- Academic Editor
Background: Breast cancer-related depression (BCRD) is strongly
associated with BC and increases recurrence and mortality. This study
investigated the role of kaempferol in the pathogenesis of BCRD and its
underlying mechanism. Methods: 4T1 mouse BC cells were treated with
corticosterone (Cort) in vitro to develop a neuronal injury model, and a
BCRD mouse model was established by injecting 4T1 cells and Cort. The effects of
kaempferol on 4T1 cells and BCRD models were measured by behavioral tests, Cell
Counting Kit-8 assay, wound healing assay, colony formation assay, Western blot
analysis, quantitative real-time PCR, hematoxylin and eosin staining,
enzyme-linked immunosorbent assay, and immunofluorescence. BCRD cells were
transfected with the cyclo-oxygenase-2 (COX-2) overexpression plasmid to study
the role of the COX-2/prostaglandin E2 (PGE2) axis in the anti-BCRD activity of
kaempferol. The connection between kaempferol and COX-2 was analyzed by molecular
docking. Results: Kaempferol reduced the viability, migration, and
clones of 4T1 cells and inhibited BC growth and depression-like behavior in mice.
Kaempferol alleviated inflammation in BCRD, decreased interleukin 1 beta
(IL-1
Breast cancer (BC) is the most common cancer among women worldwide, accounting for approximately 25% of new cancer cases in women globally. BC is also the leading of cancer-related death in women [1]. In the process of diagnosis and treatment, in addition to the changes in physical function, BC patients will also bear great psychological pressure. Studies have shown that depression and anxiety are common comorbidities in BC patients, which greatly endanger the quality of life of patients [2]. Meta-analysis showed that depression was associated with relapse and mortality in BC [3]. Therefore, understanding the mechanism behind BC-related depression (BCRD) can help prevent and block cancer development.
It has been reported that neuroinflammation is an important pathological manifestation of depression, and the levels of pro-inflammatory markers are elevated in depressed patients [4]. BC survivors showed more severe depressive symptoms with higher levels of inflammation [5]. Thus, neuroinflammation is expected to be a potential therapeutic direction for BCRD. Cyclo-oxygenase (COX)-2 is expressed in the central nervous system and is closely related to the pathogenesis of various diseases, including degenerative brain diseases, depression, and cancer [6]. COX-2 is usually induced by inflammatory factors and is responsible for regulating the secretion of prostaglandin (PG) E2 during inflammation [7]. Studies have shown that the upregulation of COX-2 and PGE2 can play an anti-neuroinflammation role [8]. Previous reports have described that COX-2 and PGE2 are upregulated in a rat model of depression, and inhibition of the COX-2/PGE2 pathway can reduce the inflammatory response in the hippocampus and effectively improve depressive behavior [9].
Kaempferol is one of the most common flavonoid aglycons in many plants and vegetables. It has been proven that kaempferol has a variety of pharmacological activities, including anti-inflammatory, anti-bacterial, neuroprotective, anti-oxidative, anti-diabetic, anti-tumor, and anti-cancer [10, 11]. Among them, kaempferol has been widely recognized as a dietary anti-inflammatory agent [12]. Research has shown that kaempferol has great potential in reducing depression-like behaviors due to its antioxidant and anti-inflammatory effects [13, 14]. Additionally, previous studies reported that the administration of kaempferol could reduce the protein abundance of COX-2 in the ischemic stroke model and Parkinson’s model [15, 16]. However, few reports focus on kaempferol attenuating the progression of BCRD via COX-2/PGE2. Therefore, in this study, we investigated the effects of kaempferol on 4T1 cells, BCRD cells, and mouse models and observed the role of the COX-2/PGE2 pathway.
The mouse breast cancer cells 4T1 (AW-CCM376) were obtained from Abiowell (Changsha, China) and maintained in Dulbecco’s Modified Eagle Medium containing 10% fetal bovine serum and 1% penicillin-streptomycin. The 4T1 cells were treated with 25, 50, and 100 µM kaempferol (N1719; APExBIO Technology LLC, Houston, TX, USA) to investigate the effects of kaempferol [17]. BCRD was mimicked in vitro, as previously described [18]. Briefly, 4T1 cells were stimulated with 10 µg/mL lipopolysaccharide (Sigma, St. Louis, MO, USA) for 6 h, and the supernatant was collected after centrifugation. The cell supernatant and 200 µM corticosterone (Cort, 50-22-6; Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China) were added to mouse hippocampal neuronal cells HT-22 (AW-CNM116, Abiowell) and cultured for 6 h to obtain BCRD cells. Then the BCRD cells were treated with kaempferol. Cells were divided into Control, BCRD, and Kaempferol groups. 4T1 cells and HT-22 cells were tested for mycoplasma. Through short tandem repeat (STR) detection, it was verified that the cell line we used was 4T1 and HT-22 cells, and there was no human-source pollution. The 4T1 (https://www.cellosaurus.org/CVCL_0125) and HT-22 cells (https://www.cellosaurus.org/CVCL_0321) used in this study were checked and found no cross-contamination.
The overexpression (oe) plasmids, oe-NC and oe-COX-2(HG-MO011198) (both from Honor Gene Co. Ltd., Changsha, China), were transfected into BCRD cells using Lipofectamine 2000 reagent (11668019; Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The sequence of COX-2 was obtained from the National Center for Biotechnology Information, and primers were designed to isolate the target gene. The target fragment was ligated into the pcDNA3.1(+) vector. After being stimulated with 25 µM kaempferol for 48 h, oe-NC and oe-COX-2 were transfected into BCRD cells. Cells were divided into BCRD, oe-NC, oe-COX-2, Kaempferol, Kaempferol+oe-NC, and Kaempferol+oe-COX-2 groups.
