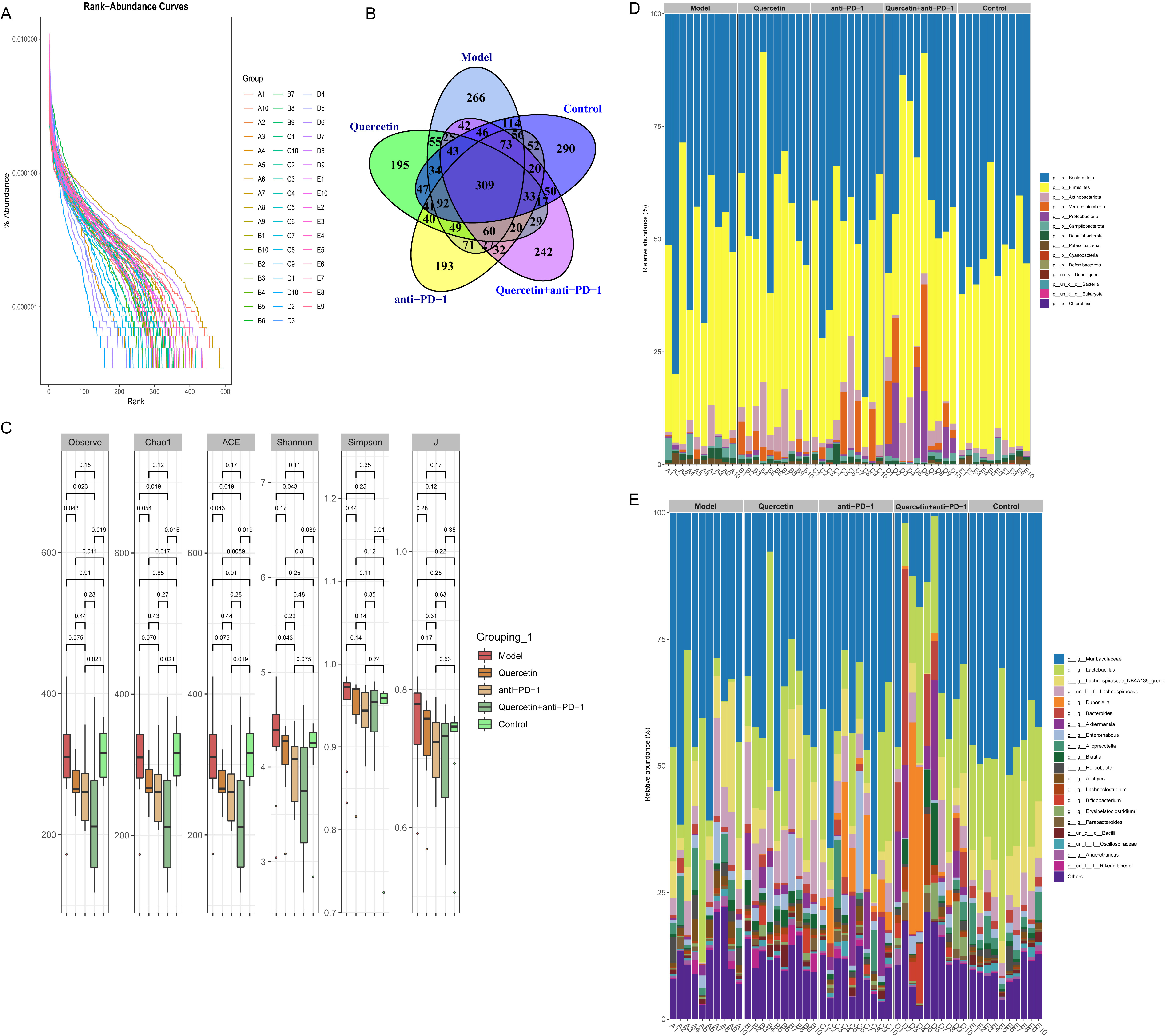
To explore the impact of combination therapy on the diversity
of GM in the orthotopically transplanted HCC model mice, 16S rRNA sequencing was
carried out on mouse fecal samples. A rank-abundance curve demonstrated increased
richness and evenness of GM in the Model group, but this decreased following
