1 College of Biological and Chemical Engineering, Changsha University, 410022 Changsha, Hunan, China
2 Department of Urology, The Second Xiangya Hospital, Central South University, 410011 Changsha, Hunan, China
3 College of Chinese Medicine, Hunan University of Chinese Medicine, 410208 Changsha, Hunan, China
†These authors contributed equally.
Abstract
Background: Age-related macular degeneration (AMD) is the most
common cause of visual disorders in the aged population and is characterized by
the formation of retinal pigment epithelium (RPE) deposits and dysfunction/death
of the RPE and photoreceptors. It is supposed that both oxidative stress and
inflammation play a critical role in the pathogenesis of AMD. The development of
therapeutic strategies against oxidative stress and inflammation in AMD is
urgently needed. Rubus suavissimus S. Lee (RS), a medicinal plant
growing in the southwest region of China, has been used as an herbal tea and
medicine for various diseases. Methods: In this project, we
evaluate the therapeutic potential of RS extract for AMD. We prepared RS extracts
from dried leaves, which contained the main functional compounds.
Results: RS extract significantly increased cell viability,
upregulated the expression of antioxidant genes, lowered the generation of
malondialdehyde and reactive oxygen species, and suppressed inflammation in
H
Keywords
- Rubus suavissimus S. Lee
- age-related macular degeneration
- retinal pigment epithelial cells
- oxidative stress
- inflammation
- high-fat diet-fed mice
Age-related macular degeneration (AMD), the most common visual disorder in
humans over 50, currently affects approximately 200 million individuals worldwide
[1]. The predominant clinical features of AMD include retinal pigment epithelium
(RPE) atrophy and the presence of extracellular deposits (known as drusen)
underneath the RPE. AMD is classified into early stage (drusen size of 63–125
µm without pigmentary abnormality), intermediate stage (drusen size
The retina has an extremely high oxygen consumption rate and produces high
levels of reactive oxygen species (ROS), resulting in the pathogenesis of retinal
degeneration, including AMD [3, 4]. In AMD, the end-products of lipid
peroxidation, for example, malondialdehyde (MDA) and carboxyethylpyrrrole (CEP),
are associated with drusen formation and RPE dysfunction [5]. CEP-modified
proteins are much more abundant in the plasma and the outer retinas of AMD
patients than in those of controls [6, 7]. Mice immunized with CEP adduct
demonstrated AMD-like pathology [8]. ROS can also oxidize cholesterol to
7-ketocholesterol (7-KC), enriched in aged RPE cells and drusen. 7-KC has been
shown to cause damage to human and rodent RPE cells. 7-KC can also enhance the
expression of proinflammatory cytokines in RPE cells, which possibly contribute
to AMD pathogenesis [9]. Accumulated evidence has shown that excessive generation
of ROS induces nuclear factor-
Rubus suavissimus S. Lee (RS) is widely distributed in Southwest China,
particularly in Guangxi and Guizhou provinces [11]. The leaves of RS contain the
natural sweetener rubusoside and have been used as a sweet tea by the local
population. Different types of functional compounds, including polyphenols,
flavonoids, diterpenes, lignans, and triterpenoids, have been identified from RS
extracts [11]. Early studies have shown that RS extract promotes adipogenesis in
preadipocytes by upregulating the expression of adipogenic transcription factors
and their target genes [12]. RS extract has been reported to lower the level of
blood glucose and to reduce both body weight gain and abdominal fat accumulation
in rats fed a high-fat diet [13]. RS extract has also been shown to decrease
lipid droplet formation in the livers of mice and hamsters fed a high-fat diet,
possibly by regulating the proliferator-activated receptor/sterol
regulatory-element binding protein (PPAR-SREBP) pathway [14, 15]. Recently, Zhang
et al. [16] reported that RS extract inhibited lipopolysaccharide
(LPS)-induced chronic inflammation in mice by decreasing the production of
proinflammatory factors, including MCP-1, IL-6, and TNF-
In this study, we preliminarily characterize the chemical constituents of RS
extract and evaluate the protective capacities of RS extract against oxidative
stress and inflammation in H
A total of 200 g dried RS leaves were ground and soaked in 90% ethanol for 3 h.
The liquid extract was filtered and dried to obtain 30.6 g of powder.
Constituents of RS extract were identified using high performance liquid
chromatography (HPLC) analysis using a Waters XBridge® BEH
C18 column (150 mm
Human RPE (ARPE-19) cells were seeded in 96-well plates (5
Total RNA was extracted from control and treated ARPE-19 cells using TRIzol
Reagent® (15596-018, Invitrogen, Carlsbad, CA, USA)
following the manufacturer’s instructions. cDNAs were synthesized with a
commercial cDNA synthesis kit (Nanjing Jiancheng Bioengineering Institute,
Nanjing, Jiangsu, China) according to the manufacturer’s protocol. The expression
of target genes was measured using a real-time PCR kit (Nanjing Jiancheng
Bioengineering Institute, Nanjing, Jiangsu, China) as described by the
manufacturer and calculated using the 2
Animal experiments were approved by the Hunan University of Chinese Medicine Animal Ethics and Welfare Committee (SYXK (Xiang) 2019-0009). C57BL/6J mice aged four weeks (50% male and 50% female) were housed in the Hunan University of Chinese Medicine Animal Unit. All mice were randomly divided into three groups (n = 8 per group); one group was fed a normal diet (ND), whereas the other two groups were fed a high-fat diet (HFD). Normal diet (Cat No. D12450B) and high-fat diet (Cat No. D12492, 78.75% normal diet, 10% corn oil, 1% cholesterol, 10% lard, and 0.25% sodium cholate) were purchased from Research Diets Inc. After 12 weeks, the ND group and one HFD group received intragastric treatment of sterilized saline daily for four weeks; the other HFD group (termed the HFD + RS group) received intragastric administration of RS extract dissolved in physiological saline (350 mg/kg/d) daily for 4 weeks. The ND group was fed a normal diet during the treatment period, whereas the HFD and HFD + RS groups were fed a high-fat diet. Animals were sacrificed after the treatment, and tissues were collected and kept in a –80 °C freezer until further analysis.
The levels of total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and triglyceride (TG) in mouse sera were measured using an automatic biochemical analyzer (HITACHI 7600, Hitachi HighTech Co., Ltd., Tokyo, Japan).
The superoxide dismutase activity, catalase activity, glutathione level, and malondialdehyde level in ARPE-19 samples and mouse samples were quantified using commercial kits (Cat No. A001-3-2, SOD; Cat No. A007-1-1, CAT; Cat No A006-1-1, GSH; and Cat No. A003-4-1, MDA; Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China) based on the manufacturer’s instructions.