To assess cell viability, we used the Cell Counting Kit-8 (CCK8) assay. In
brief, after pancreatic enzyme digestion, cells in the logarithmic growth phase
were seeded in a 96-well plate at a density of 5
The wound healing assay was performed to assess cell migration. After digestion
with trypsin, 4T1 cells were seeded in a 6-well plate at a density of 5
The colony formation assay was used to analyze cell proliferation. After kaempferol treatment, 4T1 cells (200 cells/mL) were seeded in 6-well plates and dispersed evenly. Cells were allowed to grow for the next 14 d to form colonies. After terminating the culture, the cells were fixed in 4% paraformaldehyde for 15 min, stained with crystal violet dye (AWC0333; Abiowell), and counted.
Six-week-old female BALB/c mice were purchased from Hunan SJA Laboratory Animal
Co., Ltd. (Changsha, China). As previously described, 4T1 cells (1
To assess anxiety, the open field test (OFT) was conducted. Briefly, mice were
quickly placed in the center of a square experimental box (50
To assess depressive-like states, the forced swimming test (FST) was performed. Briefly, mice were placed in clear glass vials that were 21 cm high and 16.5 cm in diameter. Water about 13 cm deep was added to the bottle, and the mice were forced to swim for 6 min. The behavior of the mice was observed for 4 min, and the immobility time was recorded. Mice were considered stationary when their head was above the water’s surface with no apparent struggle [22].
For the tail suspension test (TST), mice (1 cm from the tail tip) were suspended in the air with adhesive tape, about 60 cm above the ground. Mice were separated from each other. During the 6 min test period, the activity of the mice was observed, and the immobility time within the next 4 min was recorded [23].
Hematoxylin and eosin (HE) staining was used to assess the degree of pathological damage. Tumors from BCRD mice were collected and then subjected to fixing, embedding, and sectioning (4 µm). Sections were placed in xylene for 20 min and repeated three times. Then, the sections were hydrated through different gradients of ethanol (75–100%) and washed with distilled water. Hematoxylin (AWI0009; Abiowell) and eosin (AWI0020, Abiowell) were used for staining. Sections were dehydrated in graded alcohol (95%–100%) and placed in xylene. The morphological changes were observed and photographed using a microscope (BA210T; Motic Microscopes, Xiamen, China).
Total RNAs from cells were extracted with TRIzol (15596026; Thermo Fisher
Scientific, Waltham, MA, USA) and reversely transcribed to prepare cDNAs. The
relative expression of targets was performed by the UltraSYBR Mixture kit
(CW2601; ConWin Biosciences, Taizhou, China) and normalized to
| Gene | Sequences (5′-3′) |
| IL-1 |
F: TGAAATGCCACCTTTTGACAGT |
| R: TTCTCCACAGCCACAATGAGT | |
| IL-6 | F: GACTTCCATCCAGTTGCCTT |
| R: ATGTGTAATTAAGCCTCCGACT | |
| TGF- |
F: CTCCCGTGGCTTCTAGTGC |
| R: GCCTTAGTTTGGACAGGATCTG | |
| IL-10 | F: GTTCCCCTACTGTCATCCCC |
| R: AGGCAGACAAACAATACACCA | |
| COX-2 | F: AATACTGGAAGCCGAGCACCT |
| R: ACACCCCTTCACATTATTGCAGA | |
| PGE2 | F: TCTATGGGGCCTCCTTGCTCT |
| R: AGCACAGCCACGATAAGCAG | |
| F: ACATCCGTAAAGACCTCTATGCC | |
| R: TACTCCTGCTTGCTGATCCAC |
Total protein from mice hippocampal tissues or cell lysates was extracted with
RIPA (AWB0136, Abiowell). After protein quantification with a BCA kit (AWB0104,
Abiowell), proteins were resolved by 10% sodium dodecyl sulfate-polyacrylamide
gel electrophoresis and then electrotransferred to nitrocellulose membranes.
After blocking in 5% skimmed milk for 90 min, the membranes were incubated
overnight at 4 ℃ with the following primary antibodies: COX-2 (1:2000,
12375-1-AP; Proteintech Group Inc., Rosemont, IL, USA), PGE2 (1:300, ab217966;
Abcam, Cambridge, MA, UK), interleukin 1 beta (IL-1
According to the manufacturer’s instructions, the levels of serotonin (5-HT,
CSB-E08365m), dopamine (DA, CSB-E08661m), norepinephrine (NE, CSB-E07870m),
IL-1
The two-dimensional (2D) structure of COX-2 was downloaded from the Protein Data Bank (https://www.rcsb.org/), and the 3D structure of kaempferol was obtained from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/compound/5280863). Autodock Vina software was performed to analyze the interaction of kaempferol and COX-2, and Discovery Studio was used for analyzing and viewing the 2D and 3D images.
Mouse brain tissue underwent embedding and sectioning. After deparaffinization
and hydration, slices were placed in an EDTA buffer and heated for thermal
antigen retrieval. After cooling, the slices were washed with PBS. Then sections
were sequentially incubated in sodium borohydride, 75% ethanol, and Sudan black
solution. Slices were blocked in 5% bovine serum albumin for 1 h, then incubated
with 5-Bromo-2
Statistical analysis was conducted using GraphPad Prism 9.0 software (GraphPad
Software Inc., San Diego, CA, USA). Data are presented as the mean
To assess the effects of kaempferol on BC, 4T1 cells were stimulated with different concentrations of kaempferol (25, 50, and 100 µM). The results showed that after treatment for 24 and 48 h, kaempferol significantly reduced the viability of 4T1 cells, and the inhibitory ability was gradually increased in a dose-dependent manner (Fig. 1A). The wound healing assay showed that after 24 h and 48 h, kaempferol alleviated the migration of 4T1 cells in a dose-dependent manner (Fig. 1B,C). In addition, the number of cloned 4T1 cells was reduced after kaempferol intervention, and the effect of 100 µM kaempferol was the most prominent (Fig. 1D). These results showed that kaempferol inhibited 4T1 cell proliferation, migration, and colony formation.