combination therapy (Fig. 2A). As shown in the Venn diagram, the number of unique
microorganisms in Control, Model, Quercetin, anti-PD-1, and Quercetin+anti-PD-1
groups was 290, 266, 195, 195, and 242, respectively (Fig. 2B). Analysis of inter-group difference showed that the
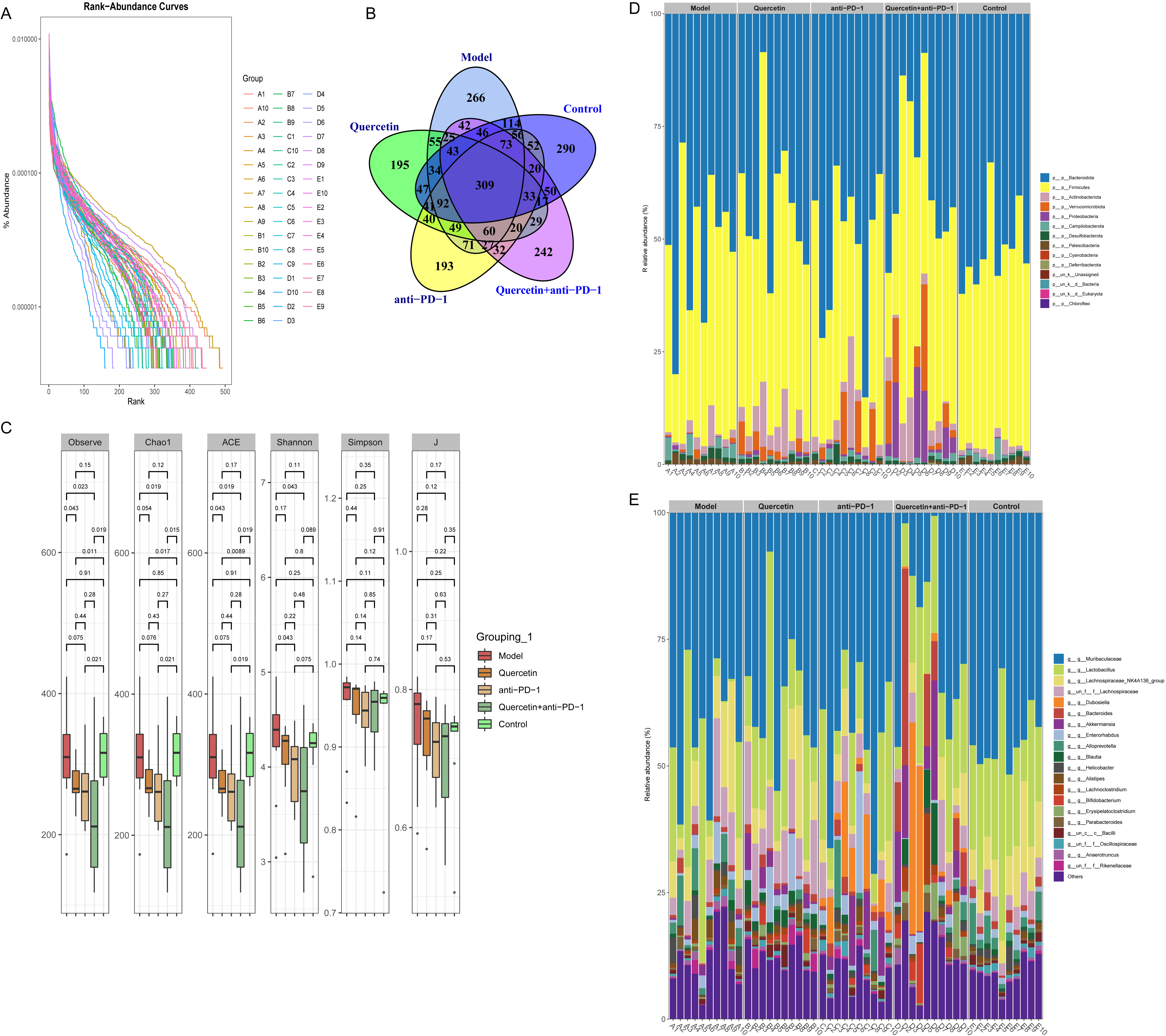 Fig. 2.
