- Academic Editor
Background: Copine 1 (CPNE1) acts as a promoter in the progression of many kinds of cancers with the exception of pancreatic cancer (PC). This research is designed to probe the function of the CPNE1-tumor necrosis factor receptor-associated factor 2 (TRAF2) axis in PC. Methods: In vivo and in vitro models of PC were constructed, and a series of biological function tests, including MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide], colony formation, flow cytometry, and immunohistochemistry, were performed. Results: The level of CPNE1 elevated dramatically in PC cells. Downregulation of CPNE1in PC cells resulted in the inhibition of colony formation and proliferation. In addition, the silencing of CPNE1 induced the G1/S arrest and apoptosis in PC cells. Additionally, TRAF2 positively interacted with CPNE1 in PANC cells. CPNE1 silencing also inhibited the growth of tumors in in vivo mouse models. Functional experiments revealed that the anti-tumor effect of CPNE1 silencing was counteracted by TRAF2 overexpression, and the tumor-promoting effect of TRAF2 overexpression was reversed by CPNE1 silencing. Conclusions: In summary, our findings indicate that the silencing of the CPNE1-TRAF2 axis restrains PC development.
Pancreatic cancer (PC) ranks fourth among all malignancies in terms of mortality [1, 2]. Due to the limitations of imaging techniques and specific biomarkers, the diagnostic sensitivity of early PC is unsatisfactory [3]. Unfortunately, although chemotherapy, radiotherapy, surgical resection, and other treatments for PC have been developed, PC patients may still exhibit various adverse reactions, tumor resistance, and tumor metastasis [4, 5]. Insufficient understanding of the complex molecular mechanism of hyperproliferation and apoptotic dysfunction is one of the key reasons for the unsatisfactory therapeutic interventions for PC [6, 7].
Copine 1 (CPNE1), belonging to the CPNE family, is a conserved protein-encoding calcium-dependent phospholipid-binding protein that evolved from protozoa to eukaryotes [8, 9]. CPNE1 consists of a C-terminal A domain and two N-terminal C2 domains, generally functioning in cell signaling transduction via interactions with intracellular proteins [10, 11]. Research has demonstrated that CPNE1 is actively involved in the tumorigenesis of several types of human cancers [12, 13, 14, 15, 16]. For instance, increased expression of CPNE1 in non-small cell lung cancer (NSCLC) was associated with poor prognosis [12, 13]. Silencing of CPNE1 contributes to inhibiting the development of osteosarcoma (OS) cells [14]. CPNE1 could promote tumor progression and chemotherapy resistance in colorectal cancer (CRC) [15] and triple-negative breast cancer (TNBC) [16].
Tumor necrosis factor receptor-associated factor 2 (TRAF2) belongs to the TRAF superfamily [17, 18, 19, 20], which has been confirmed to be associated with the occurrence of malignancies [21, 22]. TRAF2 functions as an oncogene in breast cancer (BC) to accelerate cell proliferation and repress apoptosis [21]. The progression of gastric cancer (GC) cells can be enhanced by TRAF2 overexpression [22]. Similarly, the positive effect of TRAF2 on PC development has been confirmed [23, 24]. In addition, TRAF2 generally serves as an adaptor protein [25]. Liang et al. [26] indicated that TRAF2 interacts with CPNE1 to affect the tumorigenesis of prostate cancer (PRC). However, the functional interaction between CPNE1 and TRAF2 in PC remains unclear.
In the current research, the function of CPNE1 was determined in PC cells and a tumor xenograft model. The functional interaction between CPNE1 and TRAF2 was further evaluated in PC cells. We concluded that the CPNE1-TRAF2 axis may be a potential therapeutic target for PC.
H6C7 (ScienCell, CA, USA) was used as normal pancreatic ductal epithelial cells.
Mia-PaCa-2 (MIA) and PANC-1 (PANC) were obtained from ATCC (Manassas, VA, USA).
