1 Department of Pharmaceutical Sciences, St. John's University, Queens, NY 11439, USA
2 Biomedical Sciences Program, St. John's University, Queens, NY 11439, USA
§Current affiliation: Department of Ophthalmology, Harvard Medical School, Boston, MA 02114, USA
∥Current affiliation: University of Massachusetts Medical School, Worcester, MA 01605, USA
Abstract
Background: Increasing or restoring Bone Morphogenetic Protein- (BMP-) signaling through administration of recombinant BMPs (rBMPs) has demonstrated therapeutic efficacy for treating bone fractures or to enhance repair following spinal surgeries. However, direct use of rBMPs has come up against significant obstacles like high cost and incidence of adverse effects. Recently, we reported our findings on the novel indolyl-benzimidazoles, SY-LB-35 and SY-LB-57, that fully activated BMP receptor signaling demonstrating activity profiles that mirrored rBMPs. Here, we explored the potential of these compounds to substitute for rBMPs in processes like wound healing and osteogenesis. Methods: Cell-based assays including cell viability, short- and long-term phosphorylation, protein expression, wound healing and bone differentiation assays were carried out in the pluripotent myoblast C2C12 cell line with select assays performed in multiple cell lines. Several assays included conditions in the presence of a selective inhibitor of type I BMP receptor, Activin-like kinase 2 (ALK2), or inhibitors of BMP-stimulated downstream signaling. All assays were repeated at least 3 times with replicates per condition where indicated. Statistical tests were carried out using Student’s two-tailed, t-test. Results: Sustained activation of non-canonical BMP signaling pathways was observed after 24-hour exposure to SY-LB-35 and SY-LB-57. Moreover, this treatment increased the expression of targets of BMP-mediated transcription such as the Id1 transcription factor. SY-LB-35 and SY-LB-57 promoted substantial increases in cell viability in three distinct cell types and increased the rate of wound closure in scrape-wounded C2C12 cell cultures. Cell viability and wound closure induced by SY-LB compounds required ALK2-, PI3K- and p38-dependent pathways. In contrast, responses to SY-LB compounds were not affected by ERK inhibition. Expression of bone differentiation markers beginning at 4 hours and evidence of calcium deposition detected after 21 days in C2C12 cell cultures exposed to SY-LB-35 and SY-LB-57 demonstrated the osteogenic potential of these compounds. Conclusions: The functional similarities between these novel compounds and rBMPs indicates that SY-LB-35 or SY-LB-57, acting as potent activators of BMP receptor signaling and inducers of osteogenic processes, could potentially replace rBMPs for treating BMP-related pathologies such as bone fracture repair or other wound healing processes.
Keywords
- BMP
- BMP receptors
- indolyl-benzimidazoles
- heterocycles
- wound healing
- bone growth
Bone Morphogenetic Proteins (BMPs) are a family of extracellular factors that were initially discovered for the ability to differentiate mesenchymal cells into osteoblasts and later induce ectopic bone formation [1]. Now, BMPs are known to play critical roles during embryogenesis and development, and also for maintenance of adult tissue homeostasis in many organ systems. Due to the widespread expression and importance as regulators throughout the body, deficiency in BMP production or functionality usually leads to marked defects or severe pathologies affecting cardiovascular and pulmonary, gastrointestinal, urinary, neurological, ophthalmic and musculoskeletal systems [2, 3]. Mouse knockout models targeting different components of BMP signaling leads to embryonic lethality or significant defects, emphasizing the essential developmental functions of BMPs [3, 4].
BMPs stimulate intracellular signaling in a variety of cell types such as mesenchymal cells, bone marrow stromal cells, monocytes and sensory spinal interneurons [5, 6, 7]. These proteins control important cellular processes like proliferation, differentiation, chemotaxis, axon guidance and apoptosis [8, 9, 10]. BMP dimers elicit cellular responses by simultaneously binding pairs of type I and type II serine-threonine kinase transmembrane receptors [11, 12, 13]. Ligand binding triggers assembly of receptor pairs into an active tetrameric complex [13]. In the absence of ligand stimulation, small fractions of type I and type II BMP receptors are present as pre-existing homodimers and heterodimers on the cell surface. Binding of ligand increases oligomerization of the receptors, which may induce conformational changes of the receptor molecules [14, 15]. Moreover, the composition of the tetrameric complex may impart specificity in signaling outcomes [10, 15, 16, 17].
Following activation of the tetrameric BMP receptor complex, type I BMP receptors phosphorylate downstream, pathway restricted Smads (R-Smads – Smad1, Smad5, and Smad8), which are important mediators of BMP transcriptional responses [18, 19, 20]. Phosphorylated R-Smads (p-Smads) dissociate from the receptors and form a complex with Smad4. In the nucleus, the R-Smad/Smad4 complex binds to BMP-responsive DNA elements and regulates gene expression [21, 22, 23]. BMPs also activate non-Smad intracellular signaling including p38 and Extracellular-regulated kinase (ERK) pathways, which are also responsible for regulation of gene transcription and known to play an important role in BMP-induced osteogenesis [24, 25, 26].
Because of its diverse functions and osteogenic potential, the Food and Drug Administration (FDA) approved use of human recombinant BMP2 (rBMP2) during spinal fusion and maxillary sinus reconstructive surgeries and for tibial shaft repair [27, 28]. Although stimulation of BMP receptor-dependent pathways has been shown to be beneficial, the clinical use of rBMPs poses significant obstacles [28, 29]. For example, the high concentrations of rBMP2 required to regenerate or repair bone leads to translational barriers due to high formulation costs. Moreover, clinical use of rBMPs requires potentially harmful doses to achieve efficacy and penetration of proteinaceous rBMP-based therapeutics is poor [27, 28, 29]. Thus, alternative strategies are needed to exploit the beneficial effects of BMP signaling. The cost-effective production of small molecule activators of BMP pathways, which can be formulated in large quantities with minimal difficulty and efficiently targeted to diseased or injured areas, would constitute a substantial advance in the BMP field. Such small molecules could replace rBMPs for the treatment of bone injuries or other BMP-related pathological conditions.
Our lab has recently demonstrated that select members of a novel series of small
synthetic benzimidazole compounds robustly stimulate BMP signaling and act as BMP
receptor agonists [30]. The current study extends our initial investigation of
the indolyl-benzimidazole small molecules, SY-LB-35 and SY-LB-57. Assessment of
cell viability in multiple cell types demonstrated a common cellular response to
the SY-LB compounds. In C2C12 cells, cell viability responses to SY-LB-35 and
SY-LB-57 were blocked by a selective inhibitor of Activin-like kinase (ALK2), a
type I BMP receptor subunit, as well as inhibitors to Phosphatidyl inositol
3-kinase (PI3K)- and p-38-dependent pathways, but not by an inhibitor to ERK
signaling. Amplification of cell viability and activation of non-canonical BMP
signaling were detected at picomolar concentrations of SY-LB-35 and SY-LB-57.
Interestingly, Smad phosphorylation is undetectable at these low concentrations.
In response to SY-LB compounds, Western blots showed sustained activation of p38
and ERK pathways, as well as increases in the expression of BMP-regulated
proteins, Id1 and BMP receptor type 2 (BMPR2). Wound healing assays revealed that
wound closure promoted by SY-LB compounds was dependent upon ALK2, PI3K and p38
but not ERK activity. With respect to osteogenesis, bone-related gene products,
such as
SY-LB-35 and SY-LB-57 are indolyl-benzimidazoles that were synthesized and purified by HPLC by Dr. Leonard Barasa in the laboratory of Dr. Sabesan Yoganathan at St. John’s University (Queens, NY, USA). Details of SY-LB synthesis, purification and characterization were previously reported [30, 31].
C2C12 mouse myoblast cells (CRL-1772), WEHI 274.1 (WEHI) cells (CRL-1702), and
primary Pulmonary Artery Endothelial Cells (PAECs) (PCS-100-022) were obtained
from American Type Culture Collection (ATCC®) (Manassas, VA,
USA). All three cell lines were certified to be free of mycoplasma contamination
by using the MycoStrip testing kit (#rep-mys-10) from InvivoGen (San Diego, CA,
USA) for C2C12 cells or by DAPI staining of fixed WEHI cells and PAECs. The
authenticity of the C2C12 cell line was established using ATCC Mouse Cell
Authentication Services in accordance with the Consortium for Mouse Cell Line
Authentication. Moreover, the C2C12 and WEHI 274.1 cell lines do not appear as
cross-contaminated or misidentified in either the Cell Line Authentication
Committee or the ExPASy database. Dulbecco’s Modified Eagle’s Medium (DMEM),
Penicillin/Streptomycin/Glutamine solution (PSG), Penicillin/Streptomycin
solution (PS), 0.25% Trypsin-EDTA, Ca
Cell Lysis Buffer (10
Recombinant BMP2 was purchased from R&D Systems (Minneapolis, MN, USA). The RealTime-Glo™ MT Cell Viability Assay Kit was purchased from Promega Corporation (Madison, WI, USA). PD98059 (PD), LDN193189 dihydrochloride (LDN), and SB202190 (SB) were purchased from Tocris Biosciences (Bristol, UK). LY294002 (LY) was obtained from Cell Signaling Technology (Danvers, MA, USA) and Mitomycin C (MC) was from Cayman Chemical Company (Ann Arbor, MI, USA). Recombinant mouse ALP and mouse OCN ELISA kits were obtained from Novus Biologicals (Centennial, CO, USA). p-N-Phenyl-Phosphate Substrate (p-NPP) was purchased from Life Technologies (Fredrick, MD, USA). Alizarin Red S was procured from Sigma Aldrich (St. Louis, MO, USA).
Phospho-Smad1/5(S463/465)/9(S465/467) rabbit monoclonal antibody (mAb)
(#13820S), Smad1 (D5907) XP® rabbit mAb (#6944), phospho-Akt
(Ser473) (D9E) XP® rabbit mAb (#4060), Akt (pan) (C67E7) rabbit
mAb (#4691),
Horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (#sc-2004) was obtained from Santa Cruz Biotechnology Inc. (Dallas, TX, USA). HRP-conjugated goat anti-mouse IgG (#115035068) was obtained from Jackson ImmunoResearch Laboratories (West Grove, PA, USA).