Fig. 2.The combination therapy regulated the diversity of gut microbiota (GM) in
orthotopically transplanted HCC model mice. (A) Rank-abundance curve of the
richness and evenness of GM. (B) Venn diagram of the number of GM. (C) Analysis
of
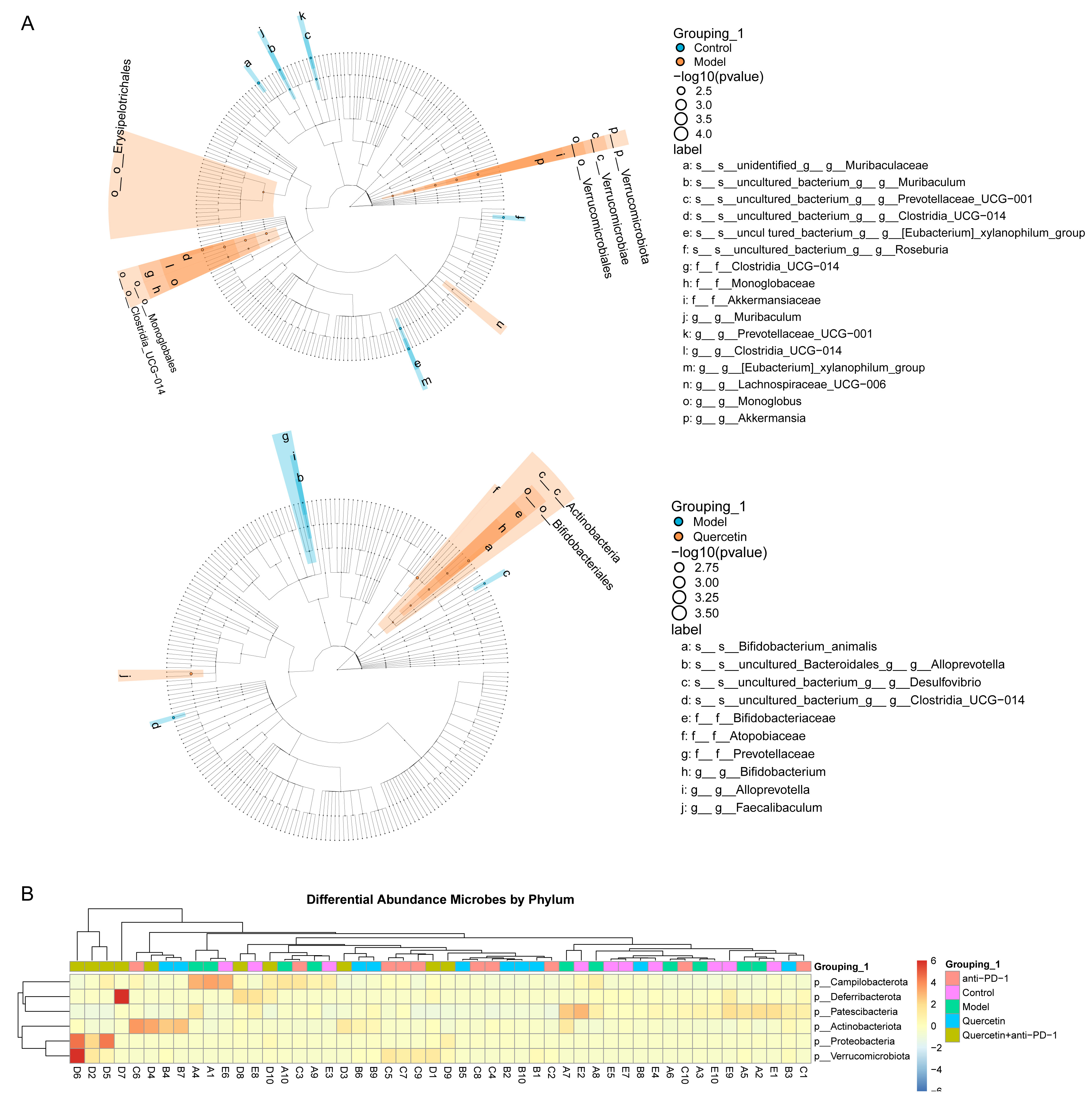
We next investigated the effect of combination therapy on the difference in GM in the HCC model. Difference analysis between groups indicated that Clostridia_UCG-014, Lachnospiraceae_UCG-006, Monoglobus, and Akkermania were enriched in the intestine of mice in the Model group. Bifidobacterium, Atopobiaceae, and Faecalibalum were enriched in the intestine of mice in the Quercetin group (Fig. 3A). Subsequently, the phylum-level analysis indicated that Campilobacterota, Deferribacterota, Patescibacteria, Actinobaciota, Proteobacteria, and Verrucomicrobiota were the key differential GM. Campilobacterota and Patescibacteria were enriched in the intestine of mice in both Control and Model groups. Deferribacterota, Actinobaciota, Proteobacteria, and Verrucomicrobiota were enriched in the intestine of mice in the Quercetin+anti-PD-1 group (Fig. 3B). These results proved that the combination therapy regulated GM variation in HCC model.