The levels of TNF-
One-way or two-way analysis of variance (ANOVA) was applied to analyze the data,
presented as the mean
The RS extract was subjected to HPLC analysis along with five standards: rutin, ellagic, hyperoside, myricetin, and rubusoside. Based on the five standards, we found that ellagic acid and rubusoside were the major compounds in the RS extract (Fig. 1), which was in agreement with previous reports [10, 16]. The three other compounds, rutin, hyperoside, and myricetin, were also detected in the RS extract at low levels. The retention times for ellagic acid and rubusoside were 13.78 min and 37.94 min, respectively. The linear regression equations based on the concentration (X, µg/mL) and the peak area (Y) for ellagic acid and rubusoside were Y = 39.9951X + 14.849 (coefficient, 0.9999) and Y = 26.1295X – 8.697 (coefficient, 0.9997), respectively. The contents of ellagic acid and rubusoside in RS leaves were 26.82 mg/g and 34.23 mg/g, respectively.
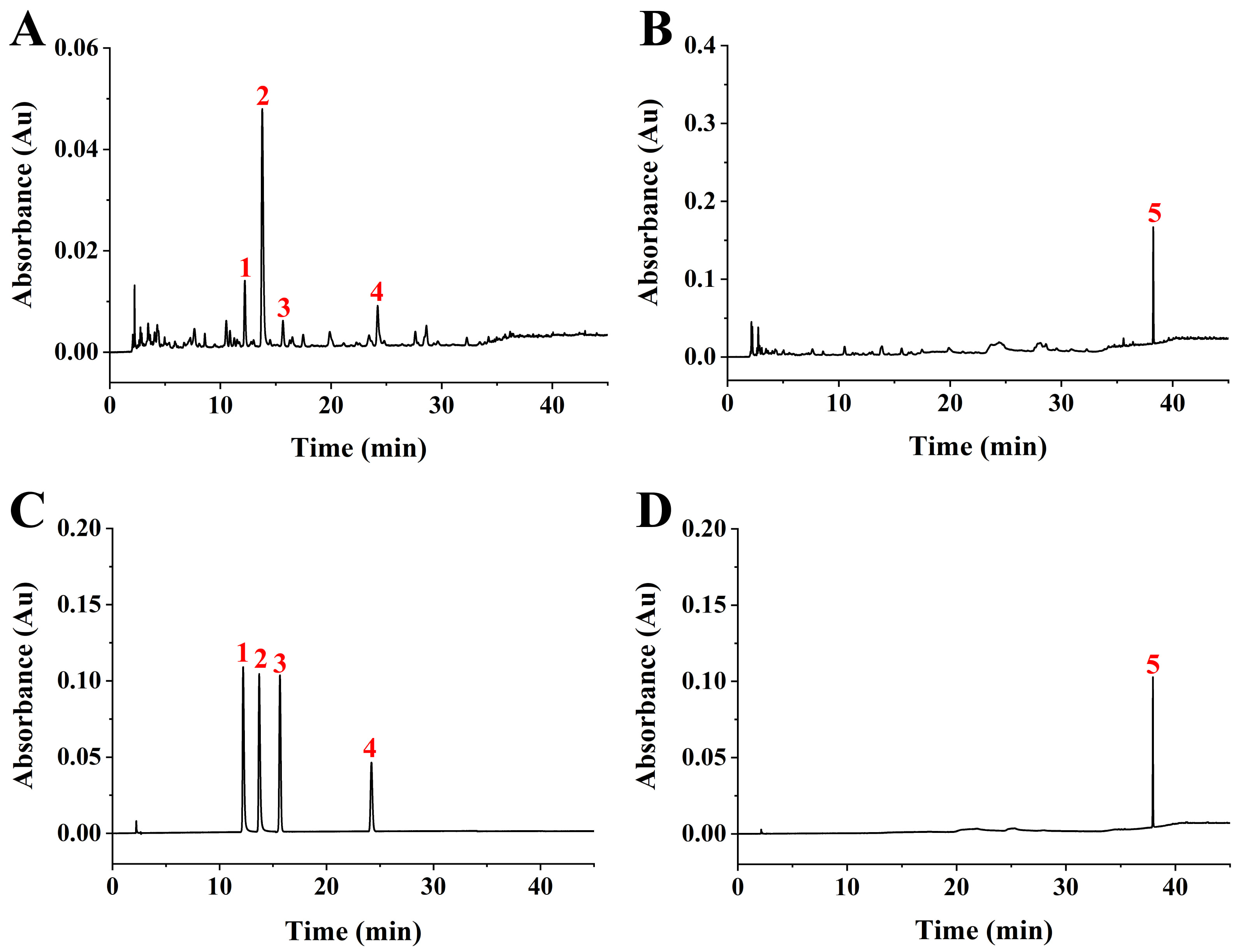 Fig. 1.
Fig. 1.High performance liquid chromatography (HPLC) analysis of Rubus suavissimus S. Lee (RS) extract. (A) HPLC chromatogram of the RS extract at 254 nm. (B) HPLC chromatogram of the RS extract at 210 nm. (C) HPLC chromatogram of standard compounds at 254 nm. 1, rutin; 2, ellagic acid; 3, hyperoside; 4, myricetin. (D) HPLC chromatogram of standard compounds at 210 nm. 5, rubusoside.
First, we treated ARPE-19 cells with H
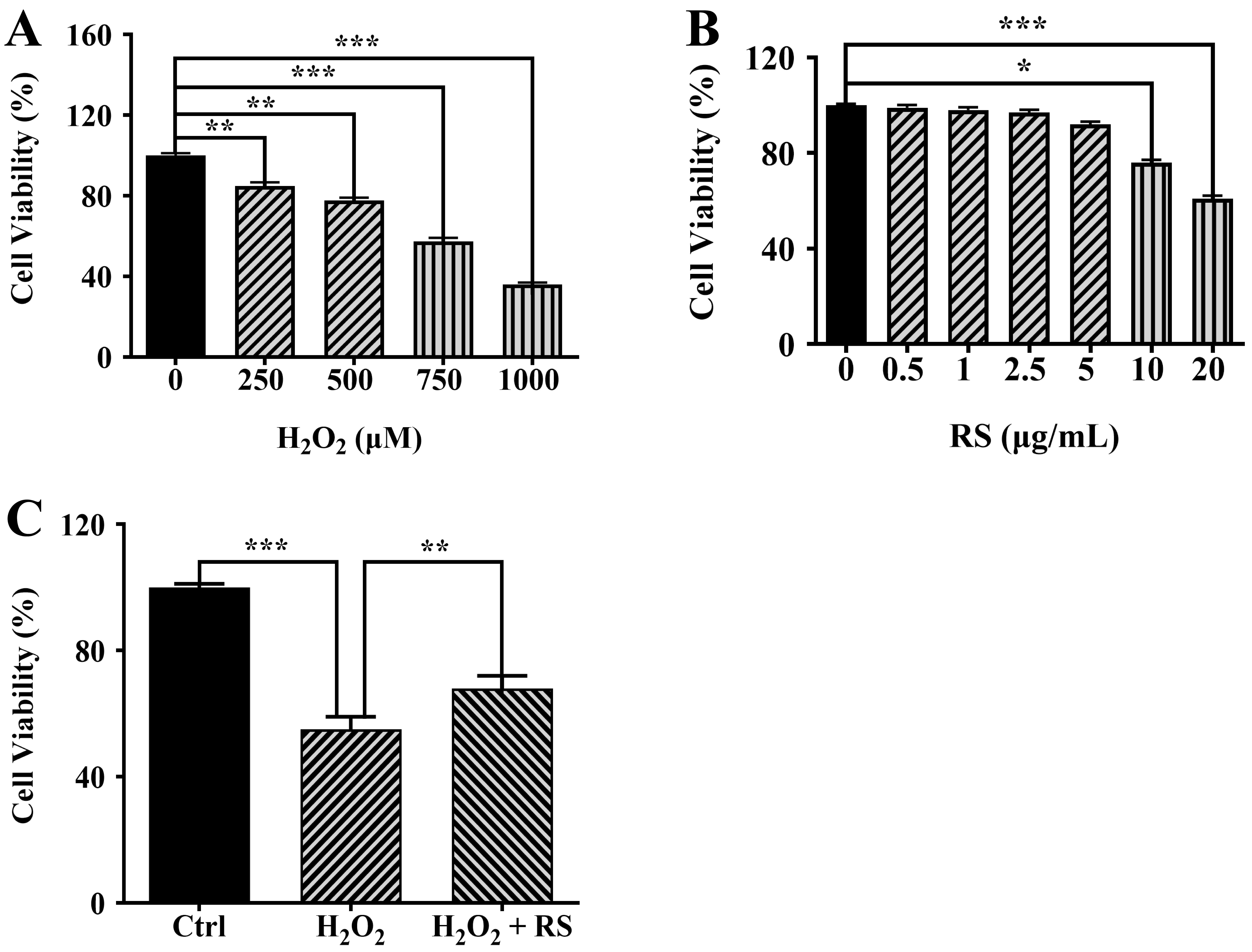 Fig. 2.