H6C7, MIA, and PANC cells were cultured in DMEM containing 10% FBS at 37
°C with 5% CO
Total RNA was extracted using an RNA extraction kit (Cwbio, Beijing, China) and
reverse transcribed using Evo m-mlv reverse transcription reagent (Accyrate
Biology, Changsha, China). SYBR green pro kit (Accyrate Biology) was used for the
RT-PCR reaction. The primers included CPNE1 forward
5
Total proteins were extracted from cells using RIPA lysis buffer (BOSTER, Wuhan, China). Then, the protein samples were separated by PAGE gel electrophoresis and transferred to nitrocellulose membranes. After being blocked with 5% nonfat milk, the membrane was incubated with primary antibodies (anti-CPNE1, Proteintech, Wuhan, China; anti-TRAF2 and anti-GAPDH, Abclonal, Wuhan, China; 1:1000 dilution) at 4 °C for 12 h. Followed by incubating with the secondary antibody (Zsbio, Beijing, China; 1:5000 dilution) at 25 °C for 30 min. The bands were visualized by a Tanon5200gel imaging system (Tanon, Shanghai, China) with corresponding ECL kit (Tanon), and quantified using Alpha Innotech software (2022 24.0.1, Kasendorf, Germany).
The transfected PC cells were plated and incubated for the right time points. Then, 20 µL MTT (Sangon Biotech) was pipetted into each well and incubated at 37 °C for another 4 h. Afterwards, 150 µL DMSO was appended. The optical density was detected at 490 nm using a microplate reader (Thermo Fisher Scientific, Waltham, MA, USA).
Cells were inoculated in 6-well plates (1000 per well) for 14 days. Then, 4% paraformaldehyde and 0.5% crystal violet were used to fix and stain the cells, respectively. Cell numbers were counted using a microscope (CKX53, Olympus, Tokyo, Japan).
For the cell cycle assay, the transfected PC cells were initially fixed with 75% ethanol for 1 h. After centrifugation at 1000 g, cells were re-suspended in D-Hank’s containing RNase (10 mg/mL) and PI (2 mg/mL). The frequencies of the cells were analyzed using a NovoCyte flow cytometer (Agilent Technologies, Santa Clara, CA, USA). The transfected PC cells were re-suspended in an appropriative buffer and stained with Annexin V-FITC and PI for the apoptosis assay. The apoptotic rate was measured via a NovoCyte flow cytometer (Agilent Technologies).
The BALB/c nude mice used for the tumor xenograft model were obtained from
Cavens (Changzhou, China). After acclimatization, mice were divided into control,
si-NC, and si-CPNE1-1 groups (n = 6). Si-CPNE1-1 or si-NC was integrated into a
lentiviral vector and transfected into PANC/MIA cells. The transfected cells (1
Tumors were fixed in 4% paraformaldehyde and cut into 4 µm sections. Sections were dewaxed in conventional xylene and dehydrated in gradient ethanol. After incubation with anti-Ki67 (Proteintech, China) and horseradish peroxidase-labeled goat anti-rabbit IgG (antibodies-online, Germany), sections were stained with DAB solution, antistained with hematoxylin, dehydrated, and sealed. The results were observed using a microscope and analyzed via Image J software (1.44, LOCI, University of Wisconsin, Madison, WI, USA).
All experiments were repeated at least three times. Data were presented as mean
The expression of CPNE1 in PC cell lines (MIA and PANC) was initially
determined. We found a relative overexpression of CPNE1 in both MIA and PANC
cells compared to that in H6C7 cells (Fig. 1A, p
 Fig. 1.