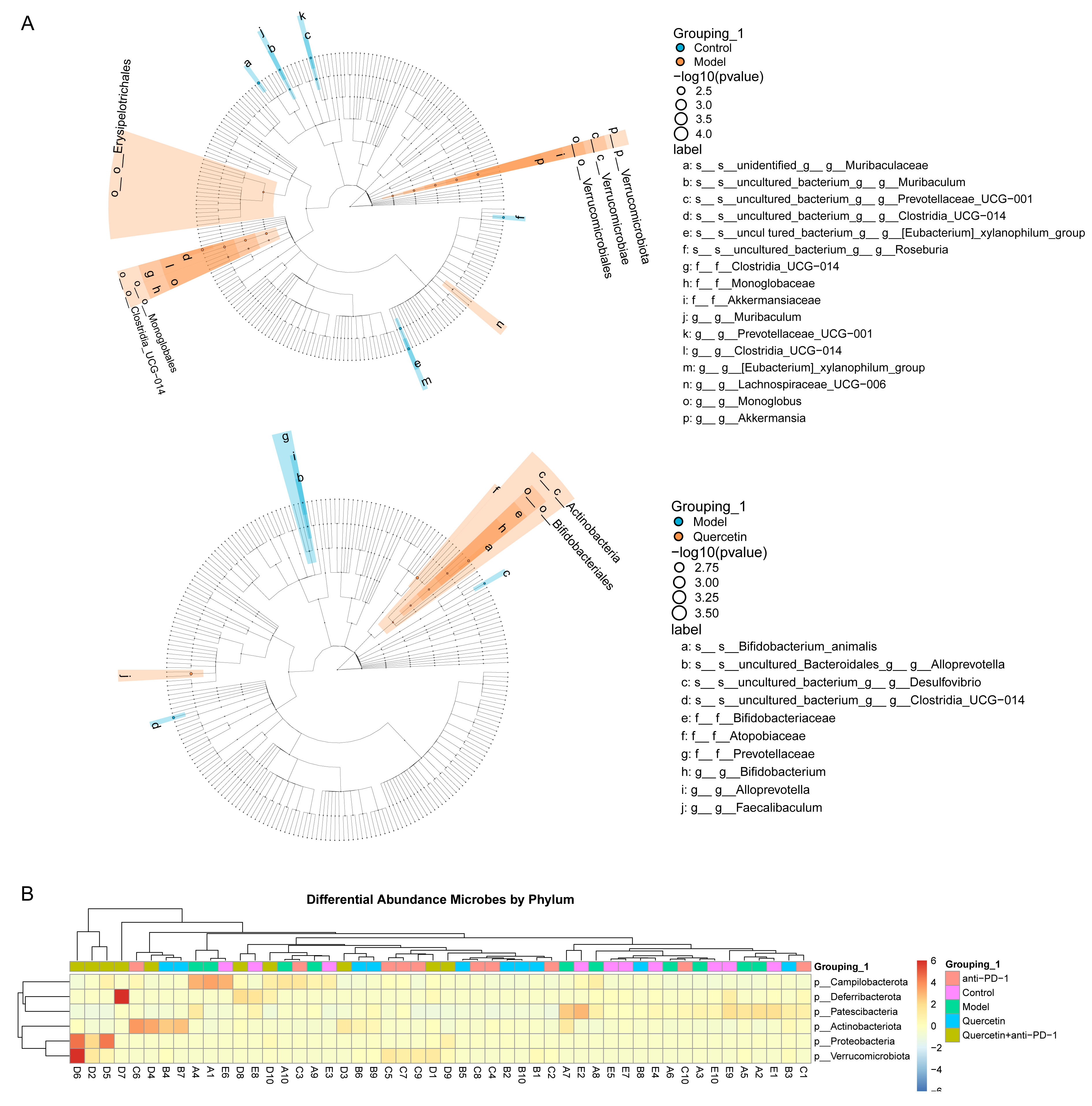 Fig. 3.
Fig. 3.The combination therapy regulated the difference of GM in orthotopically transplanted HCC model mice. (A) Lefse analysis of differential GM between groups. (B) Heatmap of the differential GM abundance between groups at the phylum level.