Fig. 2.RS reduces H
Our previous study has shown that H
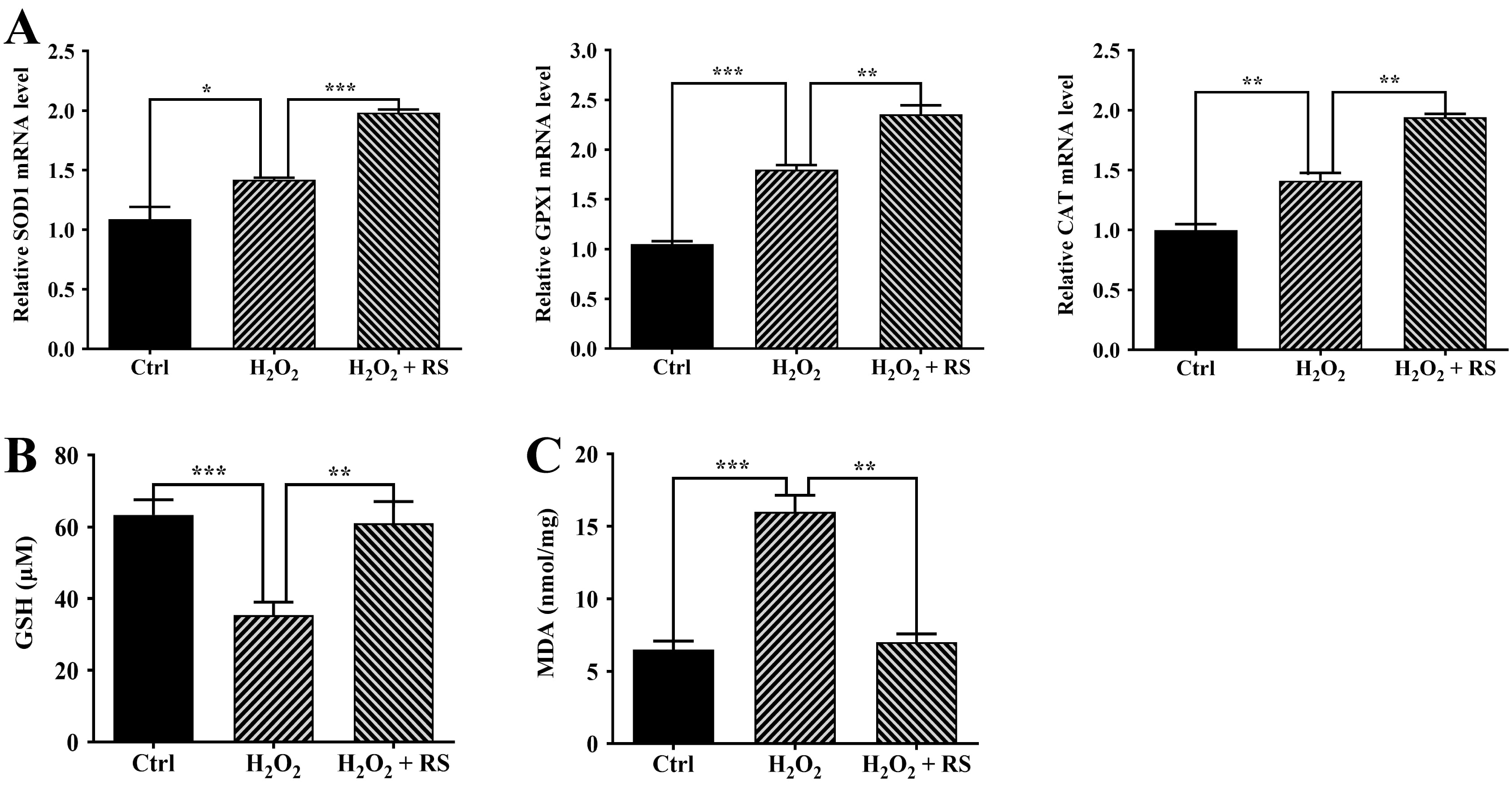 Fig. 3.