C2C12 and WEHI cells were cultured in complete growth medium (DMEM/10% FBS/1X
PSG) incubated at 37 °C in 5% CO
C2C12 cells (100 µL at 5
Cell viability of the treated cultures was obtained by using the RealTime-Glo™ MT Cell Viability Assay Kit to detect cellular luminescence with a FilterMax F5 Multi-mode Microplate Reader (Molecular Devices, San Jose, CA, USA) as previously described [30].
Cell treatments, whole cell lysate preparation and quantification of total
cellular protein were carried out as previously described [30]. Briefly, C2C12
cells (3 mL at 7.5
A detailed method for Western blotting was previously reported [30]. Briefly,
samples of whole cell lysates (20 µg) were separated on 12% TGX
Fast Cast Acrylamide gels and transferred to nitrocellulose membranes. Membranes
were blocked with 5% BSA/0.1% Tween 20/ 1
In 35 mm culture dishes, C2C12 cells (3 mL) were seeded at 7
C2C12 cells were seeded in 24-well tissue culture plates at 7
At 24-, 48- and 72-hours following treatment, 200 µL culture medium
from each well of the assay was transferred to a fresh 48-well plate. Low serum
media (200 µL) was added back to each culture to replace the
collected media. Next, an equal volume of the substrate, p-N-Phenyl-Phosphate
(200 µL p-NPP, 10 mM) prepared in Assay Buffer (2.7 g
2-Amino-2-methyl-1,3-propanediol/50 µM MgCl
At the 72-hour time point, the cultures were washed with D-PBS and trypsinized
as described above. Pelleted cells were resuspended in 500 µL D-PBS
and cell concentration was determined. A sample of 5
C2C12 cells were seeded in 35-mm dishes at 7
Duplicate solutions of OCN protein (0, 0.39, 0.78, 1.56, 3.13, 6.25, 12.5, 25 ng/mL) were used to generate a standard curve. Samples of supernatants (100 µL/ well) were transferred to the designated wells of the assay plate and the ELISA was carried out according to instructions from the manufacturer. The optical density was measured at 450 nm.
C2C12 cells were seeded in 35 mm dishes at 7
C2C12 cells were seeded at 7
Phase contrast and bright field images (20
Significance is defined as p
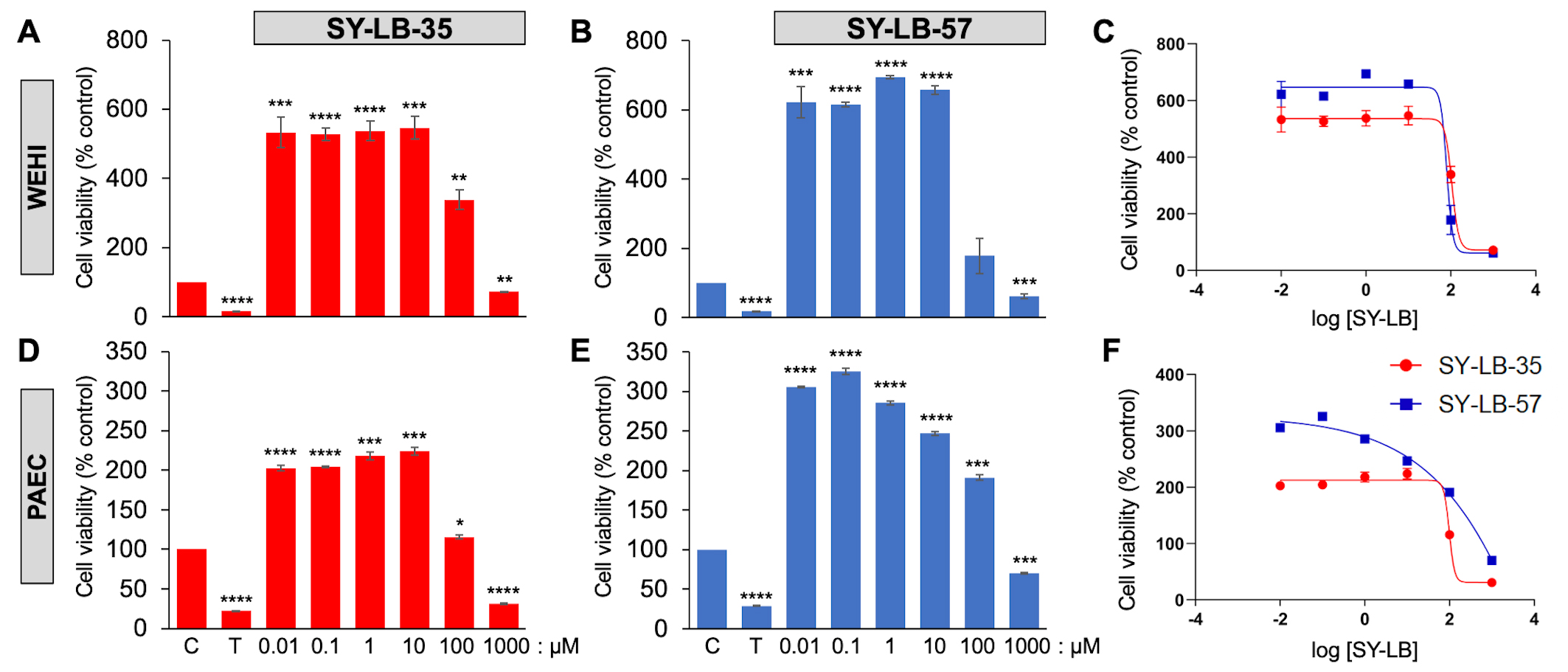
Three distinct cell types were evaluated for cell viability responses in the presence of the two benzimidazole small molecules. The C2C12 mouse myoblast cell line has been commonly used to evaluate BMP-induced responses and demonstrate robust canonical and non-canonical pathway activation evoked by rBMPs [32, 33]. Indeed, our group recently demonstrated large increases in cell viability in C2C12 cells in response to various rBMPs, including BMP2 [30]. WEHI suspension cells were originally shown to respond to a gradient of BMP2 or BMP7 in transwell chemotaxis assays [34, 35]. Later, WEHI cells were demonstrated to require select subunits of type II BMP receptors for BMP7-induced chemotaxis and growth cone collapse [36, 37]. The primary cells derived from murine pulmonary arteries (PAECs) have been targeted in a search for a cure for pulmonary arterial hypertension (PAH), since defects in BMP receptor-dependent signaling are a major cause of PAH [38]. The increases in cell viability induced by 0.01 µM to 1000 µM SY-LB-35 and SY-LB-57 in C2C12 cells were previously reported [30] and are reproduced in Supplementary Table 1. To determine if these effects were a common cellular response to these novel indolyl-benzimidazoles, WEHI cells and primary PAECs were serum-starved and exposed to 0.01 µM to 1000 µM SY-LB-35 or SY-LB-57 for 24 hours. Triton X-100 (125 µM) served as a toxic control.
In WEHI cells, cell viability response to 0.01 µM to 100
µM SY-LB-35 was significantly increased compared with control,
untreated WEHI cell cultures (Fig. 1A,C and Supplementary Table 2). In
contrast, treatment with 1000 µM SY-LB-35 caused a substantial
decrease in WEHI cell viability (Fig. 1A,C and Supplementary Table 2).
Cell viability in WEHI cells in response to 0.01 µM to 10
µM SY-LB-57 was significantly increased compared with control,
untreated cultures, however, the response to 100 µM SY-LB-57 was not
different from control (Fig. 1B,C and Supplementary Table 2). A
significant decrease in WEHI cell viability was detected in cultures exposed to
1000 µM compared with control, untreated cultures (Fig. 1B,C and
Supplementary Table 2). The IC
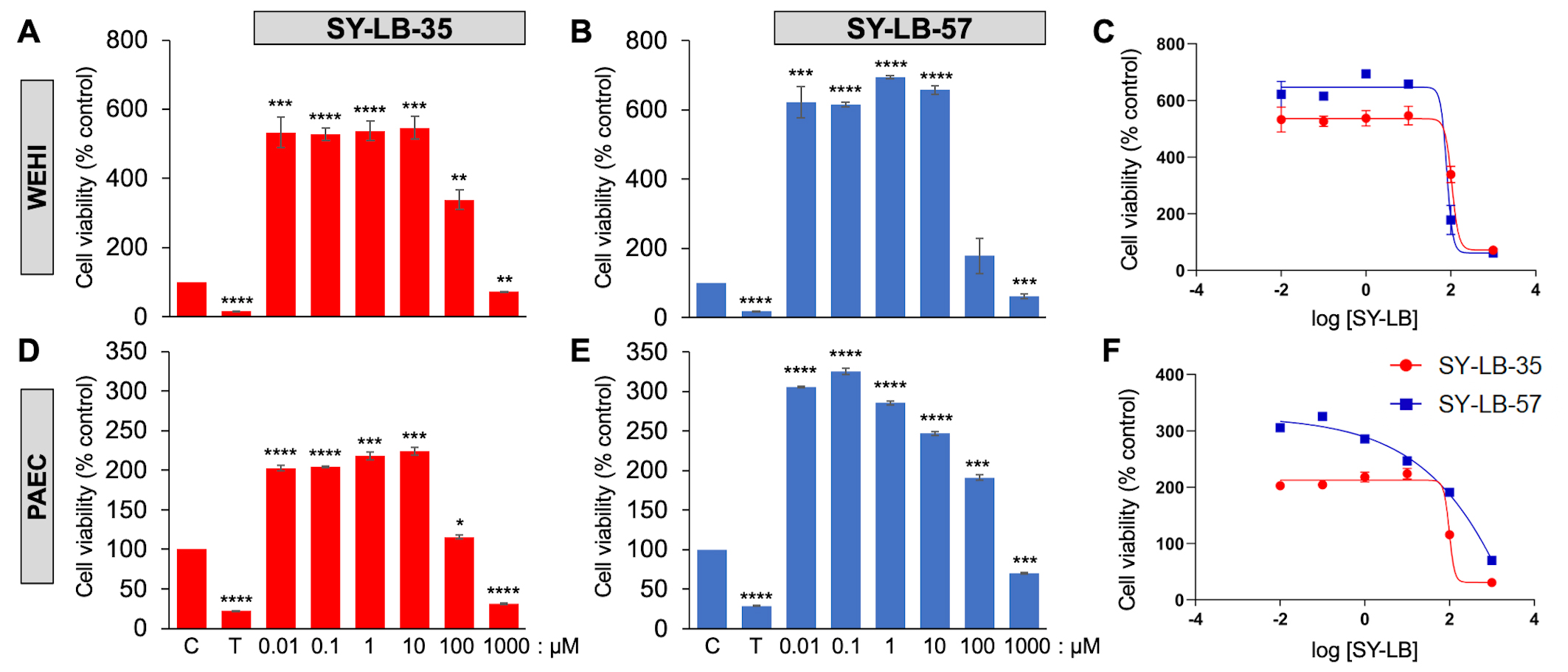 Fig. 1.