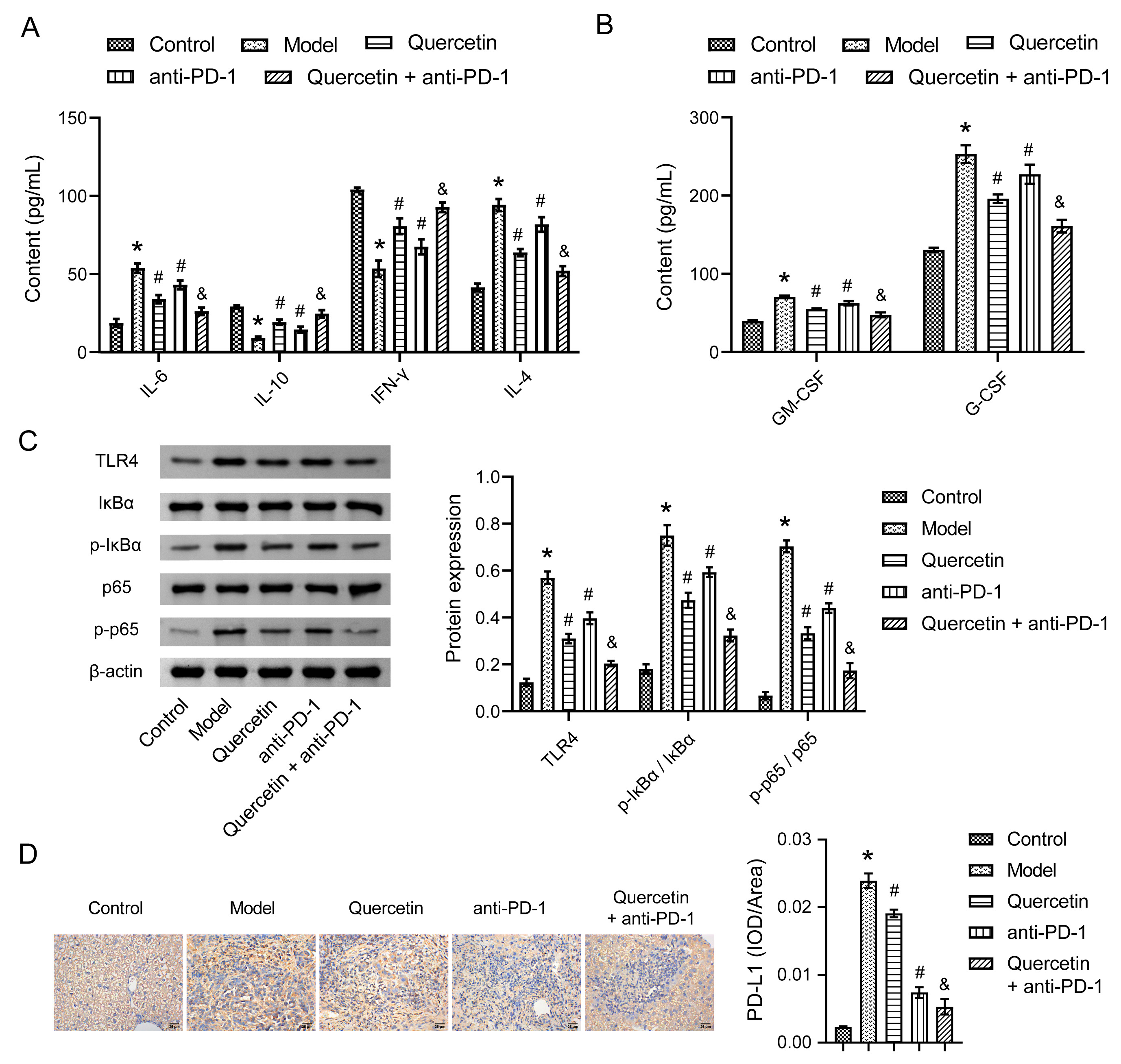
To explore the impact of combination therapy on the immunity of the
orthotopically transplanted HCC model mice, cytokine concentrations were examined
using enzyme-linked immunosorbent assay (ELISA) and Western blot. In the blood of Model mice, the levels of IL-6 and
IL-4 increased, while those of IL-10 and IFN-
 Fig. 4.