Fig. 3.Increased antioxidant capacity in retinal pigment epithelium
(RPE) cells treated with RS extract. (A) SOD1, GPX1, and
CAT expression was measured by quantitative real-time polymerase chain
reaction (qRT-PCR). (B) The basal glutathione (GSH) levels in untreated (Ctrl)
and treated ARPE-19 cells. (C) Malondialdehyde (MDA) levels in untreated (Ctrl)
and treated ARPE-19 cells. Data were analyzed using one-way ANOVA and a Tukey’s
post hoc test and displayed as the mean
Our previous study has demonstrated that H
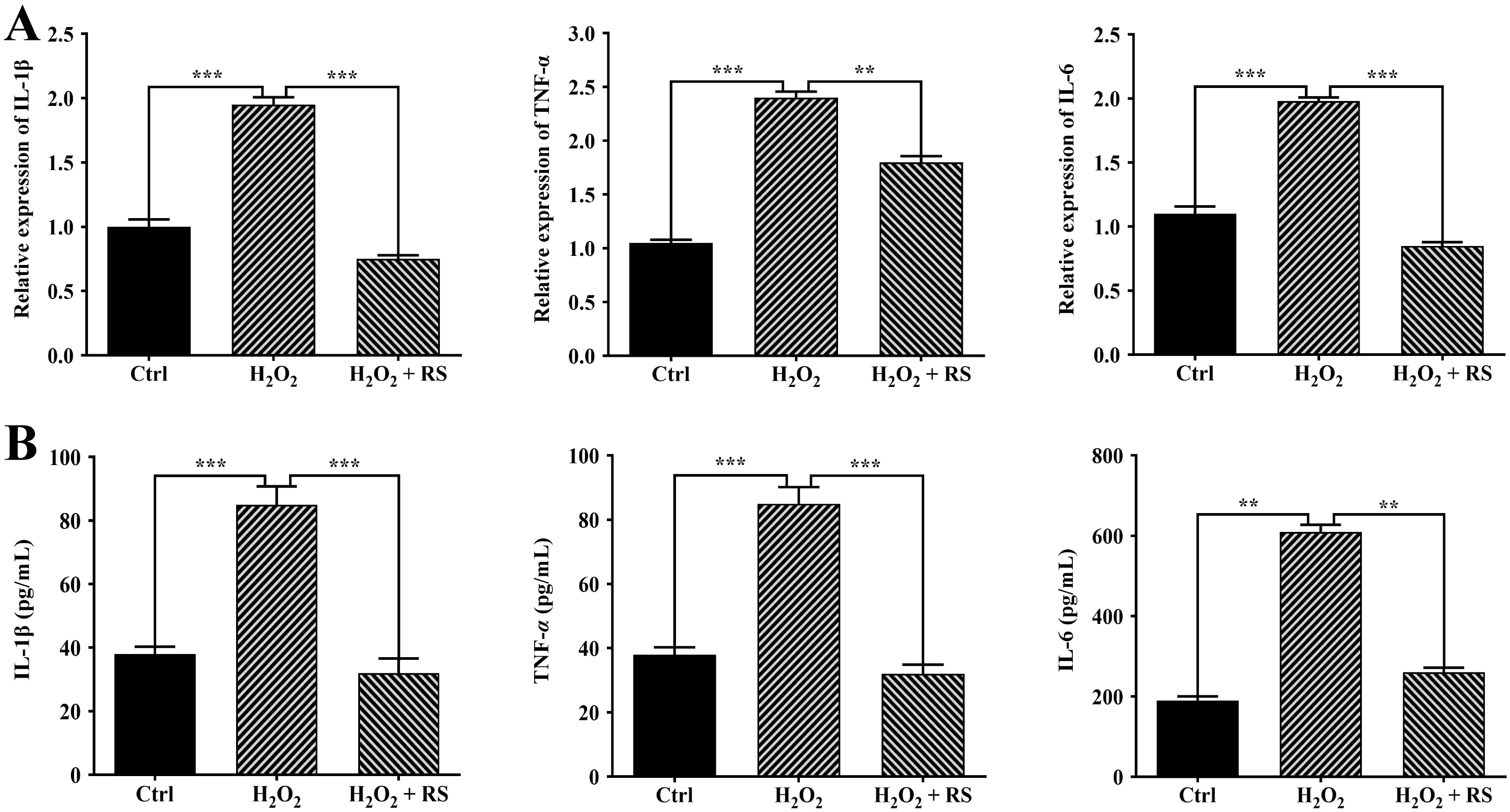 Fig. 4.
Fig. 4.Effects of RS extract on inflammation in H
To determine the function of RS extract in vivo, we established an obese mouse model. Animals were fed with ND or HFD for 12 weeks. Then, the ND group and one HFD group were administered a vehicle for four weeks, whereas the other HFD group received treatment with RS extract (HFD + RS). During the treatment phase, these animals were fed either ND (ND group) or HFD (HFD group and HFD + RS group) (Fig. 5A). Body weight was monitored during the whole experimental process. High-fat diet-fed mice showed significant weight gain after seven weeks of feeding compared to animals fed a normal diet (Fig. 5B). Administration of RS extract significantly limited weight gain in the high-fat diet-fed animals compared to the animals fed a high-fat diet alone (Fig. 5C); this is in agreement with a previous report [13].
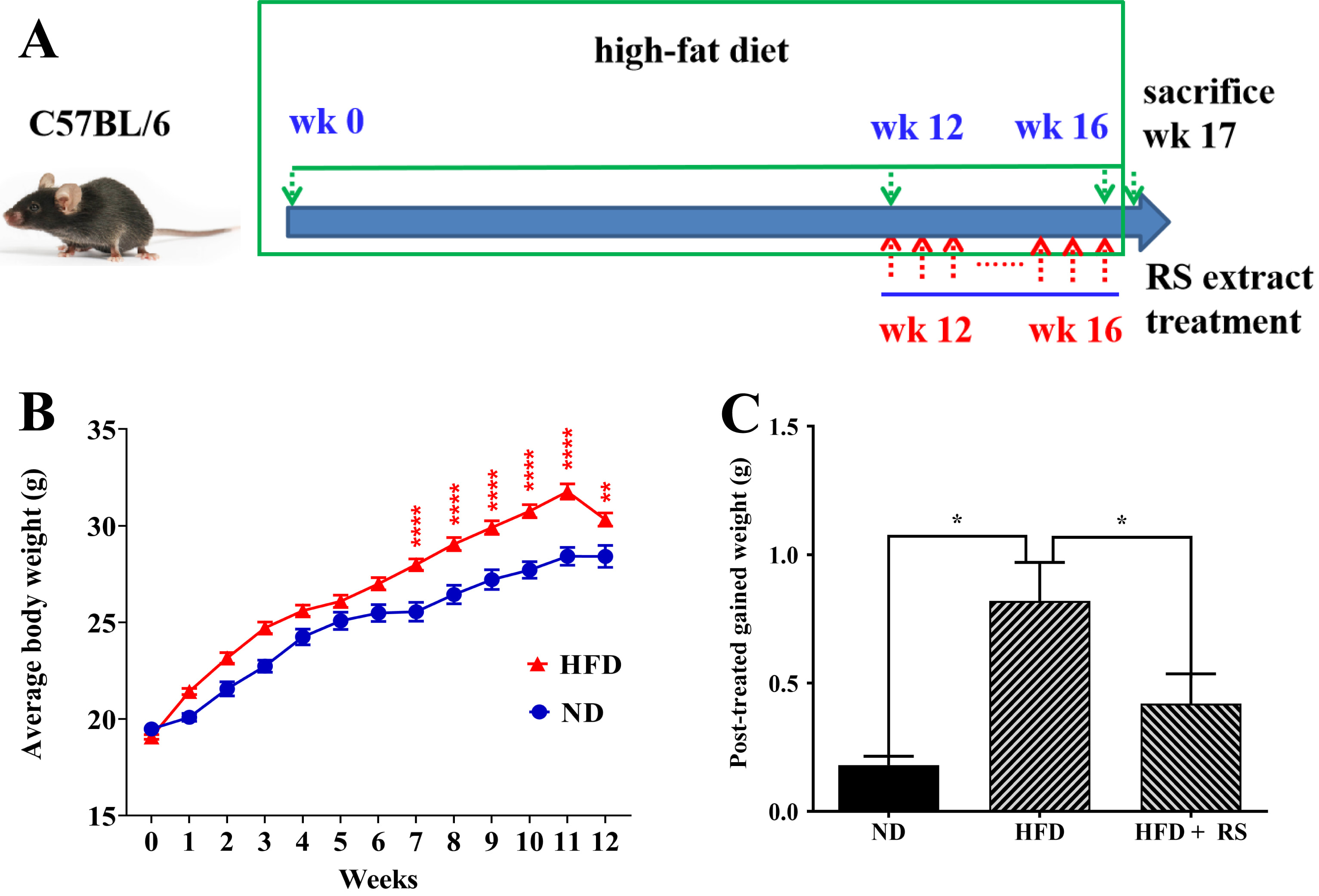 Fig. 5.