Fig. 1.CPNE1 was up regulated in PC cells. (A) The mRNA expression of
CPNE1 in H6C7 and MIA, and PANC cells. **p
We explored the influence of CPNE1 downregulation on cell proliferation in MIA
and PANC cells. The results demonstrated that compared with the si-NC group,
si-CPNE1-1/-2 significantly repressed the viability of two PC cell lines (Fig. 2A, p
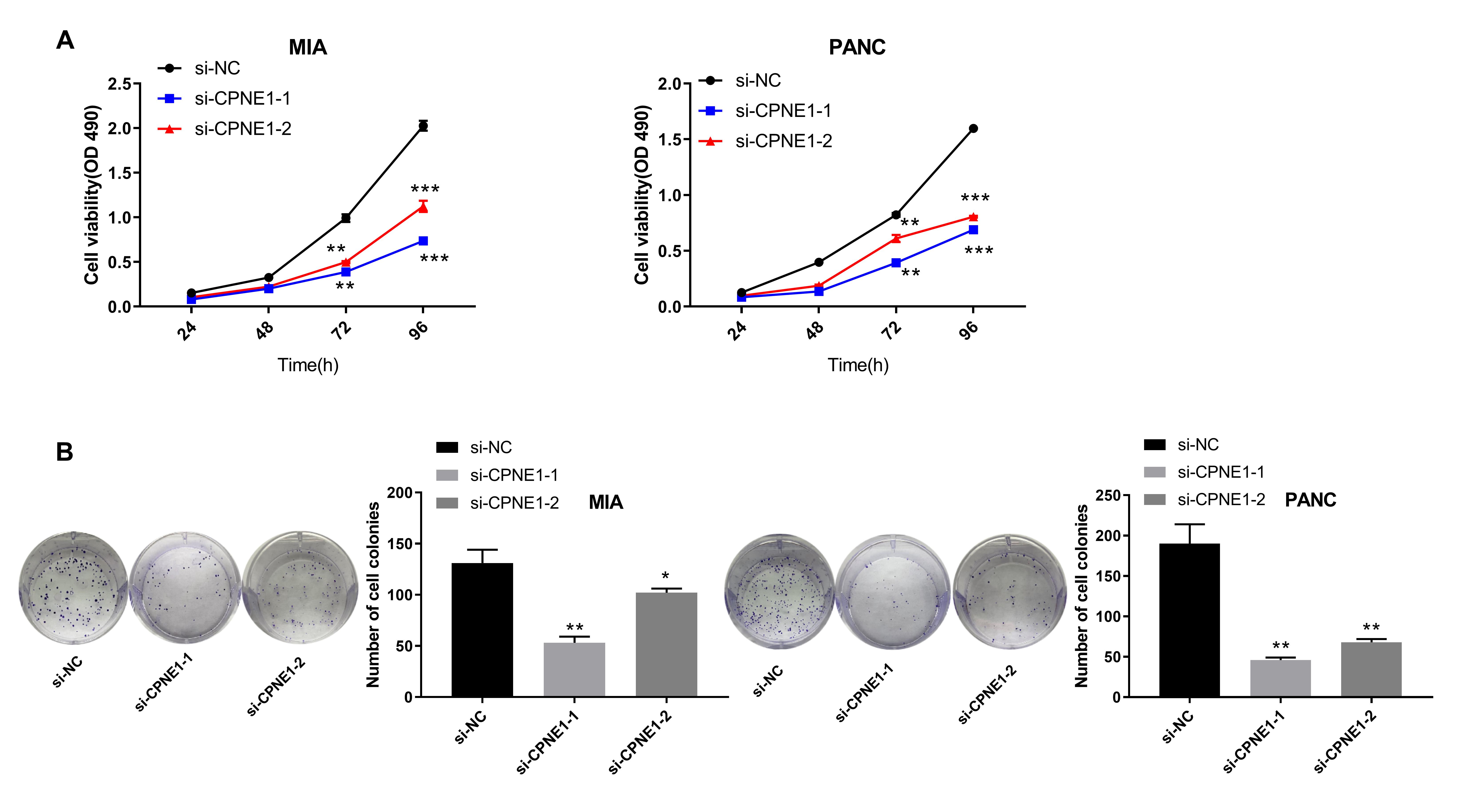 Fig. 2.
Fig. 2.Silencing of CPNE1 inhibits the proliferation of PC cells. (A)
The viability of MIA and PANC cells transfected with si-CPNE1-1/-2/NC. (B) The
number of cell colonies. *p
The influence of CPNE1 silencing on the cell cycle was further studied in MIA
and PANC cells due to its inhibiting effect on cell proliferation. Compared to
the si-NC group, the cell frequency of the G1 phase increased, whereas the cell
frequency of the S phase reduced in the si-CPNE1-1/2 groups (Fig. 3A,B, p
 Fig. 3.