Fig. 4.The combination therapy regulated the immunity of orthotopically
transplanted HCC model mice. (A) The levels of interleukin (IL)-6, IL-10, interferon (IFN)-
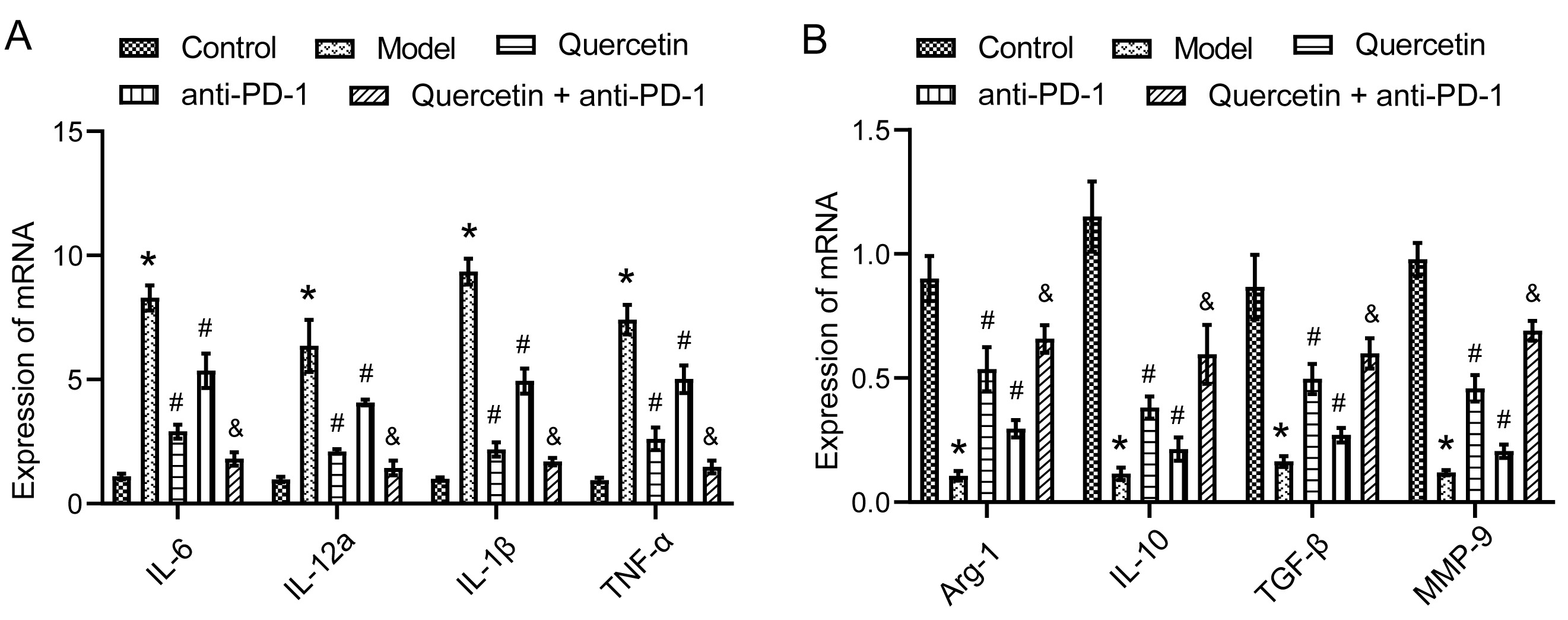
Finally, to explore the underlying mechanism
of combination therapy in regulating macrophage immunity in orthotopically
transplanted HCC model mice, RT-qPCR was conducted to analyze macrophage-related
gene expressions. M1 macrophage-related genes, including IL-6,
IL-12a, IL-1
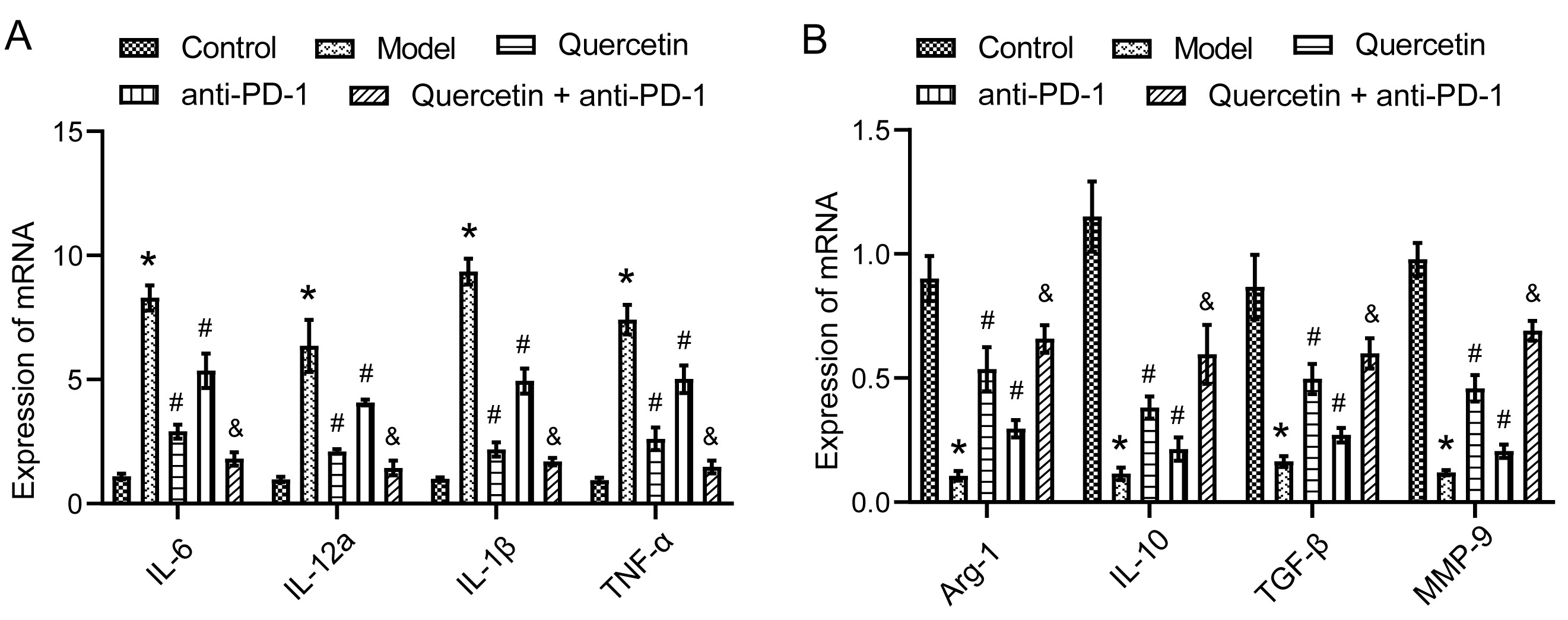 Fig. 5.
Fig. 5.The combination therapy regulated macrophage immunity in
orthotopically transplanted HCC model mice. (A) The expressions of M1
macrophage-related genes IL-6, IL-12a, IL-1