Fig. 5.RS extract reduced body weight gain. (A) Schematic of the
treatment plan. (B) Changes in body weight of C57BL/6 mice fed a normal diet (ND)
and a high-fat diet (HFD) during the experimental period. (C) Effects on body
weight gain among the ND, HFD, and HFD + RS groups. (B) Data were analyzed using
two-way ANOVA and a Bonferroni test or (C) using a one-way ANOVA and Tukey’s post
hoc test and presented as the mean
High-fat diet consumption can enhance lipid accumulation in the liver, causing increased liver weight and damage to the liver. The liver index is an important indicator of obesity in rodents. The HFD mice had markedly increased liver weight compared to the ND mice; treatment with RS resulted in a notable decrease in liver weight, although this was not significant when compared to the HFD mice (Fig. 6A,B). However, the liver index of the HFD animals was significantly higher compared to that of the ND animals. After treatment with RS extract, the index was notably decreased compared to that of the HFD groups (Fig. 6C).
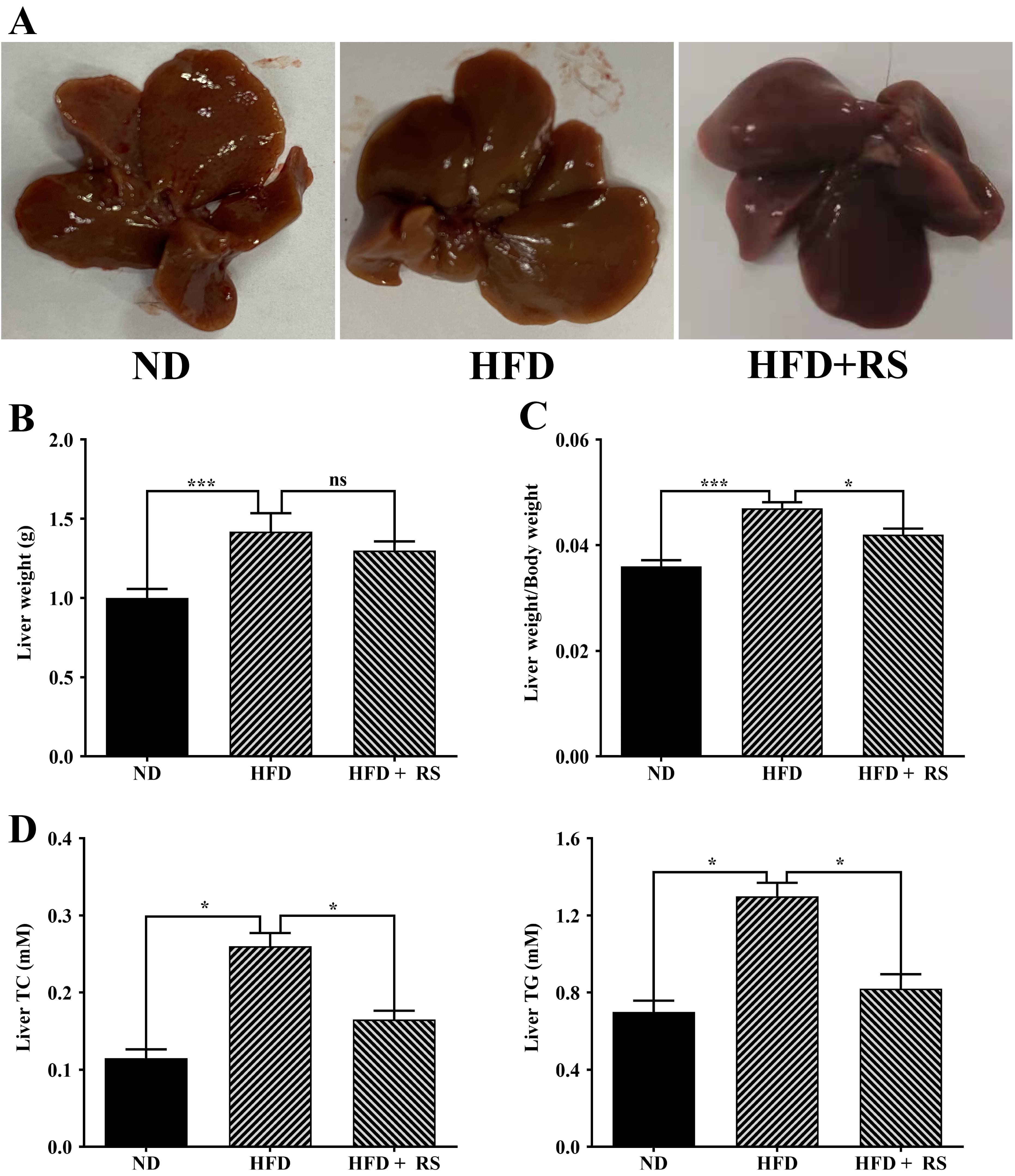 Fig. 6.
Fig. 6.Treatment with RS extract decreased the liver index and the
levels of total cholesterol and triglycerides. (A) Images of livers from mice
fed with ND, HFD, or HFD with RS extract (HFD + RS). (B) Liver weights of the
three groups. (C) Liver weight/body weight (liver index) of the three groups. (D)
Measured total cholesterol and triglycerides. ns, no significance; *p
A high-fat diet is expected to affect animal lipid metabolism. We first measured total cholesterol and glyceride in the liver of the three groups and found that a high-fat diet induced a significant increase in the total cholesterol and glyceride levels in the liver compared to that of mice fed a normal diet; treatment with RS extract reversed the high-fat diet-induced effect (Fig. 6D). We also examined cholesterol, triglyceride, LDL-cholesterol and HDL-cholesterol in mouse serum. Total cholesterol, triglyceride, and LDL-cholesterol were markedly higher in the serum of high-fat diet-fed animals, compared to animals fed with a normal diet, whereas RS extract-treated animals had a significant decrease in these lipids, compared to concentrations in high-fat diet-fed animals. HDL-cholesterol levels were significantly lowered in the serum of mice fed a high-fat diet, an effect that was reversed as a result of treatment with RS extract (Fig. 7).