Fig. 1.SY-LBs stimulate robust increases in WEHI cell viability and in
Pulmonary Artery Endothelial Cells (PAECs). The viability of WEHI cells (A,B,C) and PAECs (D,E,F) following treatment
of cultures for 24 hours with the indicated concentrations of (A,D) SY-LB-35 and
(B,E) SY-LB-57. Triton X-100 (T, 125 µM) served as a negative
control. SY-LB compounds at 1000 µM strongly reduced cell viability
when compared to control (C), untreated cells (****p
SY-LB-35 and SY-LB-57 induced significant changes in cell viability in primary
PAECs after 24 hours at every concentration tested between 0.01–1000
µM. Cell viability after exposing PAECs to SY-LB-35 was
significantly increased in response to 0.01 µM to 100
µM SY-LB-35, (Fig. 1D,F and Supplementary Table 3).
Treatment with 1000 µM SY-LB-35 substantially decreased PAEC cell
viability (Fig. 1D,F and Supplementary Table 3). Similarly, significant
increases in PAEC cell viability were detected in response to 0.01
µM to 100 µM SY-LB-57 (Fig. 1E,F and
Supplementary Table 3) and a significant decrease in PAEC viability was
measured in cultures exposed to 1000 µM SY-LB-57 (Fig. 1E,F and
Supplementary Table 3). The IC
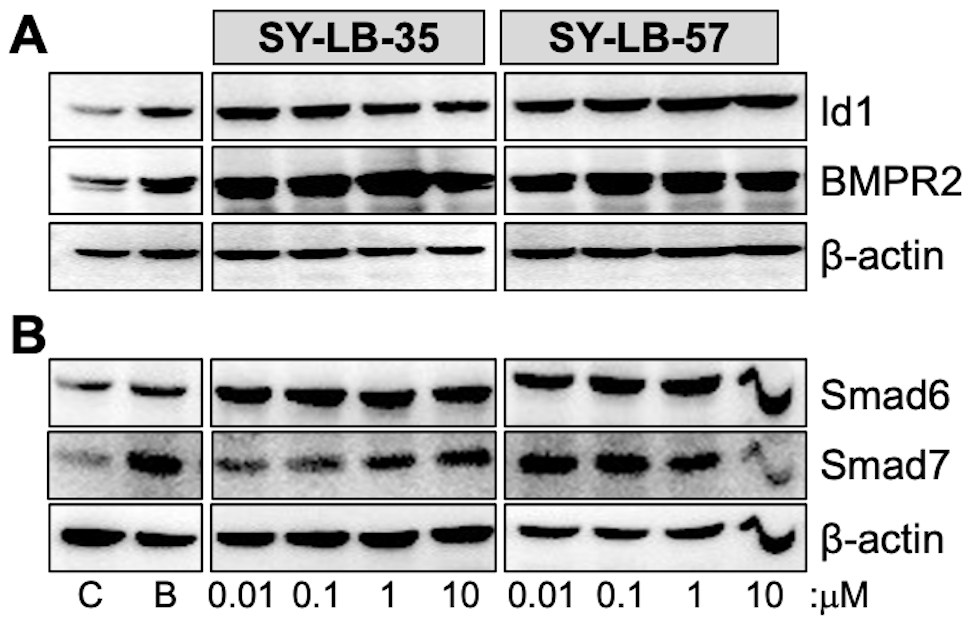
If stimulation with SY-LB-35 and SY-LB-57 induce such significant changes to
cell viability in 24 hours, then these BMP-like compounds would be expected to
promote the expression of major BMP-regulated gene products like the Id1
transcription factor, the type II BMP receptor, BMP receptor type 2 (BMPR2), and
the inhibitory Smads, Smad6 and Smad7 [39, 40, 41]. To determine if exposure to the
SY-LB compounds results in the expected upregulation of Id1, BMPR2, Smad6 and
Smad7, C2C12 cell cultures were serum-starved, then treated with BMP2 (50 ng/mL)
and 0.01 µM to 10 µM SY-LB-35 or SY-LB-57 for 24 hours.
Western blot analysis of whole cell lysates from stimulated cultures demonstrated
strong increases in Id1 and BMPR2 expression in response to BMP2 and the SY-LB
compounds relative to
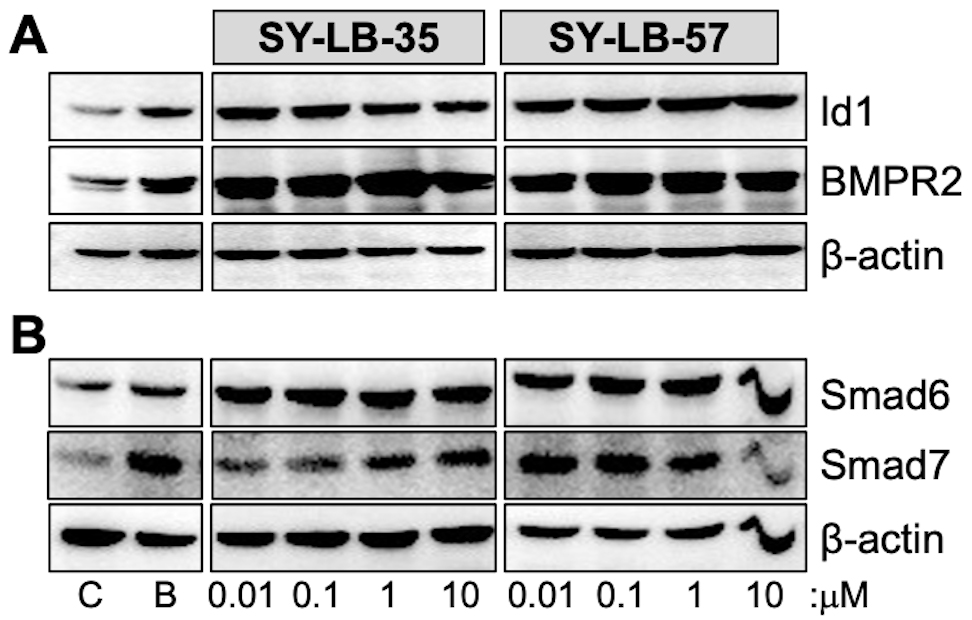 Fig. 2.
Fig. 2.Major targets of BMP-mediated transcription are upregulated by
SY-LB-35 and SY-LB-57. Serum-starved C2C12 cell cultures were stimulated with 50
ng/mL BMP2 (B), unsupplemented medium (C), SY-LB-35 (0.01–10 µM) or
SY-LB-57 (0.01–10 µM) for 24 hours. (A,B) Whole cell lysates (20
µg) were analyzed by Western blot by first probing the membranes
with antibodies that recognize (A) Id1 or (B) Smad6. The membranes were gently
stripped and re-probed with antibodies against (A) BMPR2 or (B) Smad7,
respectively. The membranes were stripped a second time and probed with
anti-
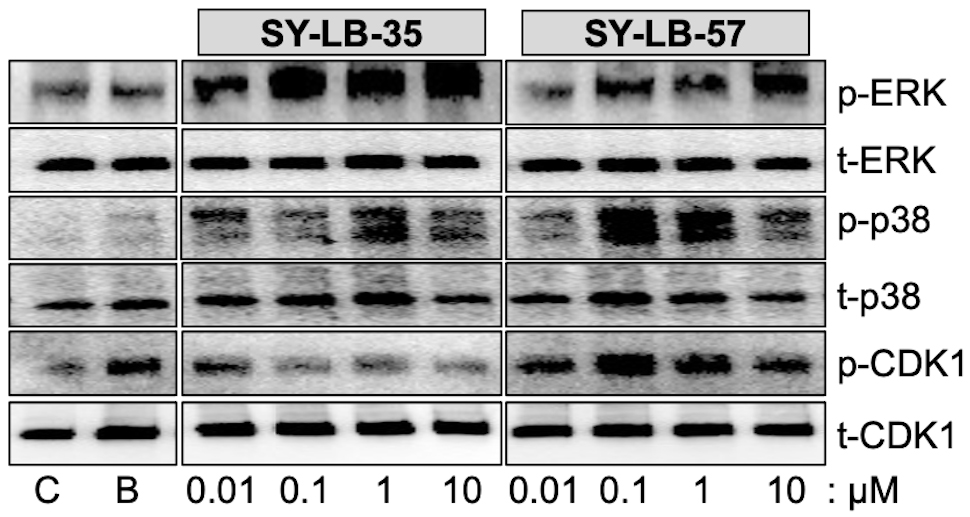
To determine if non-canonical BMP signaling pathways remain activated after 24-hour exposure to the SY-LB compounds. C2C12 cell cultures were serum-starved, then stimulated with the positive control (BMP2) or 0.01 µM to 10 µM SY-LB-35 or SY-LB-57. Whole cell lysates were analyzed by Western blot using phospho-specific and pan antibodies against p38, ERK and Cyclin-Dependent Kinase1 (CDK1). The SY-LB compounds strongly increased phospho-ERK (p-ERK) and p-p38 levels at all concentrations analyzed relative to total ERK and p38 expression levels, respectively (Fig. 3). While the enhancing effect of SY-LB-35 on p-CDK1 levels tended to decrease over time, p-CDK1 levels remained significantly higher than control after 24 hours (Fig. 3). In contrast, SY-LB-57 maintained a robust, concentration-dependent stimulatory effect on p-CDK1 levels at 24 hours at all concentrations tested (Fig. 3).