Natural compounds derived from TCM, including quercetin, are therapeutic for HCC but without side effects [19]. In addition, immune checkpoint blockade immunotherapy based on PD-1/PD-L1 has recently demonstrated encouraging outcomes in HCC treatment [9]. Multiple studies have proved that combination therapies based on quercetin or PD-1 inhibitors can effectively limit HCC progression [20, 21]. However, the exact mechanism of combination therapy for HCC remains to be explored. Herein, we have provided data obtained from mice orthotopically transplanted with HCC cells and treated with quercetin in combination with and without anti-PD-1 antibody. This study confirmed that combination therapy reshaped the TME of orthotopically transplanted HCC model mice by regulating both GM and macrophage immunity (Fig. 6).
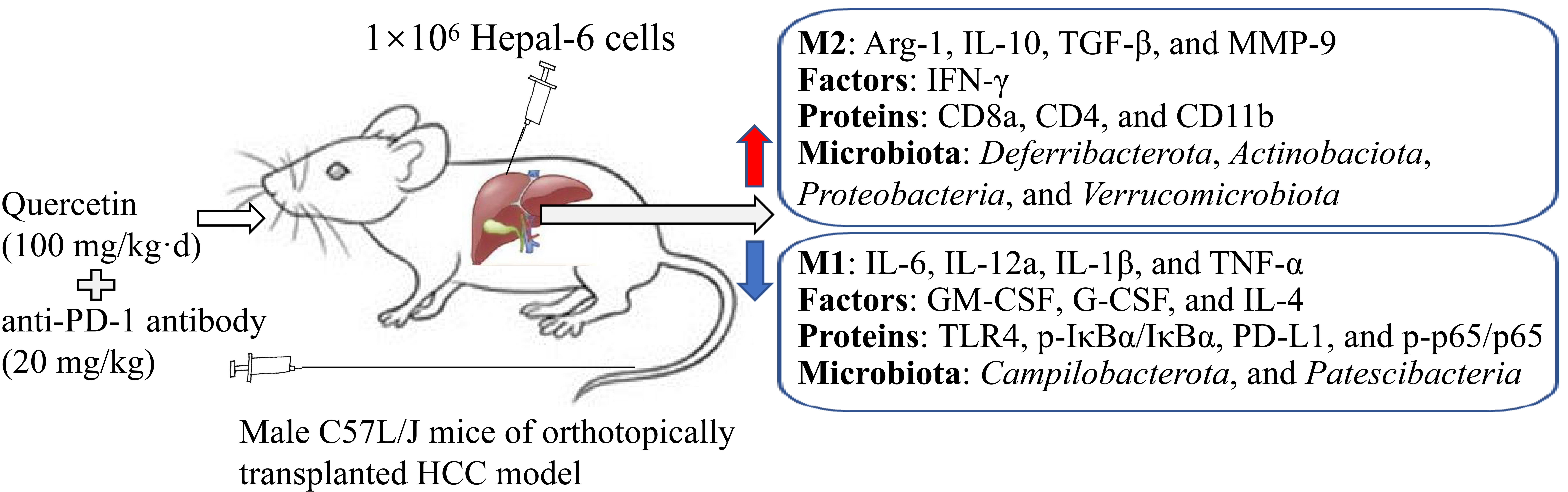 Fig. 6.