Fig. 3.Silencing of CPNE1 induces G1/S arrest in PC cells. (A) The
cell cycle of MIA cells transfected with si-CPNE1-1/-2/NC. (B) The cell cycle of
PANC cells transfected with si-CPNE1-1/-2/NC. *p
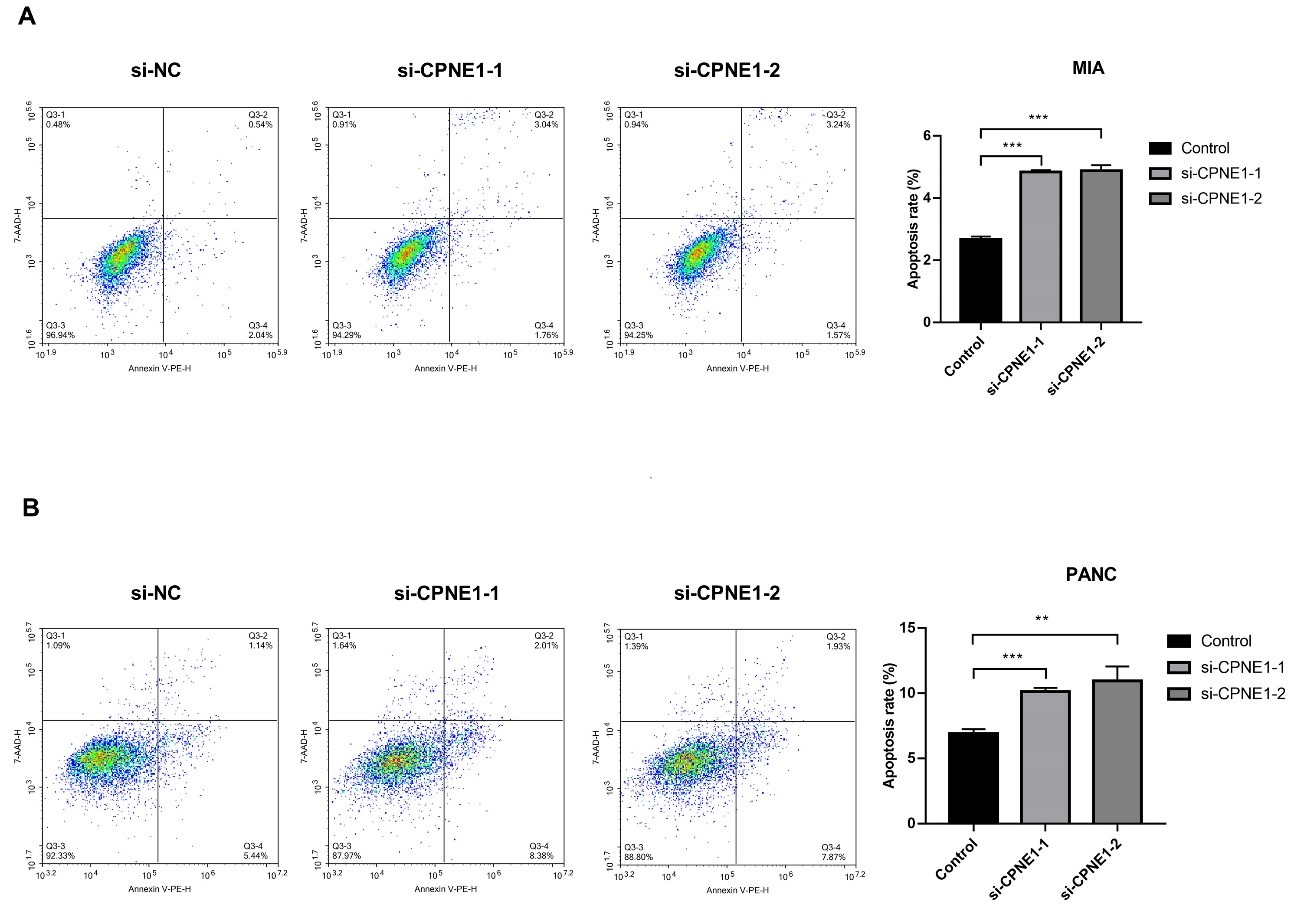 Fig. 4.