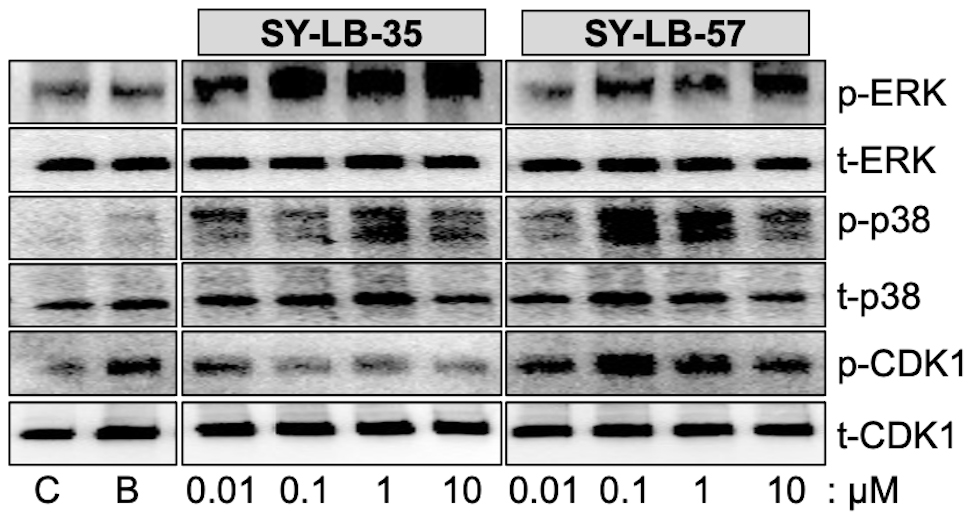 Fig. 3.
Fig. 3.SY-LB-35 and SY-LB-57 promote long-term increases in non-canonical BMP signaling pathways. Serum-starved C2C12 cell cultures were exposed to 50 ng/mL BMP2 (B) as a positive control, unsupplemented medium as a negative control (C), SY-LB-35 (0.01–10 µM) or SY-LB-57 (0.01–10 µM) for 24 hours. Whole cell lysates (20 µg) were analyzed by Western blot by first probing the membranes with phospho-specific antibodies to ERK (p-ERK), p38 (p-p38) and CDK1 (p-CDK1). The membranes were stripped and re-probed with antibodies to total cellular ERK, p38 and CDK1. Representative Western blots are shown. Full quantitative analysis of the experiment (n = 3) is reported in Supplementary Fig. 1 and Supplementary Tables 4,5,6.
Western blots from three independent experiments for each set of antibodies were
quantified and demonstrated significant increases in p-p38, p-ERK and p-CDK1 in
response to 0.01 µM to 10 µM SY-LB-35 and SY-LB-57
compared with control, untreated cultures (Supplementary Fig. 1).
Normalized results are expressed as a percentage of control, untreated cultures
(mean
Comparison of phosphorylation in response to the SY-LB compounds at 15 minutes and 24 hours revealed that levels of p-p38 are significantly greater at 24 hours than at 15 minutes (Supplementary Fig. 1A,B and Supplementary Table 4), while the profiles for p-ERK responding to SY-LB-35 and SY-LB-57 did not display such a difference (Supplementary Fig. 1C,D and Supplementary Table 5). Instead, the p-ERK profiles stimulated by both SY-LB compounds exhibited a concentration-dependent increase in ERK phosphorylation. Moreover, the percentage increases measured at 15 minutes and 24 hours in SY-LB-stimulated p38 phosphorylation at 24 hours were significantly elevated compared with 24-hour p-ERK responses (Supplementary Fig. 1A–D and Supplementary Tables 4,5).
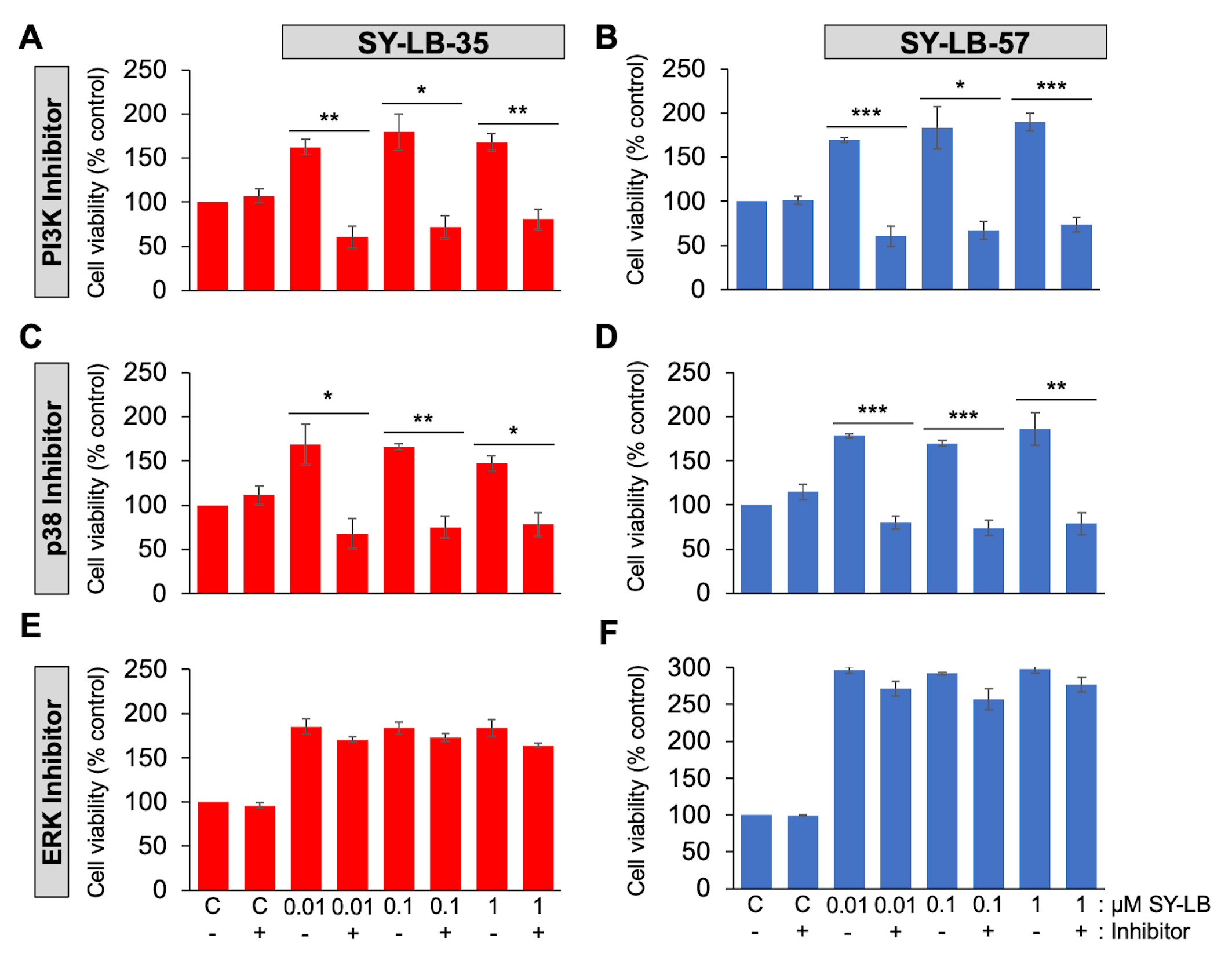
To examine the contribution of the PI3K/Akt, p38 and ERK signaling pathways on SY-LB-induced activity, cell viability assays were conducted in primary PAECs in the absence and presence of an inhibitor of PI3K (LY294002, 15 µM), p38 (SB202190, 10 µM) or ERK (PD98059, 5 µM) activity. In serum-starved PAECs stimulated for 24 hours with 0.01 µM, 0.1 µM and 1 µM SY-LB-35 or SY-LB-57, inhibition of PI3K (Fig. 4A,B) or p38 (Fig. 4C,D) signaling completely blocked SY-LB-induced increases in cell viability. In contrast, inhibition of ERK activity had no effect on the responses to SY-LB-35 or SY-LB-57 in C2C12 cell cultures (Fig. 4E,F).