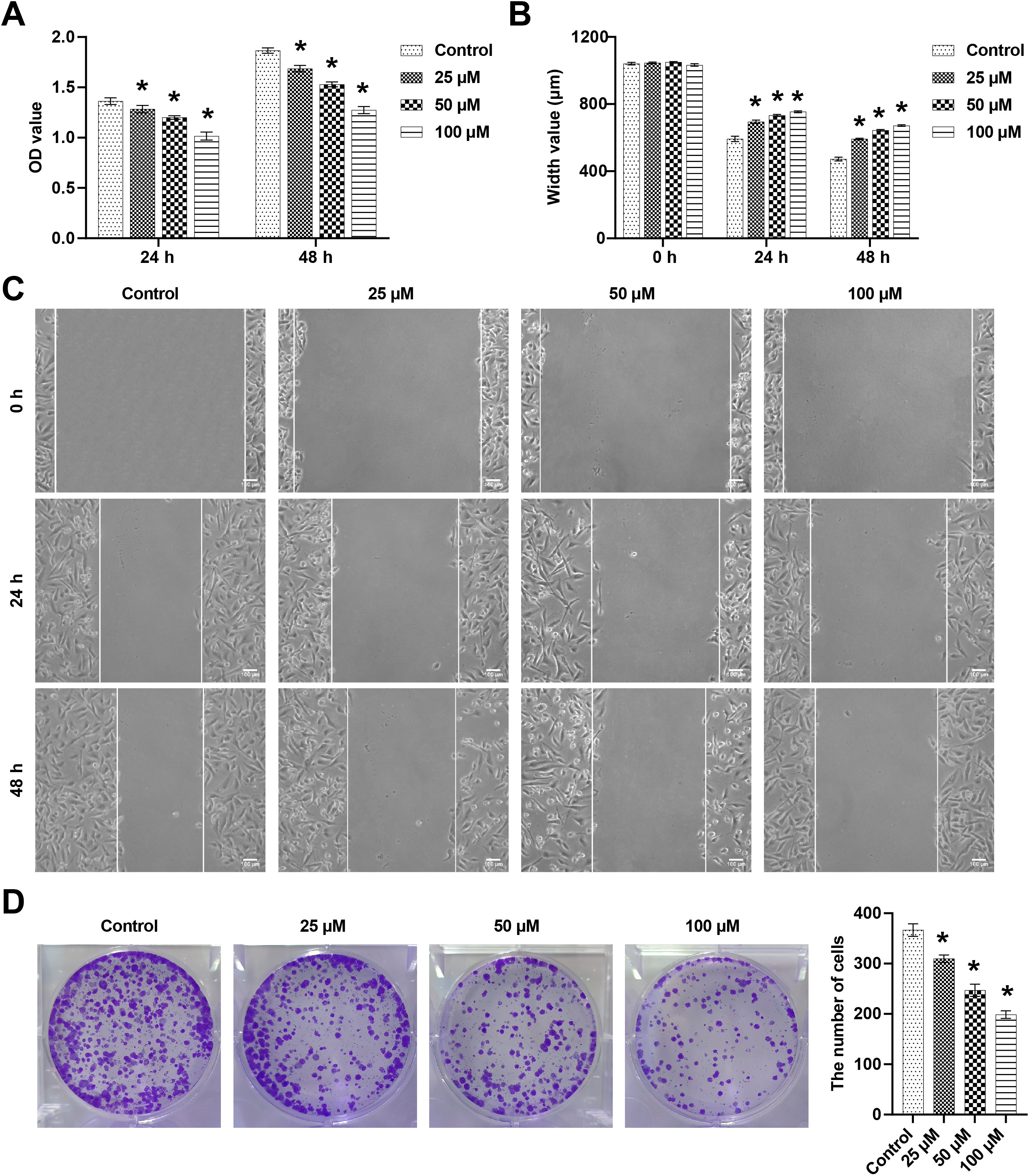 Fig. 1.
Fig. 1.Kaempferol reduced 4T1 cell viability, migration, and colony
formation. (A) 4T1 cell viability was detected by the cell counting kit-8 (CCK8)
assay at 24 h and 48 h. (B,C) The wound healing assay was performed to measure
4T1 cell migration at 24 h and 48 h. Areas not covered by 4T1 cells were measured
and counted. (D) Cell numbers were counted to assess 4T1 cell clonality. n = 3. *
p
Next, the effects of kaempferol on inflammation in neuronal cells were examined.