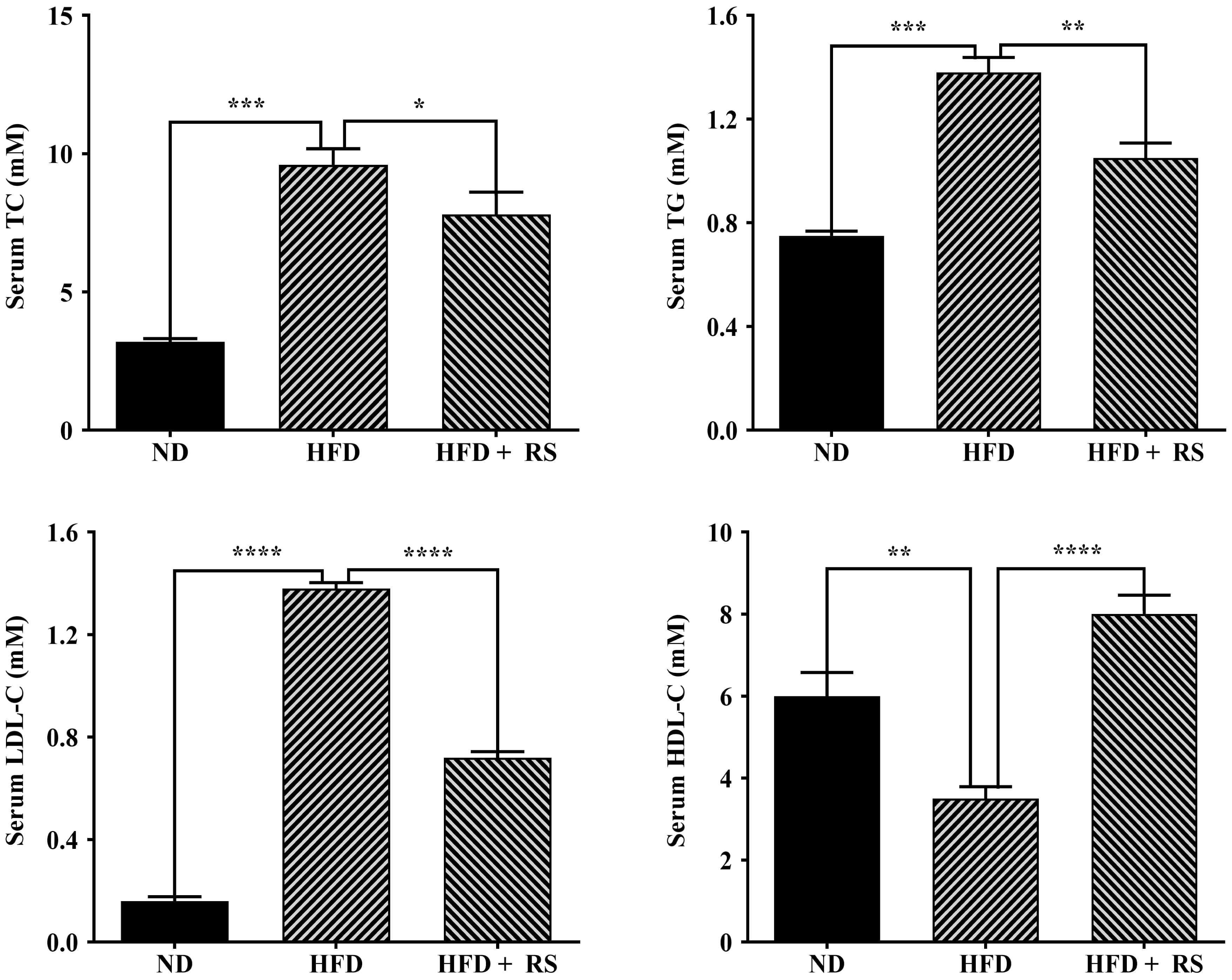 Fig. 7.
Fig. 7.Effects of RS extract on lipids in the serum of mice fed a
high-fat diet. The levels of total cholesterol (TC), triglyceride (TG),
low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein
cholesterol (HDL-C) in the serum of mice fed ND, HFD or HFD and treated with RS
extract (HFD + RS). Data were analyzed using one-way ANOVA and Tukey’s post hoc
test and displayed as the mean
Accumulated evidence has demonstrated that obesity is associated with oxidative stress [21, 22, 23]. We found a higher production of ROS, a significant decrease in Superoxide Dismutase (SOD) activity and GSH levels, and a marked increase in the MDA content in the RPE/choroid and retina of HFD-fed mice compared to ND-fed mice. Treatment with RS extract counteracted the high-fat diet-induced effects (Fig. 8).
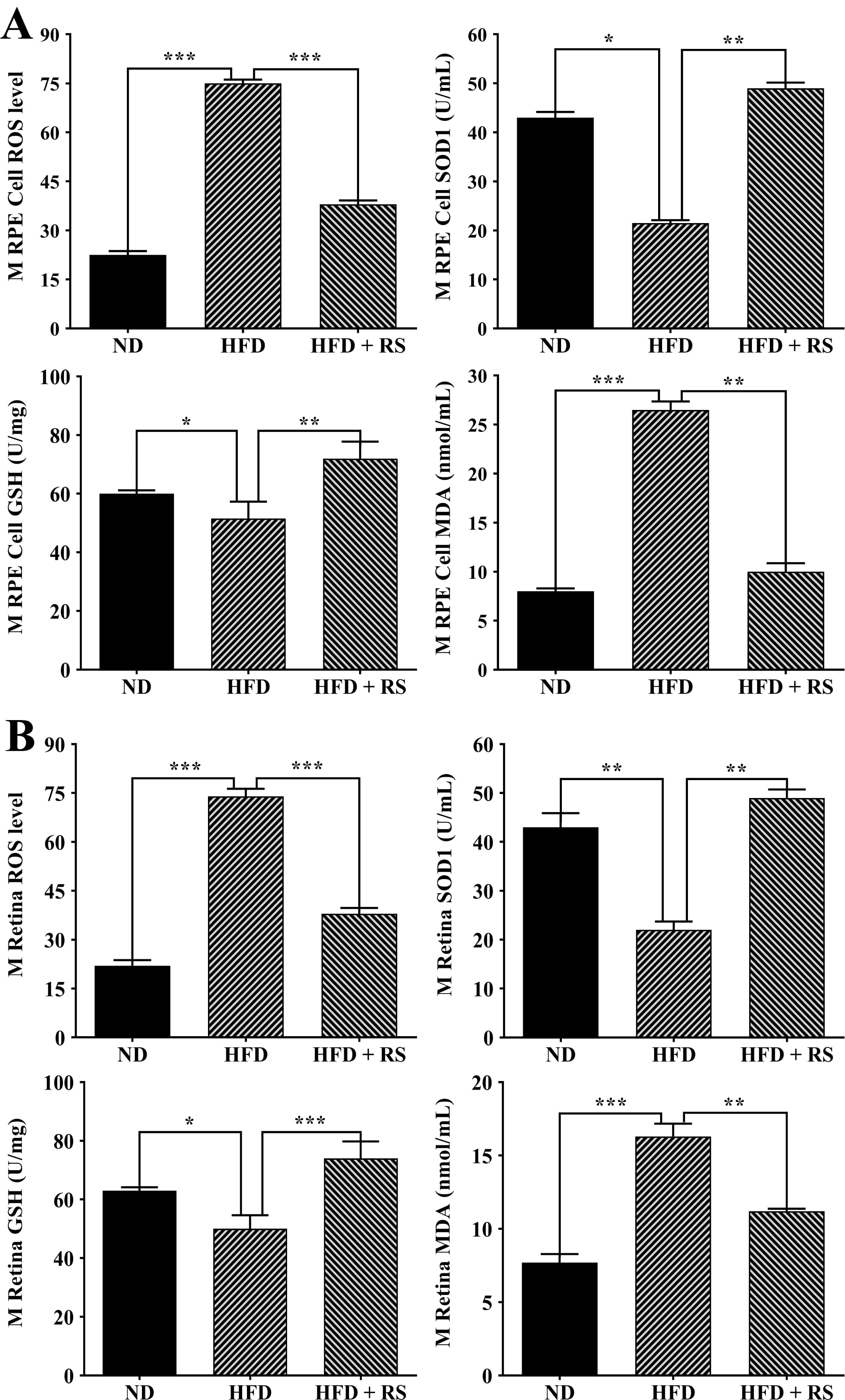 Fig. 8.