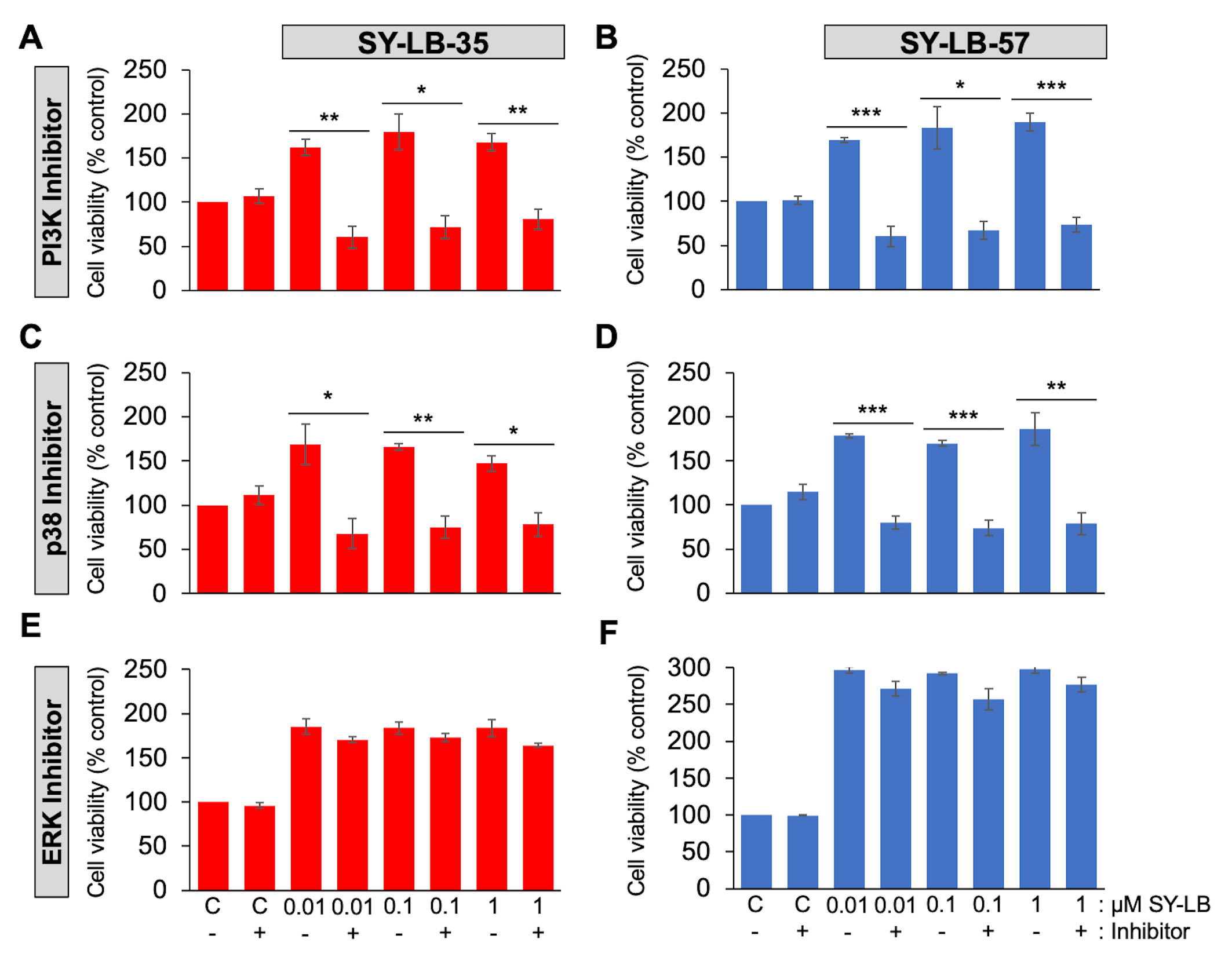 Fig. 4.
Fig. 4.Cell viability increases evoked by SY-LB-35 and SY-LB-57 are
blocked by inhibitors to PI3K- and p38-dependent signaling but not to
ERK-dependent pathways. (A–D) Cell viability in PAECs or (E,F) C2C12 cells
following treatment of cultures for 24 hours with 0.01 µM to 1
µM (A,C,E) SY-LB-35 or (B,D,F) SY-LB-57 in the absence or presence
of inhibitors to the (A, B) PI3K pathway (15 µM LY294002), (C,D) the
p38 MAPK pathway (10 µM SB202190) or (E,F) the ERK MAPK pathway (5
µM PD98059). Cultures treated with unsupplemented medium (C-) or
inhibitor alone (C+) served as controls. Cell viability increases stimulated by
SY-LBs were completely blocked by PI3K inhibition and a p38 pathway inhibitor at
every concentration tested compared with the respective control sample. The ERK
inhibitor had no effect on SY-LB-stimulated increases in cell viability. (LY(-)
vs. LY(+), SY-LB-35: 0.01 µM, 162% vs. 60%, **p = 0.0025; 0.1 µM, 179% vs. 72%, *p = 0.0110; 1
µM, 168% vs. 81%, **p
= 0.0044; SY-LB-57: 0.01 µM, 169% vs. 60%, ***p = 0.00076; 0.1 µM, 183%
vs. 67%, *p = 0.0115; 1 µM, 190% vs. 74%, ***p = 0.00083. Control
vs. LY, SY-LB-35: 100% vs. 107%, p =
0.4567; SY-LB-57: 100% vs. 102%, p = 0.7444)
(SB(-) vs. SB(+), SY-LB-35: 0.01 µM, 169%
vs. 68%, *p = 0.0237; 0.1 µM, 166% vs. 75%, **p = 0.0022; 1
µM, 147% vs. 78%, *p
= 0.0124; SY-LB-57,: 0.01 µM, 178% vs. 80%, ***p = 0.00021; 0.1 µM, 170%
vs. 74%, ***p = 0.00046; 1 µM,
186% vs. 79%, **p = 0.0086. Control
vs. SB, SY-LB-35: 100% vs. 111%, p =
0.3469; SY-LB-57: 100% vs. 115%, p = 0.1634).
(PD(-) vs. PD(+), SY-LB-35: 0.01 µM, 185%
vs. 170%, p = 0.1865; 0.1 µM, 184% vs. 173%, p = 0.2482; 1
µM, 184% vs. 164%, p
= 0.1148; SY-LB-57: 0.01 µM, 297% vs. 271%, p = 0.0730; 0.1 µM, 292%
vs. 257%, p = 0.0695: 1 µM, 298% vs. 277%, p = 0.1349. Control
vs. PD, SY-LB-35: 100% vs. 96%, p =
0.2268; SY-LB-57: 100% vs. 99%, p = 0.5046).
Cell viability is expressed as the mean
PI3K and p38 inhibitors caused significant inhibition of 0.01 µM to 1 µM SY-LB-35- and SY-LB-57-stimulated responses in C2C12 cells compared to cell viability increases in the absence of these inhibitors (Fig. 4A–D). For ERK inhibition, no significant differences were detected in SY-LB-induced cell viability responses in absence or presence of PD98059 at any concentration tested (Fig. 4E,F). There was no difference in cell viability between control cultures and cultures treated with 15 µM LY294002, 10 µM SB203190 or 5 µM PD98059 alone (Fig. 4). Decreases in BMP-induced phosphorylation of Akt, p-38 and ERK in the presence of LY294002, SB202190 and PD98059, respectively, were observed ensuring that the inhibitors were active (Supplementary Fig. 2).
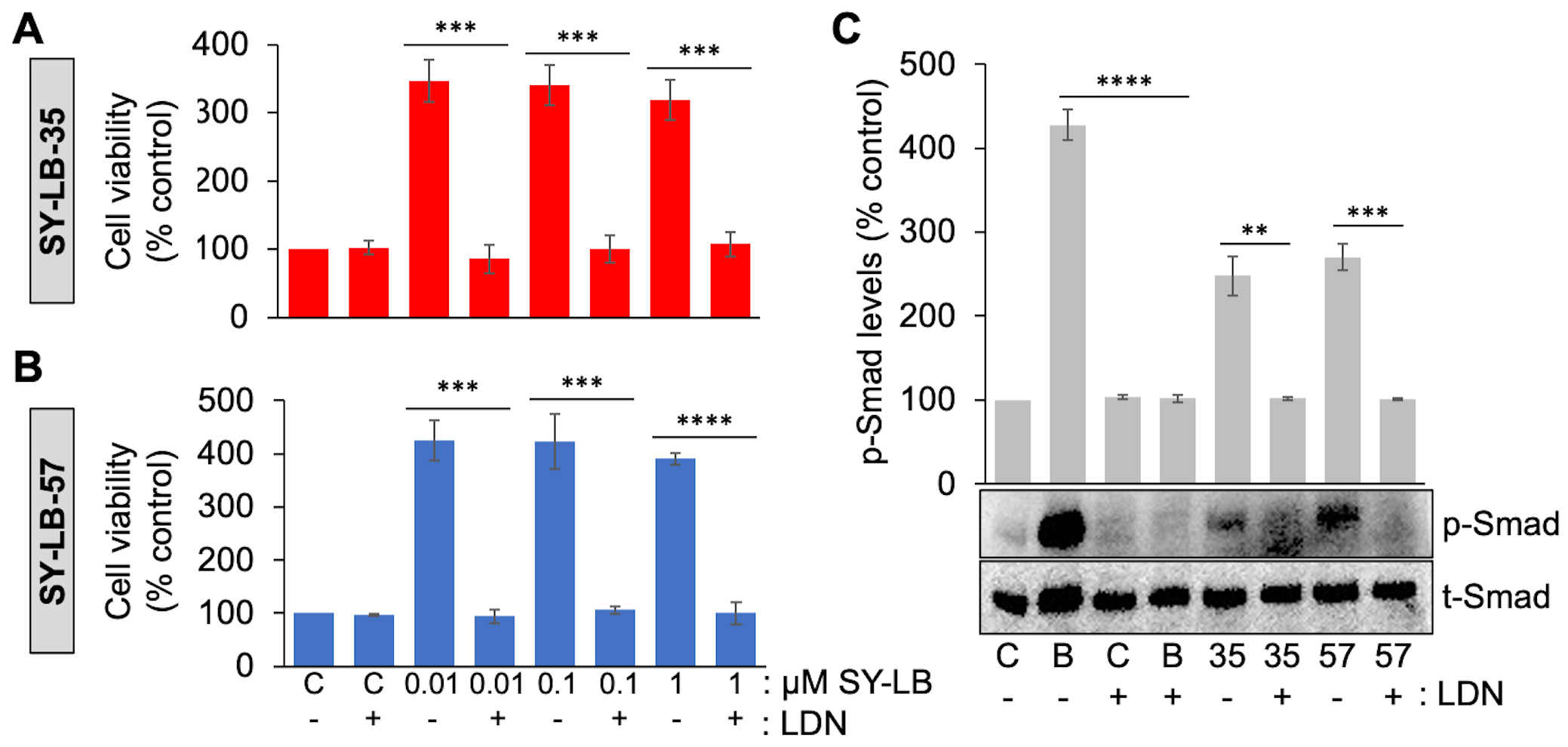
SY-LB-35- and SY-LB-57-induced cell viability increases, and Smad phosphorylation were previously shown to depend on the activity of type I BMP receptors using Dorsomorphin [30]. However, Dorsomorphin is a pan type I BMP receptor inhibitor and more selective inhibitors have since been developed including LDN193189, which selectively inhibits the type I BMP receptor subunit, ALK2 [35]. To investigate whether the SY-LB compounds stimulate increases in WEHI cell viability through a mechanism dependent upon ALK2 activity, serum-starved WEHI cell cultures were exposed to 0.01 µM, 0.1 µM or 1 µM SY-LB-35 or SY-LB-57 for 24 hours in the absence or presence of 5 µM LDN193189 (Fig. 5A,B, respectively). After 24 hours, WEHI cell responses evoked by the SY-LB compounds were completely inhibited by the presence of LDN193189 at all concentrations tested (Fig. 5A,B).