The non-toxic dose of kaempferol was determined by the CCK-8 assay. Compared with
the Control group, BCRD cells showed decreased cell viability, whereas the cell
viability was partially restored after stimulation with different concentrations
of kaempferol (10 µM, 25 µM, and 50 µM). Notably, in BCRD
cells, when the dose of kaempferol reached 50 µM, the cell viability showed
a downward trend (Fig. 2A). Therefore, we applied 25 µM kaempferol for
experiments. Subsequently, the levels of inflammatory factors were detected. BCRD
cells showed increased levels of pro-inflammatory cytokines
(IL-1
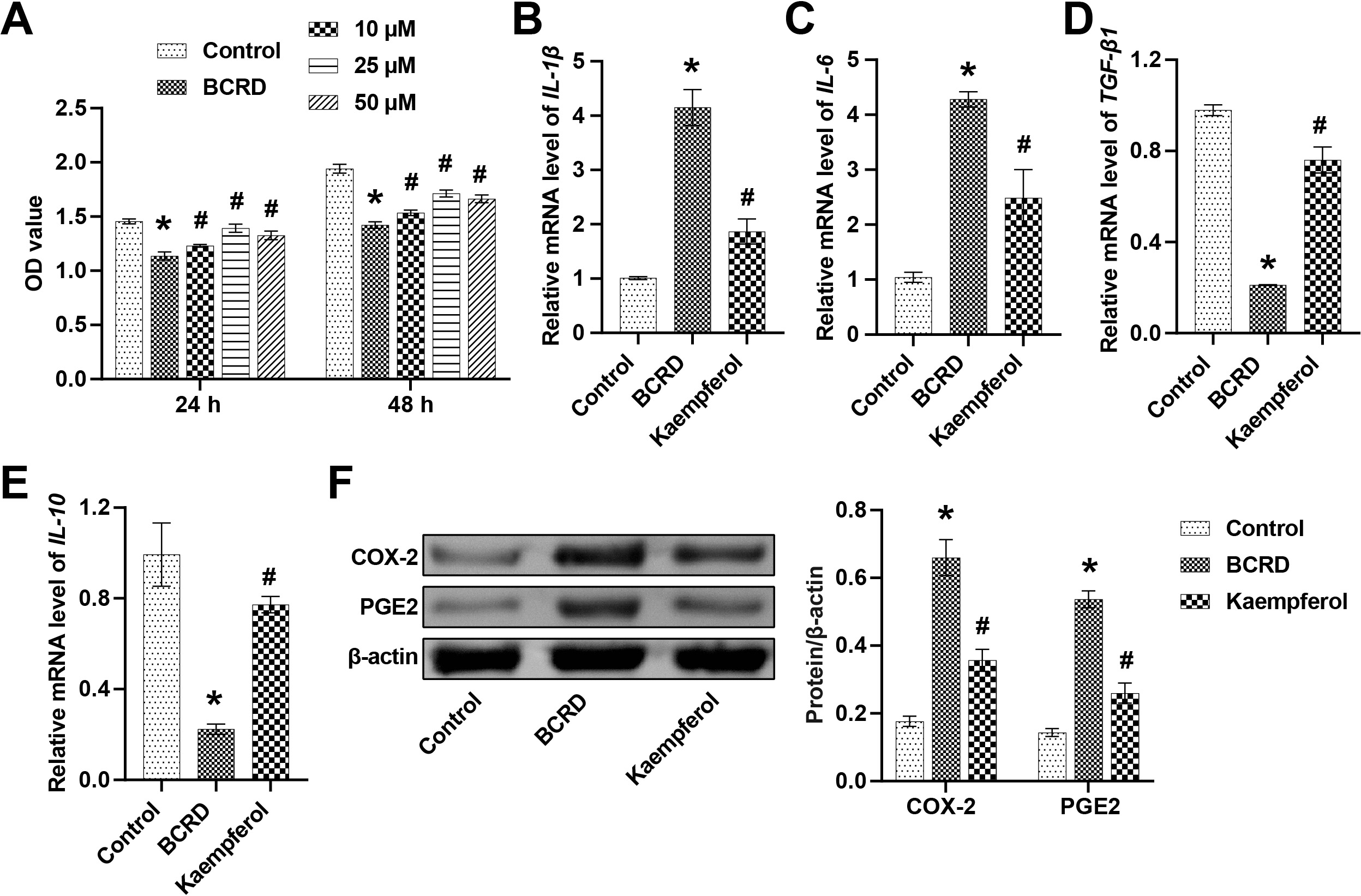 Fig. 2.
Fig. 2.Kaempferol regulated the expression of inflammatory cytokines
and cyclo-oxygenase (COX)-2/PGE2. (A) The effect of kaempferol (10 µM, 25
µM, and 50 µM) on cell viability was assessed at 24 h and 48 h. The
mRNA levels of IL-1
The role of COX-2/PGE2 in the anti-inflammatory effect of kaempferol was further explored. To confirm the kaempferol-COX-2 interaction, molecular docking was selected for analysis. Kaempferol and COX-2 had a binding energy of –9.1 kcal/mol, which indicated a strong and stable interaction between kaempferol and COX-2. In the visualized images, kaempferol bound to specific residues within the active pocket of COX-2, including PRO127, TYR2373, ARG376, GLN374, GLN2374, PHE142, PRO2538, GLY2227, VAL2228, GLY2533, ASN2537, TRP139, GLY2536, ASN-2375, GLY2225, ARG2376, and LEU2145. More specifically, kaempferol formed a Pi-Sigma conjugation at the GLN2374 residue. Kaempferol formed an Amide-Pi stacked at residue GLY2536. Kaempferol formed an unfavorable Donor-Donor at the ASN2537 residue. Kaempferol formed Pi-Alkyl and carbon-hydrogen bonds at the PRO2538 residue. Kaempferol formed Pi-Donor hydrogen and conventional hydrogen bonds at the ASN2375 residue. Kaempferol formed a conventional hydrogen bond at residues GLN374, VAL2228, and ARG2376. Furthermore, kaempferol formed van der Waals at residues ARG376, TYR2373, PRO127, PHE142, GLY2227, GLY2533, TRP139, GLY2225, LEU2145. These results suggested that kaempferol can bind to COX-2 (Fig. 3A).