Fig. 4.Silencing of CPNE1 induces apoptosis in PC cells. (A) The
apoptosis of MIA cells transfected with si-CPNE1-1/-2/NC. (B) The apoptosis of PANC cells transfected with si-CPNE1-1/-2/NC. **p
The influence of CPNE1 silencing on tumor growth was further determined in a
mouse xenograft model. The intervention of si-CPNE1-1 in MIA and PANC cells led
to a remarkably reduced tumor growth rate in mice (Fig. 5A,B, p
 Fig. 5.
Fig. 5.Silencing of CPNE1 represses the growth of tumor xenografts in
mice. (A) The tumor morphology, weight, and volume in mice after injection of
MIA cells transfected with si-CPNE1-1/NC. (B) The tumor
morphology, weight, and volume in mice after injection of PANC cells transfected
with si-CPNE1-1/NC. *p
 Fig. 6.
Fig. 6.Silencing of CPNE1 represses the proliferation of tumor
xenografts in mice. (A) The percentage of Ki67-positive cells in mice after
injection of MIA cells transfected with si-CPNE1-1/NC. (B) The percentage of Ki67
positive cells in mice after injection of PANC cells transfected with
si-CPNE1-1/NC. *p
Previous reports have confirmed an oncogene role of TRAF2 in PC [23, 24] and have
also indicated that TRAF2 acts as an adaptor protein to interact with CPNE1 in
PRC [26]. Therefore, the relationship between CPNE1 and TRAF2 was then
investigated in PANC cells. As presented in Fig. 7A, compared with the si-NC +
pcDNA-NC group, the mRNA expression of CPNE1 and TRAF2 was inhibited in the
si-CPNE1 + pcDNA-NC group, whereas it was promoted in the si-NC + pcDNA-TRAF2
group (p
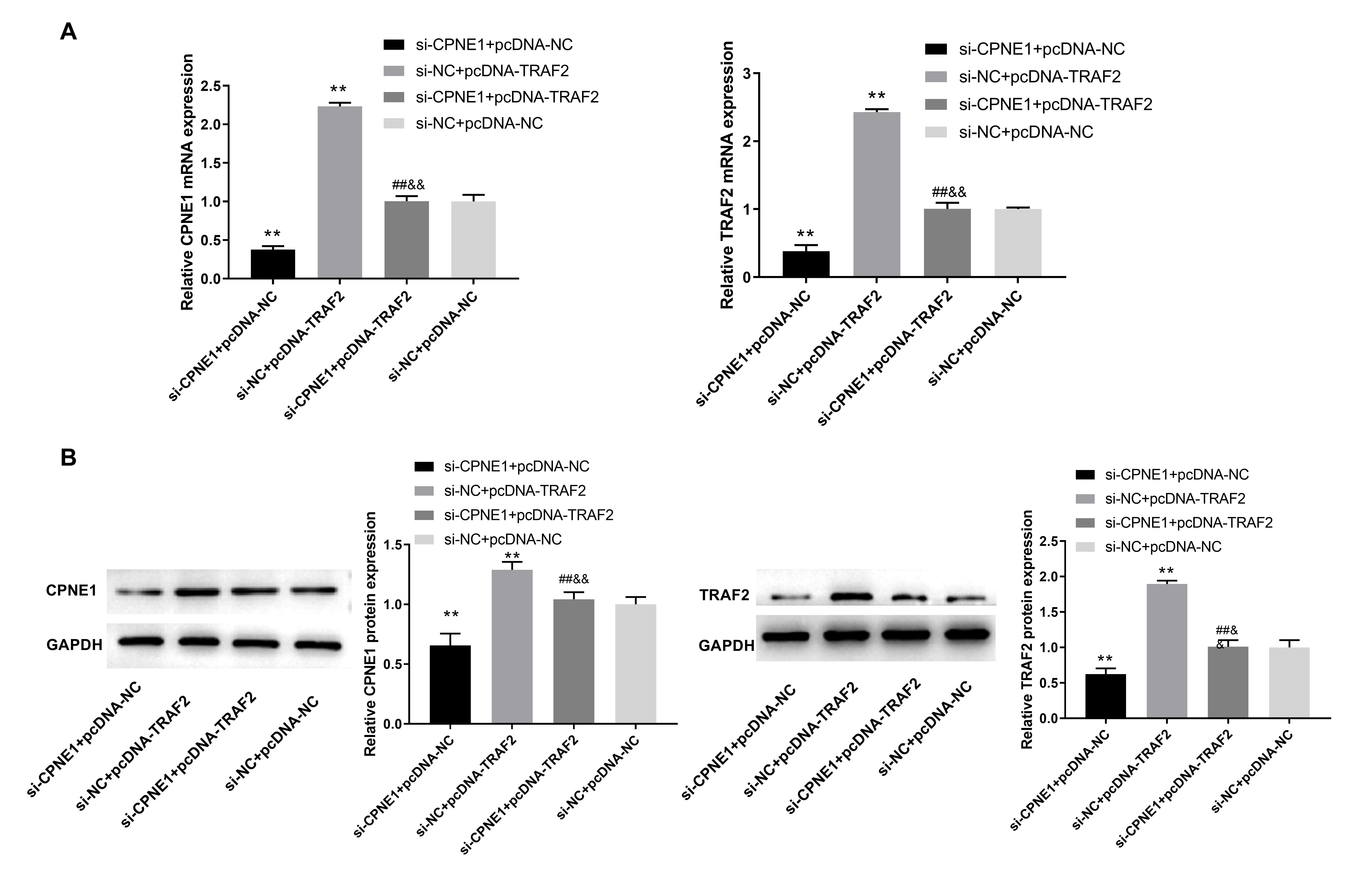 Fig. 7.
Fig. 7.CPNE1 interacts with TRAF2 in PC cells. (A) The mRNA expression
of CPNE1 and TRAF2 in transfected PANC cells. (B) The protein expression of CPNE1
and TRAF2 in transfected PANC cells. PANC cells were transfected with si-CPNE1 +
pcDNA-NC, si-NC + pcDNA-TRAF2, si-CPNE1 + pcDNA-TRAF2, and si-NC + pcDNA-NC,
respectively. **p
Relevant cellular function experiments in PANC cells further validated the
interaction between CPNE1 and TRAF2 in PC progression. The results indicated that