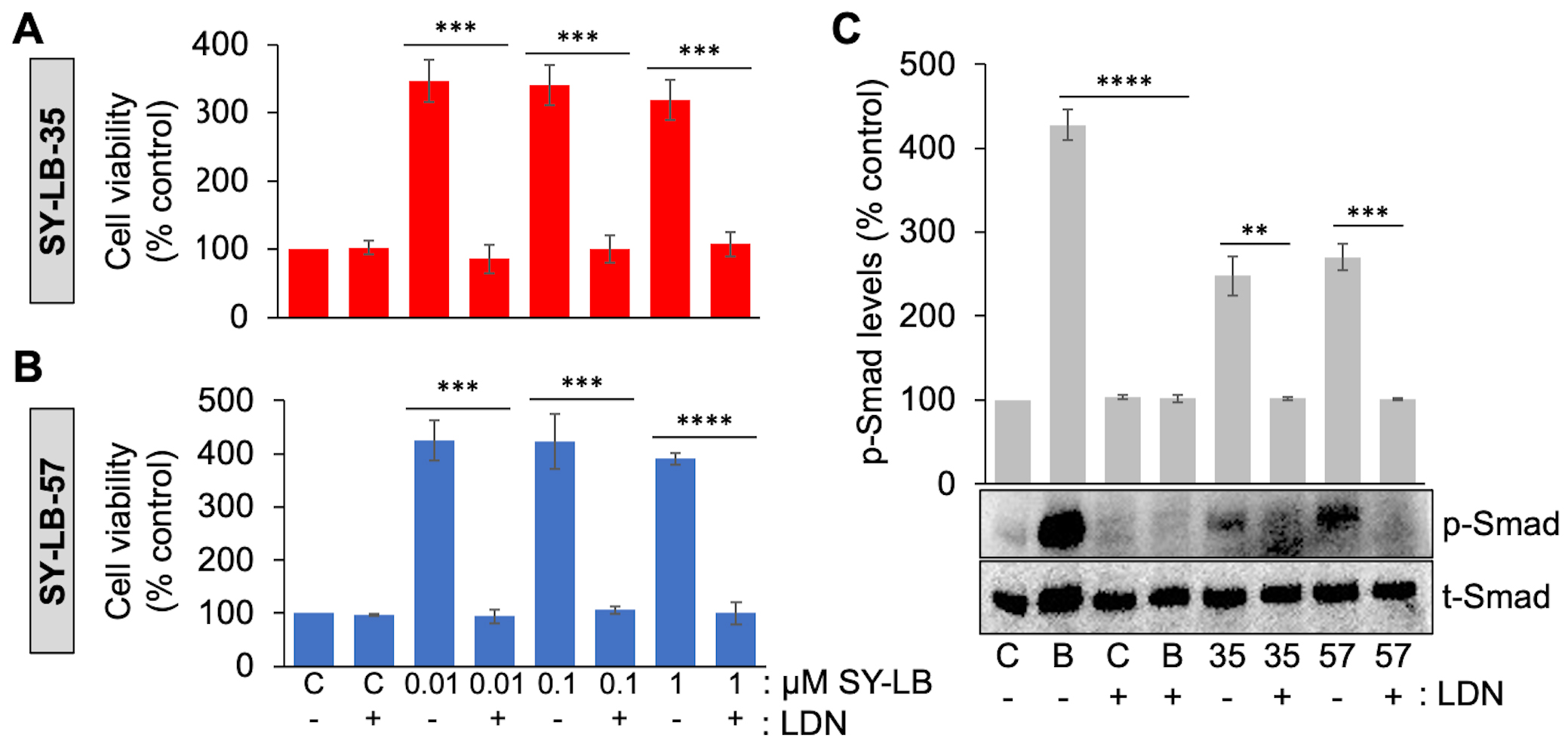 Fig. 5.
Fig. 5.Increases in cell viability and Smad phosphorylation levels
depend on ALK2 activity. (A, B) Cell viability in WEHI cells following treatment
of cultures for 24 hours with 0.01 µM to 1 µM (A)
SY-LB-35 or (B) SY-LB-57 in the absence or presence of an inhibitor of ALK2
activity (5 µM LDN193189 (LDN)). Cultures treated with
unsupplemented medium (C-) or inhibitor (C+) alone served as controls. Cell
viability increases stimulated by SY-LB-35 or SY-LB-57 were completely blocked by
the ALK2 inhibitor at every concentration tested compared with the respective
control sample. LDN(-) vs. LDN(+), SY-LB-35: 0.01 µM, 347% vs. 85%, ***p = 0.00028; 0.1
µM, 340% vs. 85%, ***p
= 0.00031; 1 µM: 319% vs. 85%,
***p = 0.0004; SY-LB-57: 0.01 µM, 425%
vs. 94%, ***p = 0.00015; 0.1 µM,
423% vs. 106%, ***p = 0.00046; 1
µM, 359% vs. 97%,
****p = 2.69
To demonstrate the activity of the ALK2 inhibitor and to confirm the dependence
of SY-LB-35 and SY-LB-57 on ALK2 activity specifically, C2C12 cells were starved,
pre-treated with 5 µM LDN193189 and exposed to BMP2 (50 ng/mL) or
the SY-LB compounds at 1 µM for 30 minutes. C2C12 whole cell lysates
were analyzed by Western blot using antibodies against p-Smad and total Smad
(t-Smad) (Fig. 5C). Levels of p-Smad normalized to t-Smad expression in the
absence and presence of LDN193189 is reported as the mean
The endogenous, extracellular BMP antagonist, Noggin, binds BMPs with high affinities and has a marked preference for BMP2 and BMP4 over BMP7 [42, 43]. The Noggin/BMP interaction prevents BMPs from binding to cell surface receptors, thus serves to regulate active BMP levels and, consequentially, BMP receptor signaling [43]. To determine whether SY-LB-35 or SY-LB-57 are antagonized by Noggin, C2C12 cells were starved and pre-treated for 1 hour with recombinant Noggin (400 ng/mL), followed by treatment with the positive control, BMP2 at 50 ng/mL and 1 µM SY-LB-35 or SY-LB-57 for 30 minutes. Western blot analysis of p-Smad and t-Smad levels in C2C12 whole cell lysates reveals that in the absence of Noggin, Smad phosphorylation levels stimulated by SY-LB-35 and SY-LB-57 at 1 µM were increased significantly compared with control, untreated cells (Supplementary Fig. 3A). The p-Smad responses to BMP2, SY-LB-35 and SY-LB-57 were eliminated by pre-treatment with Noggin (Supplementary Fig. 3A).
To determine whether concentrations greater than 1 µM SY-LB-35 or SY-LB-57 can overcome the blockade of Smad signaling by Noggin, C2C12 cells were starved and exposed to SY-LB compounds at 10 µM, 100 µM and 1000 µM in absence or presence of 400 ng/mL Noggin for 30 minutes. Treating C2C12 cells with SY-LB-35 or SY-LB-57 at concentrations greater than 10 µM can overcome the antagonism of Smad phosphorylation by Noggin (Supplementary Fig. 3B,C, respectively).
To investigate potential crosstalk between the canonical and non-canonical BMP
signaling pathways, RNA interference experiments were carried out to knockdown
the level of Smad4 as a vital player of the canonical pathway. C2C12 cells were
transfected with empty vector as a negative control or a lenti-viral shRNA
plasmid targeting Smad4 and incubated for 48 hours [36]. Down-regulation of Smad4
expression relative to
Smad4 shRNA-transfected C2C12 cell lysates were stimulated with 50 ng/mL BMP2 or 1 µM SY-LB-35 and probed for Smad6 and Smad7 expression (Supplementary Fig. 4C). In unstimulated cultures, a significant increase in Smad6 and Smad7 expression levels was detected in response to Smad4 shRNA transfection alone (Supplementary Fig. 4C), which was significantly amplified by 30-minute exposure to BMP2 or SY-LB-35, compared with control, untransfected, untreated C2C12 cell cultures (Supplementary Fig. 4C).