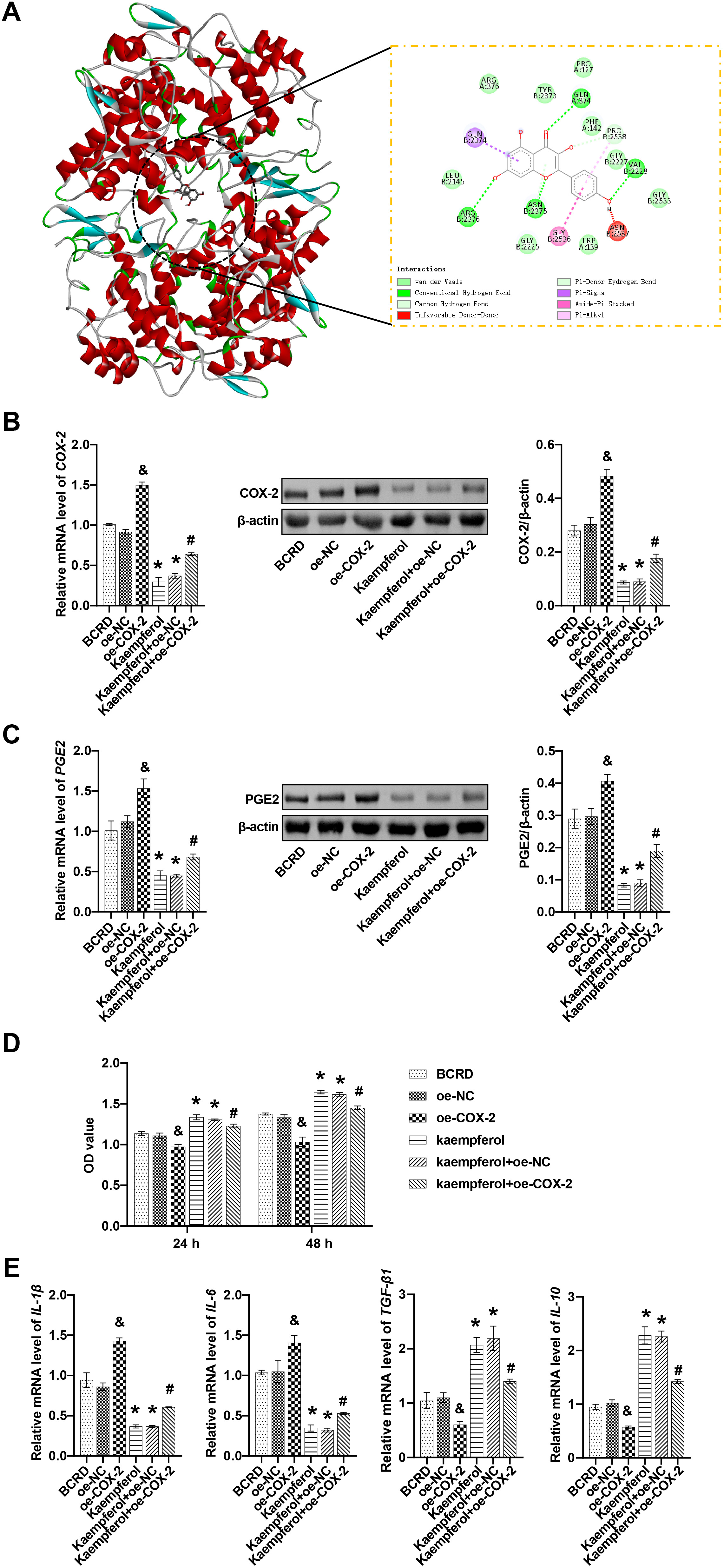 Fig. 3.
Fig. 3.Kaempferol inhibited the COX-2/PGE2 pathway to relieve
neuroinflammation. (A) Molecular docking revealed the binding of kaempferol to
COX-2. (B) The mRNA and protein levels of COX-2 were measured. (C) The mRNA and
protein levels of PGE2 were measured. (D) The effect of COX-2 on cell viability
was detected. (E) The effects of COX-2 on the levels of IL-1
Next, BCRD cells were transfected with the overexpression plasmid oe-COX-2. Our
results showed that compared with the oe-NC group, oe-COX-2 increased the
expression of both COX-2 and PGE2 in BCRD cells. By contrast, treatment with
kaempferol reduced the expression of COX-2 and PGE2 in BCRD cells compared with
the BCRD group. Compared with Kaempferol+oe-NC, oe-COX-2 elevated the expression
of COX2 and PGE2 in BCRD cells after kaempferol intervention. Compared with the
oe-COX-2 group, kaempferol inhibited the expression of COX2 and PGE2 (Fig. 3B,C).
In addition, oe-COX-2 decreased BCRD cell viability compared to the oe-NC group,
whereas Kaempferol enhanced BCRD cell viability. The Kaempferol+oe-COX-2 group
exhibited reduced BCRD cell viability compared to Kaempferol+oe-NC. Kaempferol
decreased cell viability compared with the oe-COX-2 group (Fig. 3D). Compared
with the oe-NC group, oe-COX-2 increased IL-1
We developed a mouse model of BCRD to confirm the therapeutic effect of kaempferol in vivo. Compared with the BCRD group, kaempferol promoted the growth of mice (Fig. 4A). Compared with the BCRD group, kaempferol significantly inhibited the volume and weight of BC tumors (Fig. 4B,C), suggesting that kaempferol had certain anti-BC effects on BCRD mice. Next, the tumor structure of BC was observed. As shown in Fig. 4D, the tumors of kaempferol-treated BCRD mice showed reduced inflammatory cell infiltration, clear cell structure, and tight arrangement, showing that kaempferol protected the pathological tumor structure of BCRD mice.