Fig. 8.RS extract treatment enhanced the antioxidant capacity of the
RPE/choroid (A) and retinas (B) of mice fed a high-fat diet. The levels of
reactive oxygen species (ROS), GSH, and malondialdehyde (MDA) and SOD activity
were measured in RPE/choroid and retinas of ND, HFD, and HFD + RS mice. Data were
analyzed using one-way ANOVA and Tukey’s post hoc test and expressed as the mean
Further investigation showed that high-fat diet-fed animal serum also had significantly higher levels of ROS and MDA, decreased SOD activity, and low levels of GSH compared to those of animals fed a normal diet. In RS extract-treated animal serum, ROS production was notably decreased, SOD activity and GSH levels were significantly increased, and MDA was markedly decreased compared to that of animals fed with HFD alone (Fig. 9).
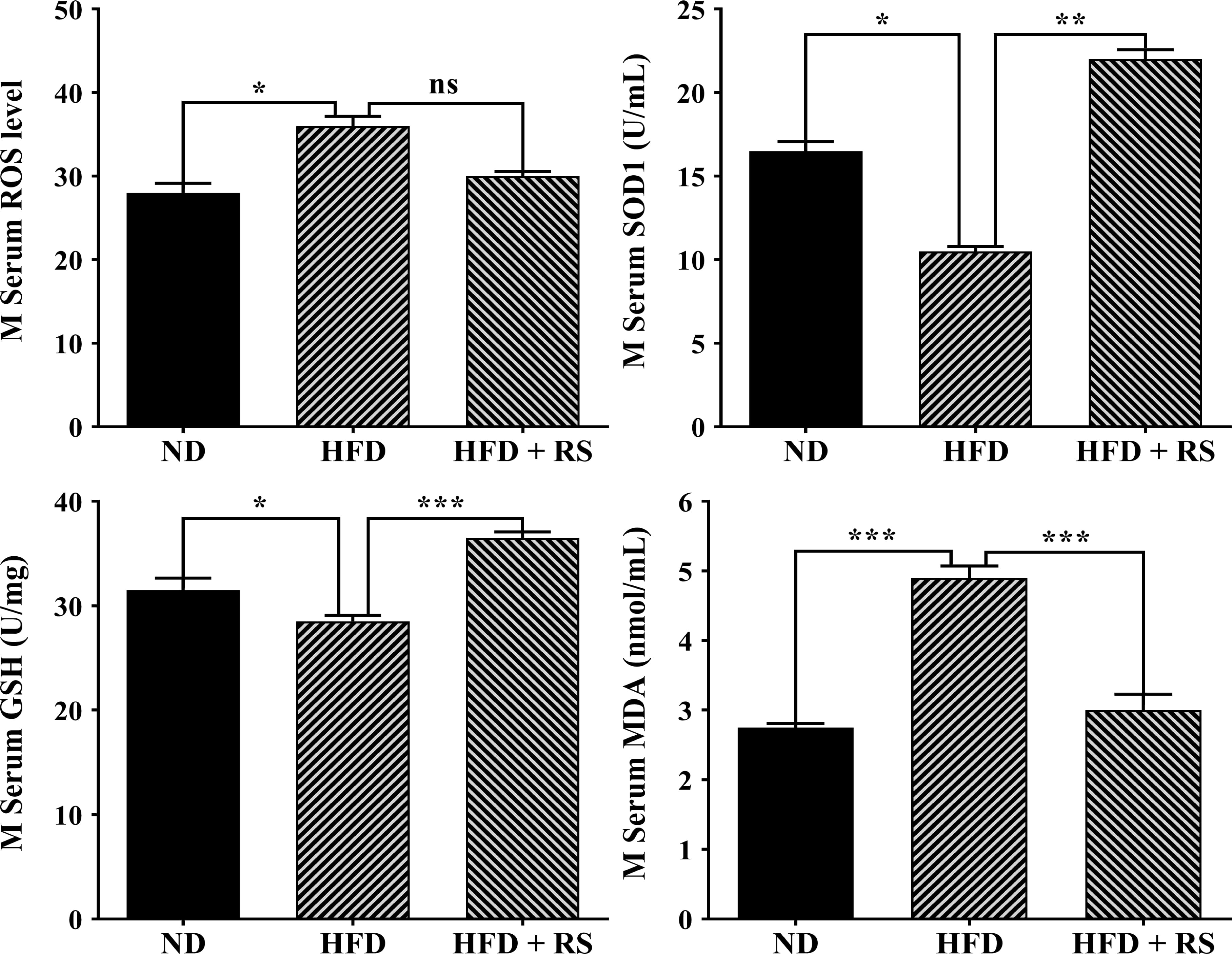 Fig. 9.