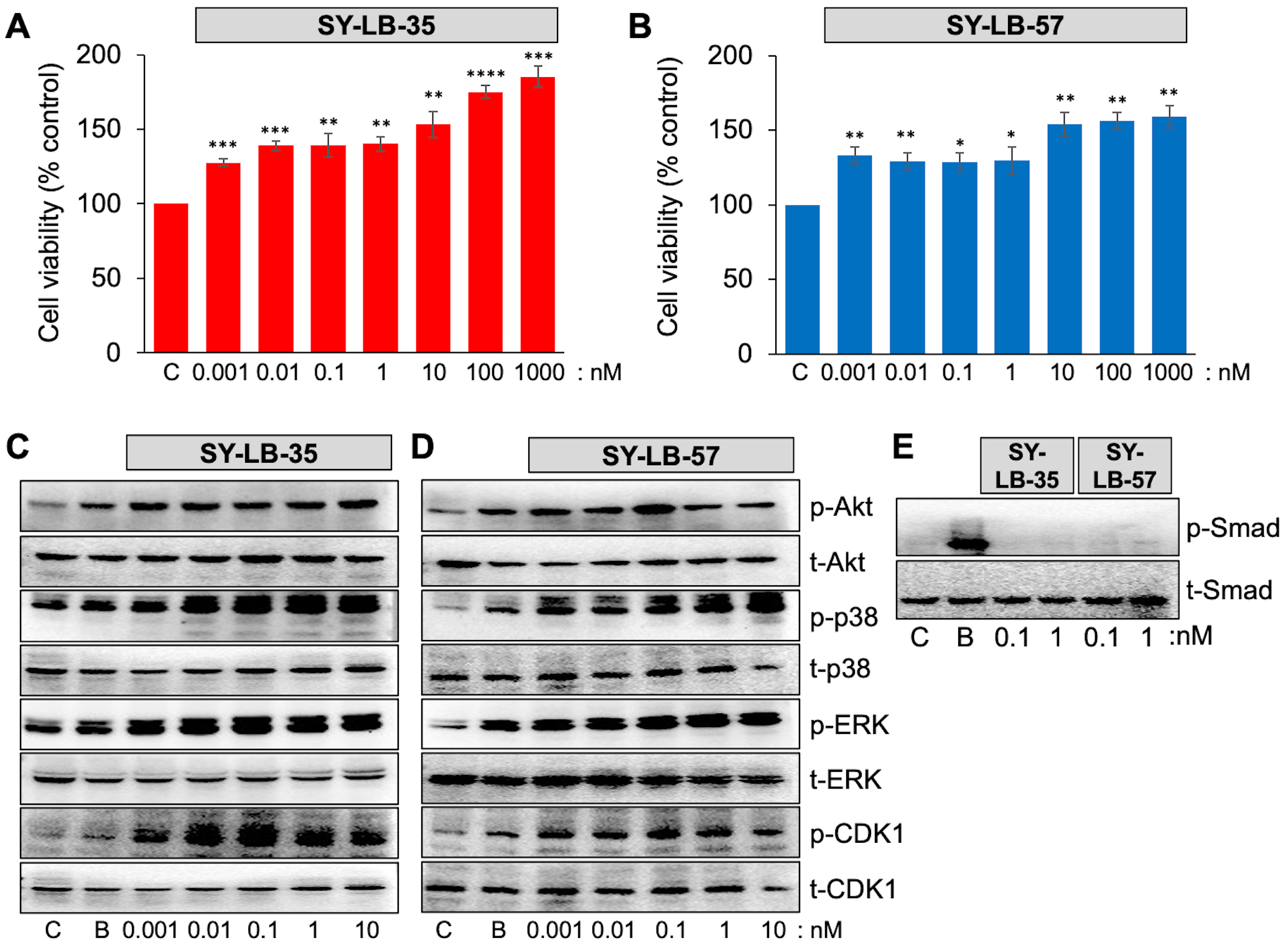
To determine the lower limit for increasing cell viability, serum-starved C2C12 cell cultures were exposed to SY-LB-35 and SY-LB-57 over a very low concentration range (0.001–1000 nM) for 24 hours. SY-LB-35 and SY-LB-57 continued to stimulate significant increases in cell viability at concentrations as low as 1 pM (Fig. 6A,B). Moreover, concentration-dependent reduction of cell viability was observed as the concentrations decreased. The cell viability induced by SY-LB compounds demonstrated significantly higher percentages of cell viability than the control, untreated cells at all tested concentrations (Fig. 6A,B).
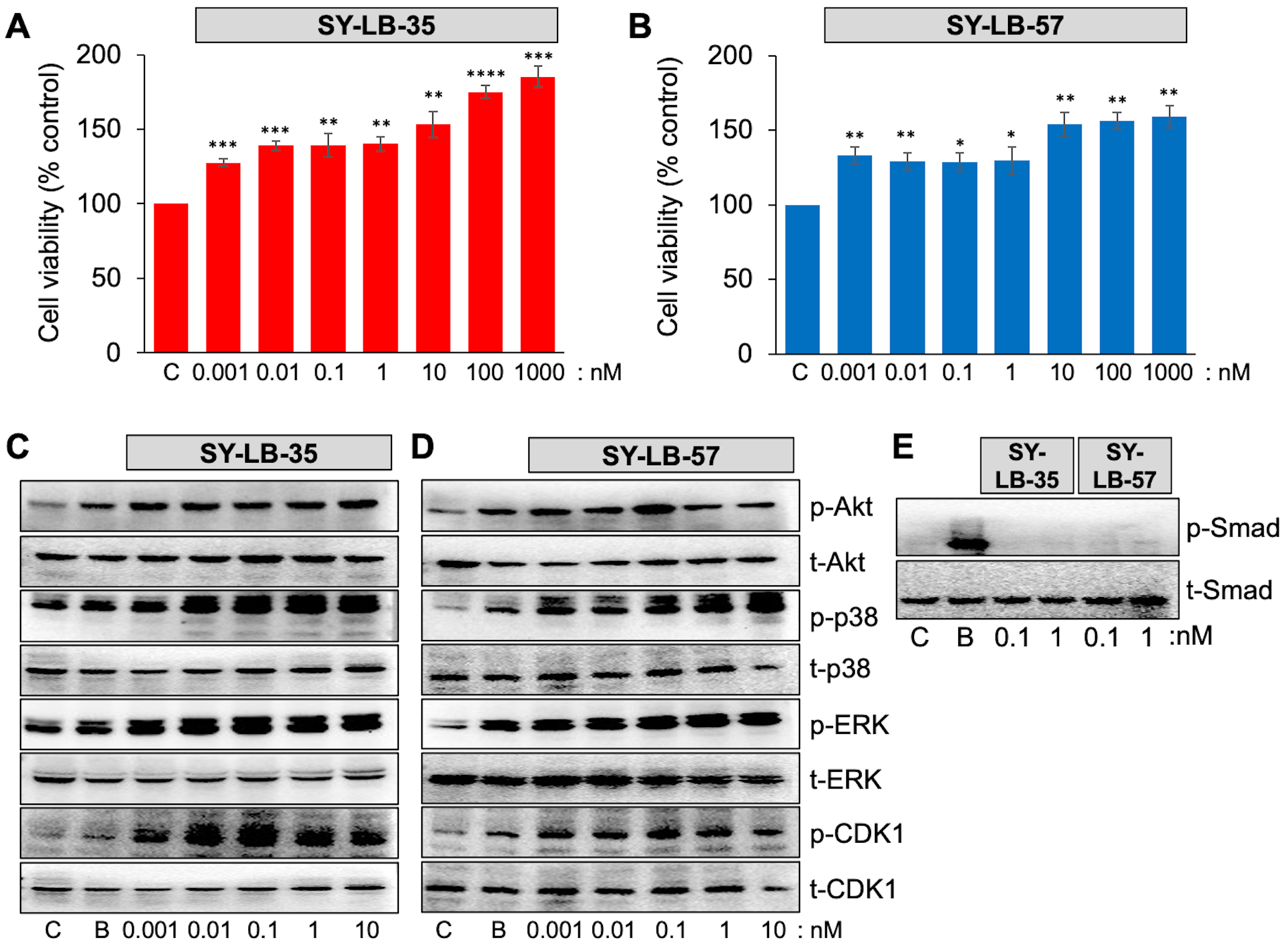 Fig. 6.
Fig. 6.SY-LBs stimulate increases in cell viability and non-canonical
signaling, but not canonical, Smad signaling at picomolar concentrations. (A,B)
Cell viability in C2C12 cells following treatment of cultures for 24 hours with
0.001 nM to 1000 nM of (A) SY-LB-35 and (B) SY-LB-57. SY-LB compounds
significantly increased cell viability across the entire concentration range
compared with control (C), untreated cells. Cell viability is expressed as the
mean
The canonical and non-canonical BMP signaling pathways were analyzed by Western blot of C2C12 cell lysates following 15- and 30-minute treatment with picomolar concentrations of SY-LB-35 or SY-LB-57. BMP2 at 50 ng/mL was used as a positive control. Low concentrations (0.001–10 nM) of SY-LB-35 (Fig. 6C) and SY-LB-57 (Fig. 6D) were able to stimulate phosphorylation of Akt (as a readout for PI3K activity), p38, ERK, and CDK1 at concentrations as low as 1 pM. In contrast, Smad phosphorylation was not detected in response to SY-LB-35 or SY-LB-57 at either 100 pM or 1 nM following a 30-minute stimulation (Fig. 6E).