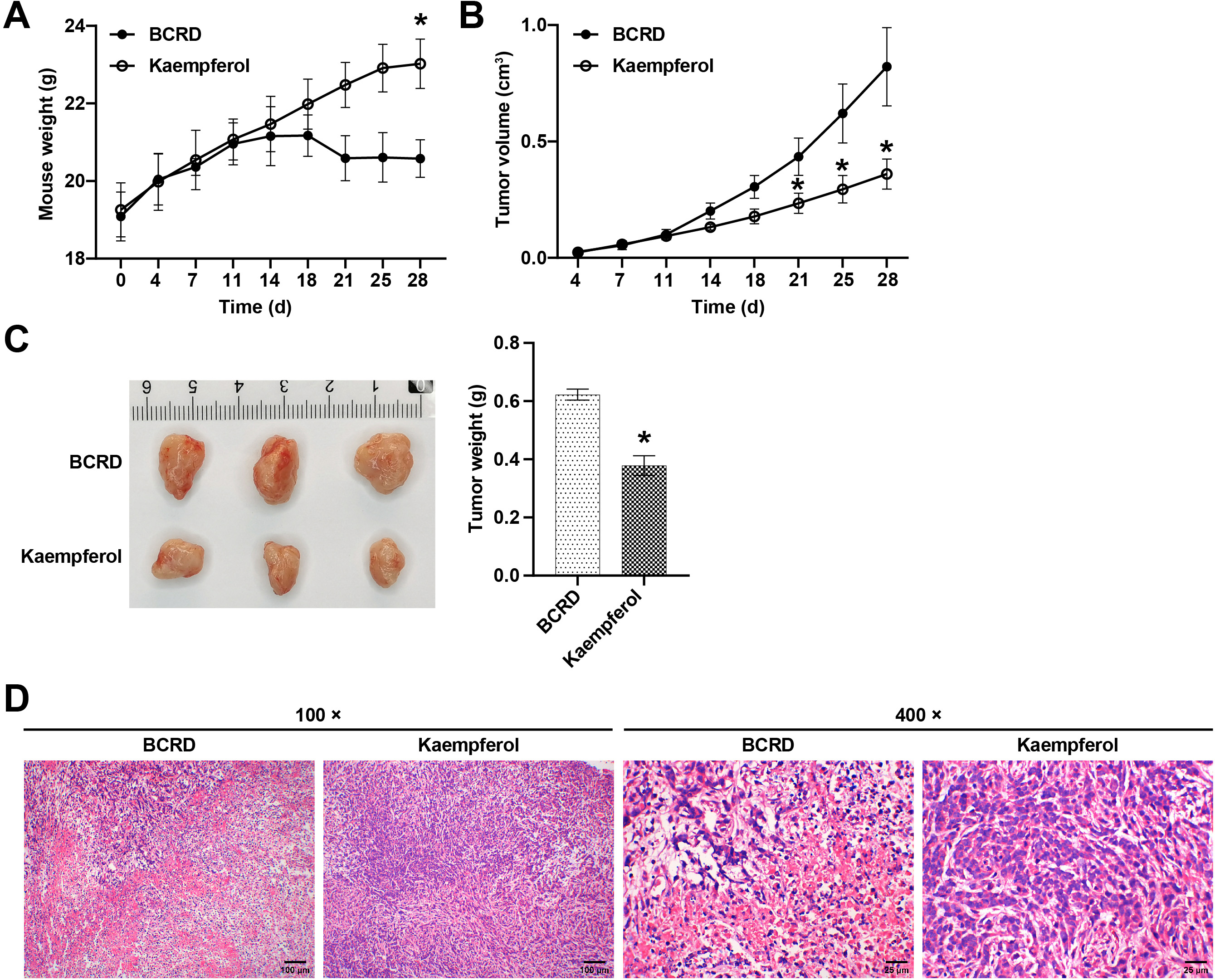 Fig. 4.
Fig. 4.Kaempferol inhibited the growth of BC and neuroinflammation in
BCRD mice. (A) Within 28 days of modeling, the mouse weight in each group (n =
5) was recorded. (B) Within 28 days of modeling, the tumor growth in each group
was recorded. (C) On the 28th day, the tumors of mice in each group were
photographed and weighed. (D) Representative images of the pathological phenotype
in each group were presented. n = 3. * p
The effect of kaempferol on the depressive behavior of mice in the BCRD group was assessed by behavioral studies. The results of the FST showed that the immobility time of BCRD mice increased, and kaempferol effectively reduced the immobility time of BCRD mice (Fig. 5A). The OFT showed that the total distance of mice in the BCRD group was significantly reduced, but kaempferol reversed this trend (Fig. 5B). Kaempferol inhibited the stress-induced increase in immobility time during the TST (Fig. 5C). Major neurotransmitters (NTs) in depression include (5-hydroxy tryptamine, 5-HT), dopamine (DA) and norepinephrine (NE). Compared with the Control group, 5-HT, DA, and NE levels were downregulated in the BCRD group, while kaempferol partially restored their levels (Fig. 5D). Taken together, these findings demonstrated that kaempferol contributed to reducing depression-like behaviors in BRCD mice.
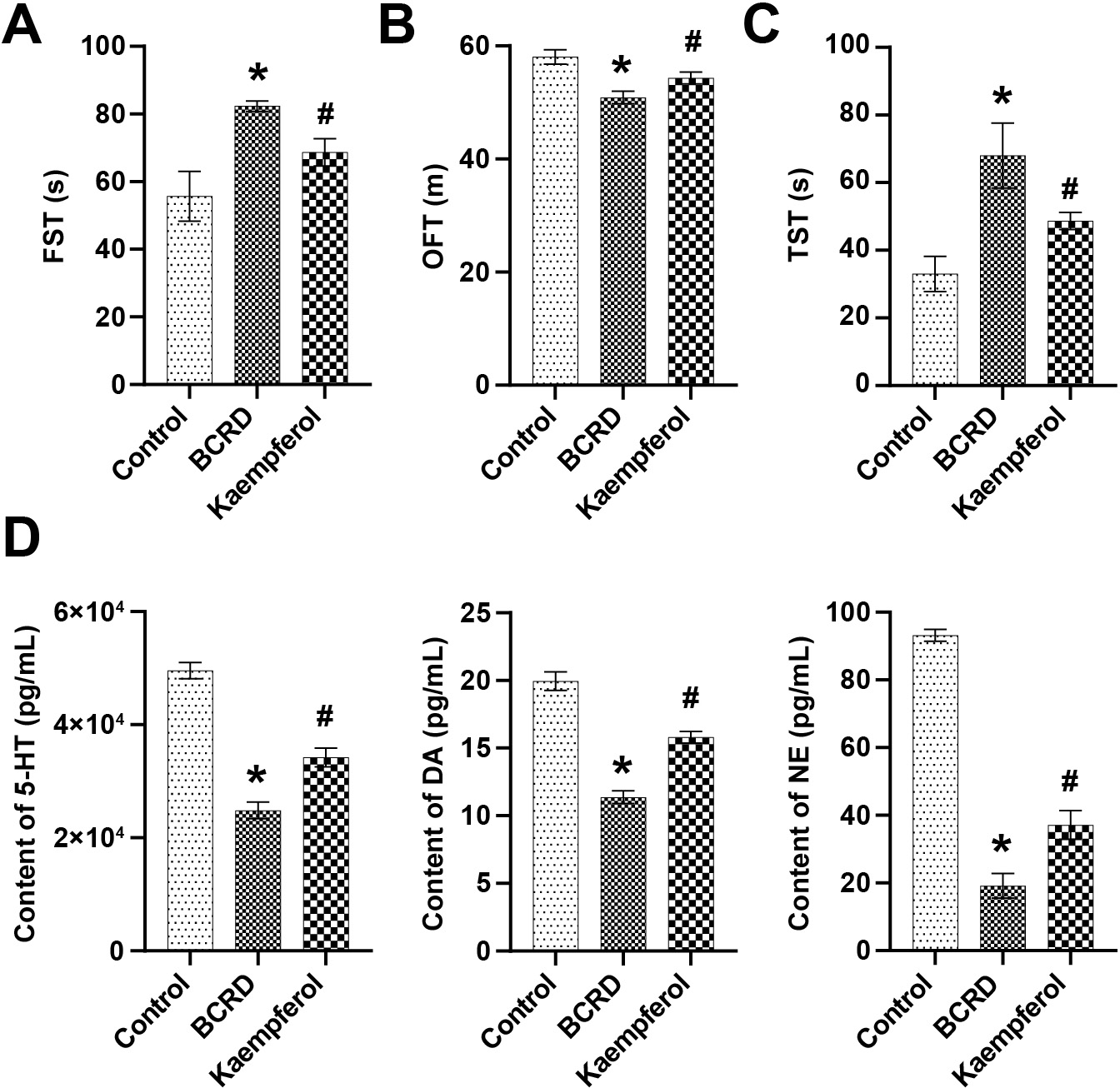 Fig. 5.
Fig. 5.Kaempferol improved depression in BCRD mice. (A) Forced
swimming test (FST). (B) Open field test (OFT). (C) Tail suspension test (TST).
(D) The contents of 5-hydroxy tryptamine (5-HT), dopamine (DA), and norepinephrine (NE) were examined by ELISA. n = 3. * p
IL-1
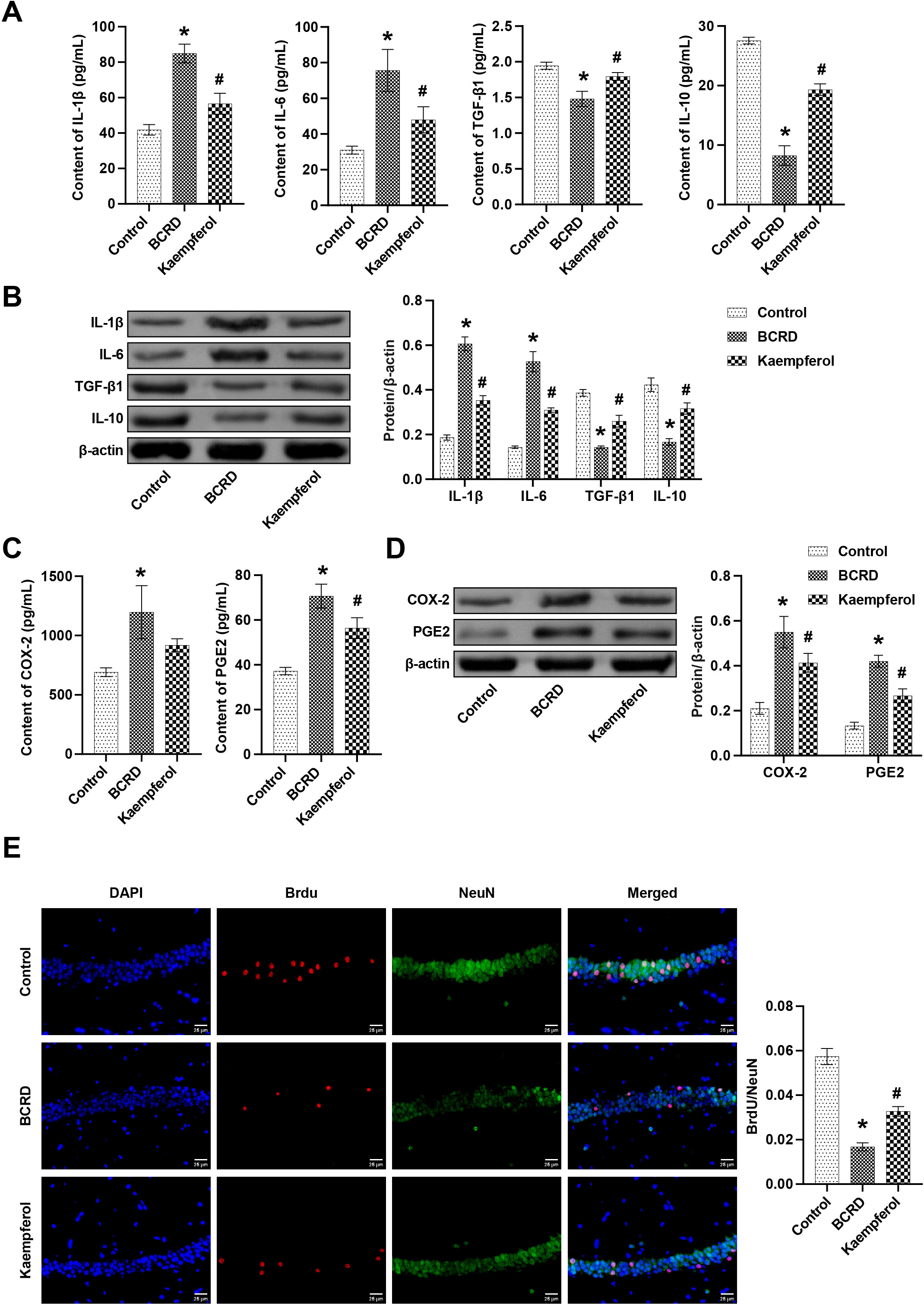 Fig. 6.