Fig. 6.The combination therapy of quercetin and anti-PD-1 antibody in HCC mice was achieved through the regulation of GM and macrophage immunity.
The anti-tumor effects of quercetin in HCC models have been demonstrated in both in vitro and in vivo studies [13]. After quercetin treatment in mice, liver tissue injury and inflammatory infiltration were reduced in mice [22]. Here, H&E staining showed that cell necrosis, fibrosis, and inflammatory infiltration were reduced in the liver tissues of mice receiving combination therapy, thus the progression of HCC was significantly inhibited. The results also presented that combination therapy of quercetin and anti-PD-1 antibody had a stronger anti-cancer effect than either quercetin or anti-PD-1 antibody alone. It is well-established that TME plays a critical role in tumor occurrence and progression [23]. Besides, PD-1 inhibitors work by activating tumor infiltration of lymphocytes to inhibit tumor growth [24, 25]. Here, Western blot displayed low expressions of CD8a, CD4, and CD11b in liver tissues of HCC mice, which were considerably elevated in response to combination therapy. This suggested that combination therapy could restore immune recognition and attack within the TME, thereby enhancing anti-tumor immune response. These results further proved that the combination therapy limited further progression of HCC by activating an anti-tumor immune response.
Increasingly, studies have illustrated the
participation of GM in the occurrence and progression of HCC through the
engagement of the gut-liver axis. Hence, reshaping the homeostasis of GM holds
significant potential to delay HCC progression [7]. Clinical studies on HCC
patients found that the relative abundance of Firmicutes in the GM is
significantly decreased, while that of Bacteroidetes is significantly
increased relative to healthy controls [26]. Moreover, the
Firmicutes/Bacteroidetes ratio serves as an ecological
imbalance indicator of GM. Here, the
In HCC, TAMs are crucial components of TME and are intimately linked to poor
patient prognosis [28]. Studies on HCC show that upregulated expressions of both
IL-4 and IL-6 are related to TAMs infiltration and HCC metastasis [29]. Others
have discovered that apigenin can induce apoptosis in HCC cells by suppressing
IL-4 expression. In addition, MiR-98 can inhibit HCC progression by increasing
IL-10 expression and inhibiting TAMs [30]. IFN-
This study only investigated the combination therapy of quercetin and PD-1 inhibitor for HCC. In TCM, many components exhibit therapeutic effects on HCC. In the future, the combined therapeutic effects of different active components with PD-1 inhibitors can be explored, and their specific therapeutic mechanisms of action through modulating the gut-liver axis can be elucidated. This will provide a theoretical basis for investigating the pathogenesis of HCC as well as developing new drugs.
Here, we found that combination therapy reshaped the TME of HCC mice. The therapeutic effect was paralleled by the regulation of the GM and macrophage immunity. This study suggested that TCM ingredients in combination with PD-1 inhibitors could be a potential new strategy for HCC treatment.
Data can be provided upon request.
RW designed the research study. RW, JX, TZ, ST, YW, ZZ and ZH performed the research. RW and JX analyzed the data. RW wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The animal experiment was approved by the Ethics Committee (Hidden by assistant editor according to double-blind principile) (No. ZYFY20210320).
Not applicable.
This research was funded by Youth program of Natural Science Foundation of Hunan Province, grant number 2021JJ40407; Youth project of Hunan Administration of traditional Chinese Medicine, grant number 202101; and Outstanding youth project of Hunan Education Department, grant number 20B451.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.