si-CPNE1 + pcDNA-TRAF2 reversed the promotion of TRAF2 overexpression and the
inhibition of CPNE1 silencing on cell proliferation (Fig. 8A,B, p
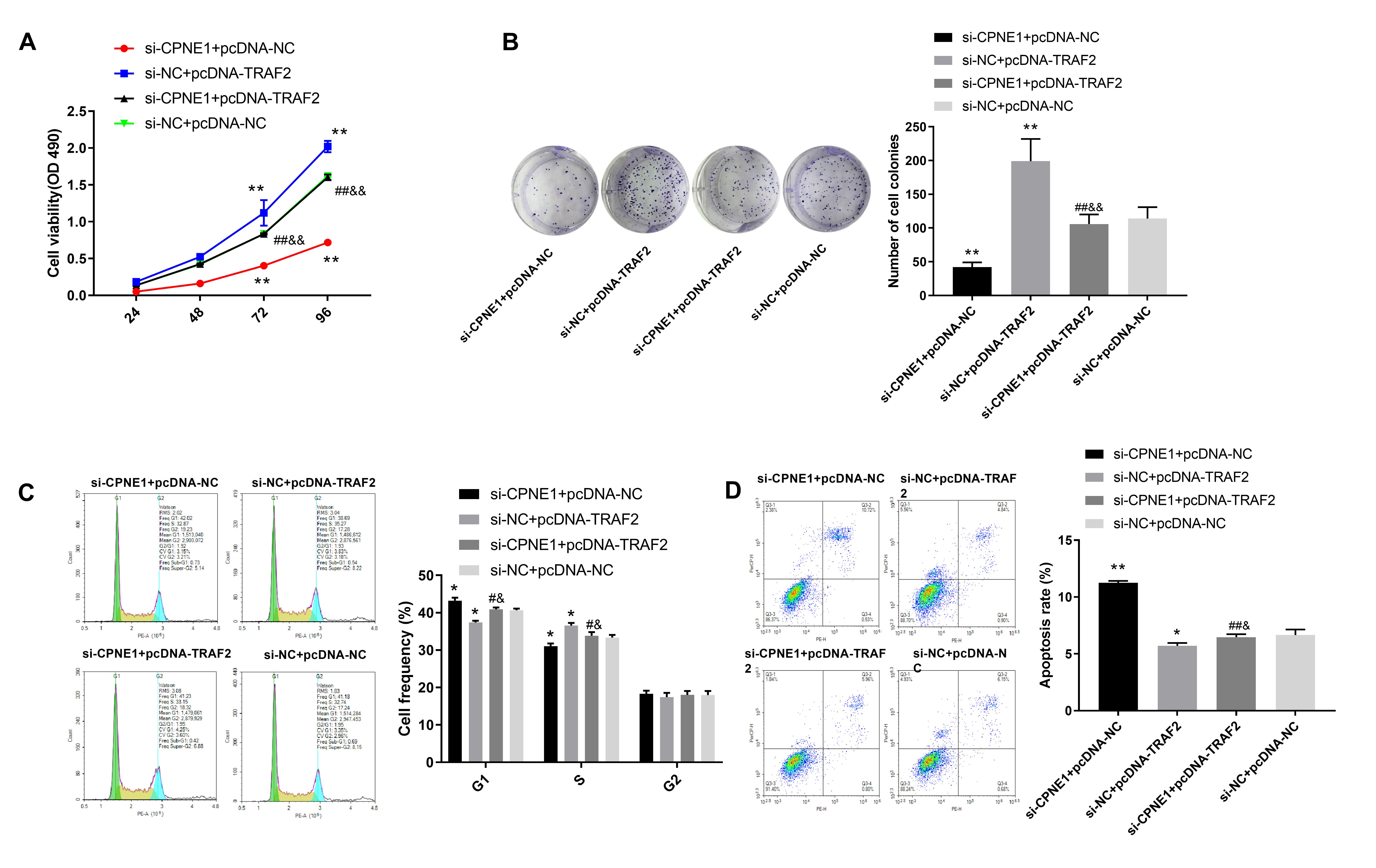 Fig. 8.
Fig. 8.Silencing of CPNE1 suppresses the proliferation and accelerates
PC cell apoptosis via interaction with TRAF2. (A,B) The viability of
transfected PANC cells. (C) The cell cycle of transfected PANC cells. (D)
Apoptosis of transfected PANC cells. PANC cells were transfected with si-CPNE1 +
pcDNA-NC, si-NC + pcDNA-TRAF2, si-CPNE1 + pcDNA-TRAF2, and si-NC + pcDNA-NC,
respectively. *p
PC is a malignant endocrine and digestive system tumor with a high incidence rate and mortality. According to incomplete statistics, approximately 448,000 new pathologies and 430,000 deaths occur yearly [27]. In addition to a late diagnosis, limitations still exist despite developing therapies for PC [4, 5]. Therefore, an in-depth exploration of the molecular mechanisms will contribute to better PC treatment.