Fig. 9.RS extract mediated redox homeostasis in mouse sera. The levels
of ROS, GSH, and MDA and the SOD activity were detected in the serum of mice fed
a normal diet (ND), high-fat diet, or high-fat diet with concurrent treatment
with RS extract. Data were expressed as the mean
Growing evidence indicates that obesity can activate inflammatory signaling
pathways, such as the NF-
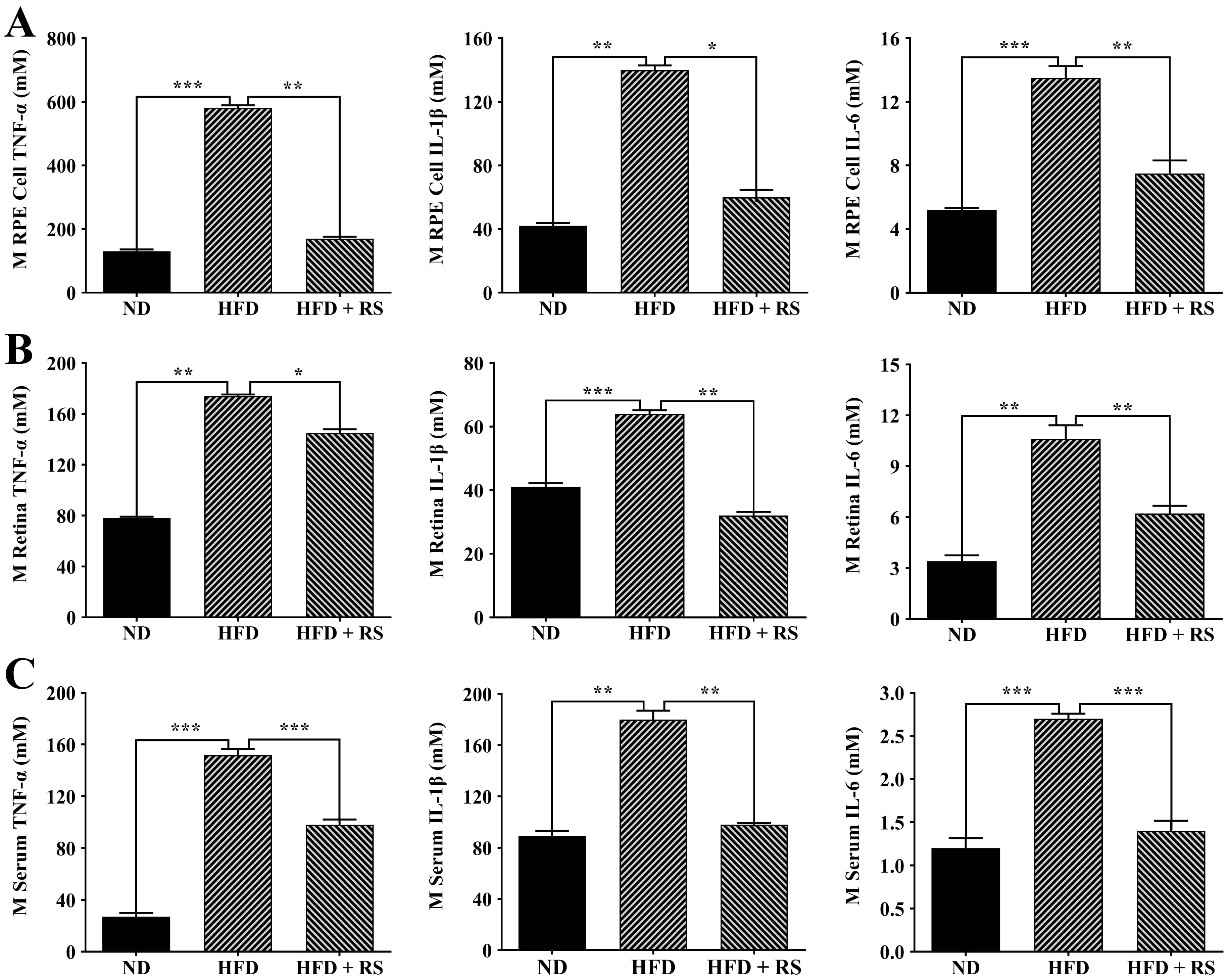 Fig. 10.
Fig. 10.Effect of treatment with RS extract on the inflammatory
cytokines TNF-
In this study, we investigated the protective effects of RS extract against
oxidative stress and inflammation in H
A recent study identified 69 compounds from RS extract, including gallic acid,
ellagic acid, rubusoside, kaempferol, rutin, and caffeic acid, possibly mediating
lipid metabolism [11]. Based on the standards, we identified five predominant
compounds: ellagic acid, rubusoside, rutin, myricetin, and hyperoside. The roles
of these compounds in cellular function and diseases have been widely
investigated. Ellagic acid (EA) has demonstrated a wide range of activities,
particularly antioxidant and anti-inflammatory activities [29]. EA contains four
hydroxyl groups that can scavenge both superoxide and hydroxyl groups. EA has
been shown to inhibit oxidative stress and inflammation in chemical-induced
neurodegenerative disorders [29]. Dietary EA inhibited inflammation and
attenuated retinal pathology in a diabetic retinopathy rat model [30]. Rubusoside
has been shown to enhance antioxidant enzyme activities, inactivate the
NF-
Obesity is associated with late AMD and is possibly involved in the progression of AMD [43]. Genome-wide association studies have shown that variants in lipid homeostasis genes (ABCA1, CETP, APOE, and LIPC) are associated with AMD [44]. High-fat/cholesterol diets also result in AMD-like retinal pathology in animals [45, 46]. Excessive lipids taken up from the intestine in a high-fat diet are stored in the liver or accumulate in peripheral tissues via blood circulation, which results in systemic and local oxidative stress and inflammation [24]. Previous investigations have demonstrated that high-fat diets caused significantly increased levels of cholesterol and glyceride in mouse liver, serum, retina, and RPE/choroid; high-fat diet-fed mice also had markedly increased levels of proinflammatory cytokines in sera, retina, and RPE/choroid [22, 23]. In the current study, a high-fat diet also had similar effects on lipids, oxidative stress, and inflammation, whereas RS extract counteracted these effects. Other researchers have reported that RS extract decreased the levels of serum cholesterol and triglycerides in rats and hamsters fed a high-fat diet [13, 15]. In fact, individual compounds identified in RS extract have been shown to mediate lipid metabolism. For example, rubusoside mediates lipid metabolism in hamsters fed a high-fat diet [47]. Recently, researchers have begun to investigate the underlying mechanisms of lipid metabolism mediation by RS extract. The prediction by Jiang et al. [11, 15] that the effects of RS extract on lipid homeostasis may be associated with 20 signaling pathways, including the PPAR/SREBP pathway, has been experimentally confirmed. The effect and molecular mechanisms of RS extract on lipid metabolism in retina and RPE/choroid require further investigation.
VEGF is a critical player in the development of wet AMD, and anti-VEGF antibodies are commonly utilized in wet AMD treatment [2]. Oxidative stress can upregulate VEGF expression in human RPE cells [48]. A high-fat diet also increases VEGF expression in retina and RPE/choroid of rodents [49, 50]. RS extract has been shown to attenuate VEGF expression in preadipocytes, with its antiangiogenic function partially dependent on one of its compounds, gallic acid [13, 51]. The other RS compounds, rutin, myricetin, and hyperoside, have also exhibited antiangiogenic activities against cancer [52, 53, 54]. Therefore, it would be worthwhile to examine the antiangiogenic activity of RS extract in a laser-induced neovascularization wet AMD mouse model [9].
In conclusion, RS extract suppressed H
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Investigation, ML, SW, YW, JZ, JC, XP, QY and ZZ; conceptualization, ZZ and ZT; writing - original drafting and revising, ML and ZZ. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The animal work of this study was approved by the Animal Ethics and Welfare Committee, Hunan University of Chinese Medicine (SYXK (Xiang) 2019-0009, approved date 10 January 2019).
Not applicable.
This work was partially supported by the Changsha platform and talent plan (KQ2203002); the Changsha Natural Science Foundation (KQ2202400); the Natural Science Foundation of Hunan Province (2021JJ30761, 2023JJ30090); the Key R&D Program of Hunan Province, China (No. 2022WK2008); the International and Regional Science and Technology Exchange Project of Hunan Association for Science and Technology (No. 2023SKX-KJ-08); and the Scientific Research Fund of Hunan Provincial Education Department (No. 22A0596, No. 22B0820).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.