Fig. 6.Kaempferol improved neuronal damage in BCRD mice, at least in
part through COX-2/PGE2 signaling. The levels of IL-1
Compared with healthy women, BC patients have a significantly increased risk of depression, and identifying depression can help improve the mental status and quality of life in BC patients [24, 25]. Studies have shown that depression has a pathogenic effect on BC, so adjuvant therapy for depression has become a potential direction for the prevention and treatment of BC [20, 26]. Currently, medication, electroconvulsive therapy, and cognitive-behavioral therapy are commonly used to treat mental illnesses, including depression [27]. Due to drug interactions or significant toxicity, the tolerance is reduced, and the therapeutic effect is not ideal. The development of natural products of plant origin may be a safer and more effective alternative therapy [28]. Currently, the role of some natural products in the treatment of depression has been discovered, such as Isoliquiritin [29], Baicalin [30], and Kaempferol [31]. In addition, kaempferol is reportedly a therapeutic agent for BC [32]. Therefore, we speculated that kaempferol could be used to treat BCRD.
In our study, kaempferol downregulated the viability, migration, and clone of
4T1 cells. After kaempferol treatment, tumor growth in BCRD mice was
significantly alleviated. In behavioral tests, BCRD mice lost interest in
exploring novel environments and had increased inactivity time during the TST and
FST, which was reported in our previous study [20]. Conversely, kaempferol
effectively increased the total journey and decreased the immobility time in BCRD
mice. These results suggest that kaempferol has a certain therapeutic effect on
BCRD. Neuroinflammation is widely recognized to be involved in the pathology of
depression [33]. Improvement of neuroinflammation can reverse depressive behavior
in many cases involving inflammatory factors fluctuations. Our study showed that
IL-1
COX-2 is an inflammatory mediator, and COX-2 inhibitors are considered to be
promising anti-inflammatory and antidepressant drugs [35, 36]. Previous studies
have shown that curcumin may exert antidepressant effects, at least in part by
limiting COX-2 signaling to modulate factors such as postsynaptic transmission
and cell viability [37]. Another study demonstrated that puerarin reduces COX-2
expression, thus helping to restore intestinal mucus barrier dysfunction and
neuroinflammatory hyperactivation and ultimately mitigating depression-like
behavior [38]. In the present study, we demonstrated that the abundance of COX-2
was increased in the BCRD model. Kaempferol restricted the expression of COX-2,
which is consistent with previous reports [39]. Overexpression of COX-2 decreased
the viability of BCRD cells, upregulated IL-1
A growing number of reports have proposed that blocking COX-2 activity improves stress-induced depression-like behavior, a process involving decreasing PGE2 [36]. COX-2 is a rate-limiting enzyme responsible for the final conversion of arachidonic acid (AA) to PGE2 [42]. More specifically, AA is converted to the unstable intermediate prostaglandin H2 (PGH2) via COX, which is further converted to PGE2 by terminal PGE2 synthases (PGES). After synthesis, PGE2 binds to receptors known as prostaglandin E receptors (EP1-4), which are coupled to G proteins and participate in the regulation of inflammation [43, 44]. Therefore, it appears that COX-2 catalyzes the production of PGE2, and PGE2 then binds to receptors to activate downstream signaling pathways. Due to financial and time constraints, the more intricate regulatory connection between COX-2 and PGE2 remains to be further explored, which will be further investigated in our future studies. Our report showed that the abundance of PGE2 was increased in the BCRD model. Kaempferol and overexpression of COX-2 effectively decreased its expression, suggesting that kaempferol inhibited BCRD progression, at least in part through the COX-2/PGE2 signaling pathway. COX-2 exerts most of its functions through its metabolite PGE2 and is also involved in various signaling pathways such as nuclear factor kappa B, extracellular signal-regulated kinase, phosphoinositide 3-kinase/AKT, and cyclic AMP/protein kinase A to modulate neurological function [45, 46]. COX-2 plays a critical role in depression by regulating gut microbiota, mitochondrial function, hippocampal neuronal damage, and the hypothalamic-pituitary-adrenal axis [8]. Therefore, further investigation is needed to elucidate the mechanisms by which kaempferol ameliorates BC development and depression-like behaviors via COX-2. Additionally, the development of BCRD has been linked to intestinal flora dysbiosis, neuronal pyroptosis, and immune responses [18, 47]. However, whether kaempferol targets other biological processes to regulate BCRD progression still needs more experimental evidence.
To summarize, our study supported the role of kaempferol in anti-BCRD, at least in part through the COX-2/PGE2 signaling pathway, to regulate neuroinflammation, neurogenesis, and neurotransmitters. Our findings broaden kaempferol-based therapeutic strategies and provide potential directions for treating BCRD.
The datasets used during the current study are available from the corresponding author on reasonable request.
YS designed the research study. QZ, YHa, YHe, YF, and HY performed the research. YHa and YC analyzed the data. QZ wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work to take public responsibility for appropriate portions of the content and agreed to be accountable for all aspects of the work in ensuring that questions related to its accuracy or integrity.
Animal experiments were approved by the Ethic Committee of Hunan Cancer Hospital & The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University (No. SBQLL-2021-034).
We would like to thank Hunan Cancer Hospital & The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University for their administrative and technical support.
This research was funded by National Natural Science Foundation of China (No. 82104846); Scientific Research Project of Hunan Provincial Health Commission (No. 202103101959 & 202204015380); and Hunan Cancer Hospital Climb Plan (No. 2020NSFC-B001).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