CPNE1 is a key member of the CPNE family, which is up regulated in a series of cancers, such as NSCLC [12, 13], OS [14], CRC [15], and TNBC [16]. This study demonstrated that CPNE1 was also up regulated in PC cell lines. This result suggests that CPNE1 may be an oncogene in PC. Until now, multiple studies have shown that CPNE1 depletion could inhibit the proliferation and induce the apoptosis of cancer cells. Research has found that the downregulation of CPNE1 attenuates the viability but triggers the apoptosis of CRC cells [15]. Moreover, the proliferation is repressed, and apoptosis is enhanced in TNBC cells by the transfection of sh-CPNE1 [16]. In this study, we found that the transfection of CPNE1 silencing remarkably reduced proliferation and expedited apoptosis of PC cells. The inhibition of hyperproliferation caused by CPNE1 silencing may be useful for blocking PC progression. Further studies confirmed that silencing of CPNE1 increased PC cells in the G1 phase and reduced those in the S phase. This result was consistent with a previous NSCLC study [13] and suggests that the downregulation of CPNE1 can lead to G1/S arrest in PC cells. In summary, silencing CPNE1 may inhibit PC progression by blocking malignant proliferation in vitro. Consistent with the experimental results in vitro, si-CPNE1-1 repressed the growth of tumor xenografts. Therefore, we conclude that the knockdown of CPNE1 can retard PC development by inhibiting tumor growth.
Increasing evidence has confirmed that the C2 domains of CPEN1 are crucial for interacting with intracellular proteins, especially for phospholipid and calcium-related proteins [11, 28, 29]. TRAF2 is an oncogene that has been confirmed to modulate cell proliferation and apoptosis in PC [23, 24]. TRAF2 generally functions as an adaptor protein to link upstream receptors to downstream molecules [20]. CPEN1 can bind to different intracellular proteins through its C-terminal A structural domain, and TRAF2 is an adaptor protein [26]. Notably, a previous study by Liang et al. [26] found that TRAF2 affects the tumorigenesis of PRC by interacting with CPNE1. Based on the above phenomena, we speculated that TRAF2 may also interact with CPNE1 in the tumorigenesis of PC. As expected, TRAF2 overexpression reversed the effect of si-CPNE1 on CPNE1 expression, and CPNE1 silencing reversed the effect of pcDNA-TRAF21 on TRAF21 expression. These results indicate that TRAF2 positively interacted with CPNE1 in PANC cells. Furthermore, the following functional validation experiments also confirmed our expectations. Taken together, we conclude that silencing of CPNE1 may repress cell proliferation and induce apoptosis in PC through interacting with TRAF2.
There were several limitations of this study. First, other malignant behaviors such as migration, invasion, and drug resistance in PC cells must be studied except for excessive proliferation. Second, the interaction between CPNE1 and TRAF2 is not verified in vivo. In future studies, an in-depth study of the direct mechanistic relationship between CPNE1 and TRAF2 should be performed. Third, the downstream mechanism of CPNE1/TRAF2 requires further exploration. However, this study verified CPNE1-TRAF2’s role in PC development and provided novel targets for clinical treatment.
The current study illustrated an increased expression of CPNE1 in PC cells. Silencing of CPNE1 repressed cell proliferation and induced apoptosis via interaction with TRAF2 in PC. Both CPNE1 and TRAF2 may be potential molecular targets for the treatment of PC.
Data and materials to support the findings of this study is available on reasonable request from the corresponding author.
YS and ZLi designed the research study. YS, BS and ZY performed the research. YS, ZLi, LX and ZLu analyzed the data; YS, AL, and LX wrote the manuscript. AL and YZ performed the data collection. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work to take public responsibility for appropriate portions of the content and agreed to be accountable for all aspects of the work in ensuring that questions related to its accuracy or integrity.
All animal experiments were strictly followed the Guide for the Care and Use of Laboratory Animals and approved by the ethical committee of Affiliated Hospital of Shandong University of Traditional Chinese Medicine, which the approval number is JN.No202110125c0040930[016].
Not applicable.
This research was funded by Special Education Fund of Shandong Province, Introduction and Training team of Outstanding Young Talents of Shandong Province “Clinical Teaching Reform and Innovation Practice Team of Integrated Chinese and Western Medicine”, grant number 005-505012.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