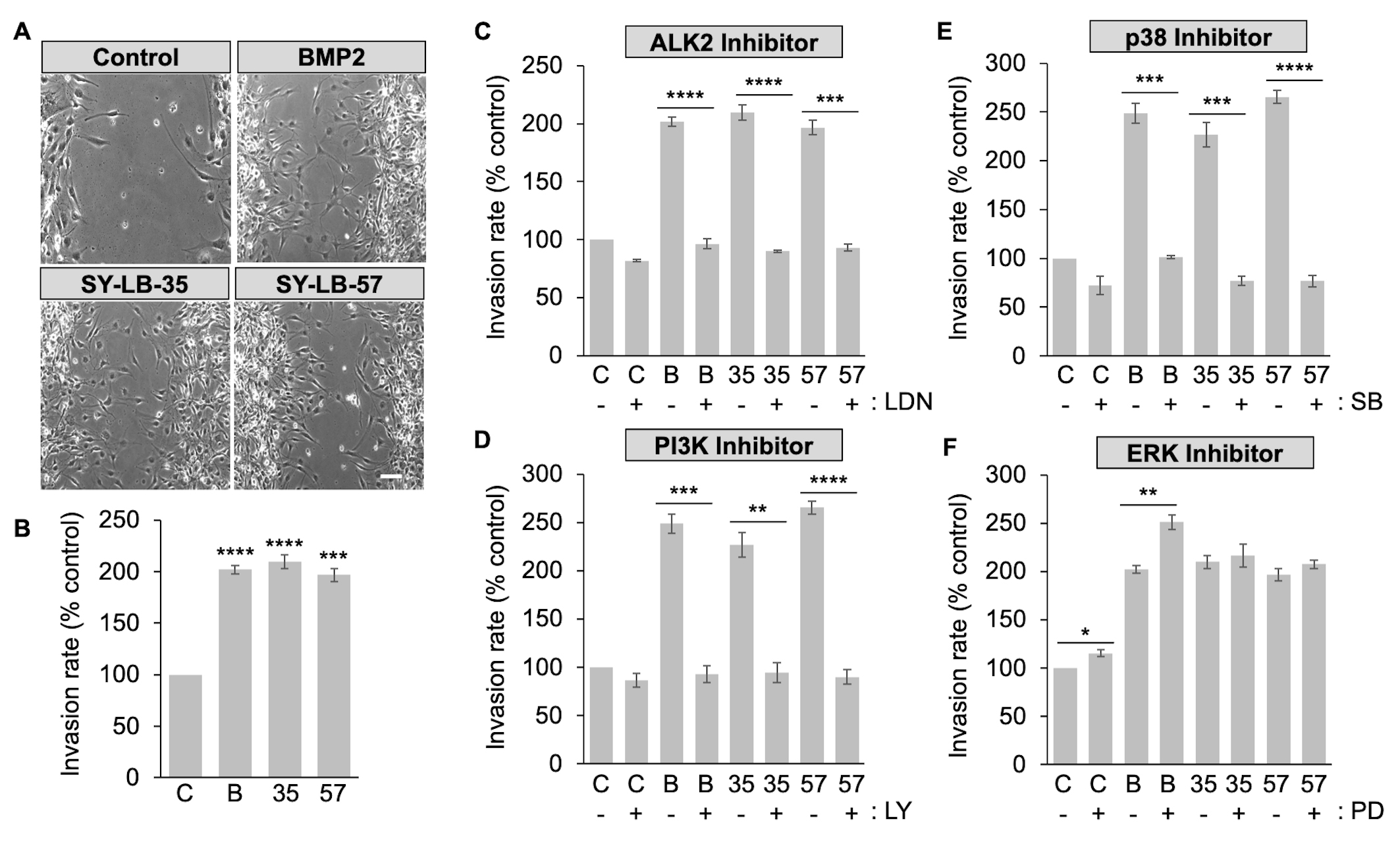
To explore how SY-LB-35 and SY-LB-57 impact cell migration, in vitro wound healing/scratch assays were conducted. C2C12 cell monolayers were scrape-wounded followed by exposure to BMP2 (50 ng/mL) or 1 µM SY-LB compounds for 18 hours. The wounds were documented at 0-hour and 18-hour time points to assess wound closure over time. Migration of cells into the wounded area was quantified by counting the number of cells in the scratch area at 18 hours (Fig. 7A,B). Significantly more C2C12 cells invaded the scratched area in the presence of BMP2 or the SY-LB compounds compared with control, untreated cultures (Fig. 7A,B).
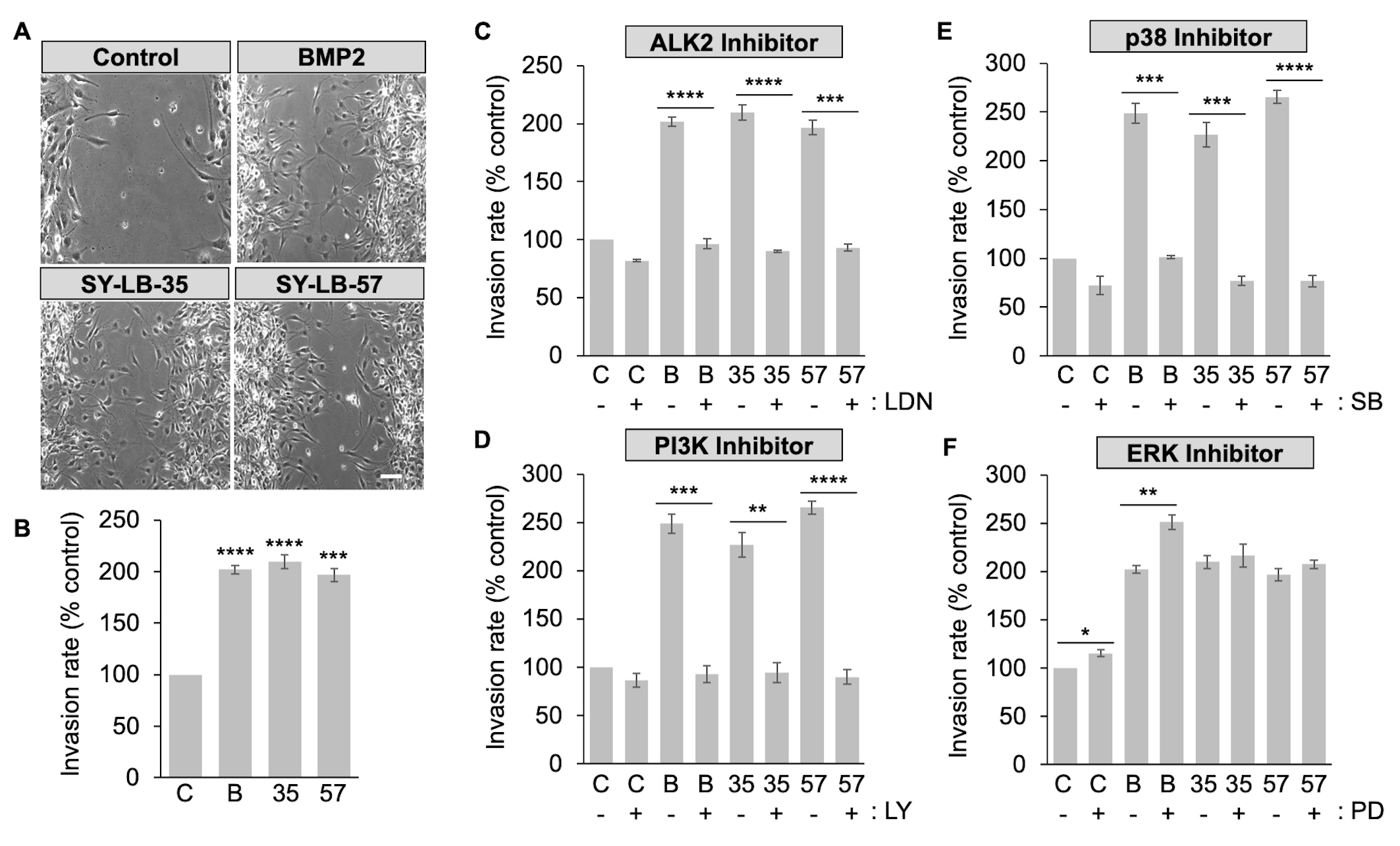 Fig. 7.
Fig. 7.SY-LB-promoted wound healing is dependent upon ALK2, PI3K and
p38 but does not rely on ERK signaling. (A) Representative phase contrast images
of C2C12 scratch assays incubated for 18 hours in the presence of 50 ng/mL BMP2
and 1 µM SY-LB-35 or SY-LB-57. Control cultures were maintained in
unsupplemented medium. The wounds were examined at 0- and 18-hour time points to
assess wound closure over time. BMP2 and SY-LB compounds promote invasion of
C2C12 cells into the wounded area, where few cells have entered the wounded area
in control cultures after 18 hours. (B) A quantitative assessment of wound
healing shown in (A) was carried out by counting the number of cells invading the
wounded area after 18 hours. The Invasion rate is expressed as the number of
cells invading the wound as a percentage of invading cells in control (C-),
untreated cultures. BMP2 (B, 50 ng/mL, SY-LB-35 (35, 1 µM) and
SY-LB-57 (57, 1 µM) significantly increased the migration of C2C12
cells into the scrape-wounded area compared with control (mean
To explore the contribution of ALK2 activity to SY-LB-induced wound healing, in vitro scratch assays were carried out as described above. The monolayers were scrape-wounded followed by addition of BMP2 (50 ng/mL) or the SY-LB compounds at 1 µM in absence or presence of the selective ALK2 inhibitor, LDN193189, at 5 µM. Representative images at 18 hours are shown in Supplementary Fig. 5. LDN193189 treatment alone had a small, but significant effect on the Invasion rate of C2C12 cells (Fig. 7C and Supplementary Fig. 5). Mimicking the effect of LDN193189 on BMP2-evoked C2C12 cell migration, the presence of LDN193189 completely blocked C2C12 cell invasion into the wounded area promoted by the SY-LB compounds (Fig. 7C).
To assess the involvement of PI3K/Akt, p38 and ERK signaling pathways in SY-LB-induced cell migration, C2C12 cells were scrape-wounded and incubated with BMP2 (50 ng/mL) and 1 µM SY-LB compounds for 18 hours in the absence or presence of inhibitors to PI3K (15 µM LY294002), p38 (10 µM SB203190) and ERK (5 µM PD98059). Representative images at 18 hours are shown in Supplementary Fig. 5. Inhibition of PI3K and p38 had a profound effect on C2C12 cell migration and significantly eliminated SY-LB-induced increases in Invasion rates (Fig. 7D,E, respectively). LY294002 had no effect on the Invasion rate of control, untreated C2C12 cell cultures (Fig. 7D). Inhibition of p38 with SB203190 produced a minor but significant decrease in the Invasion rate of control, untreated cultures (Fig. 7E).
In contrast to the effects of PI3K and p38 inhibition on C2C12 cell invasion rates, inhibition of the ERK pathway alone significantly increased the Invasion rate in control, untreated cultures and in cultures treated with BMP2 (Fig. 7F). PD98059 had no significant effect on C2C12 cell migration evoked by SY-LB-35 or SY-LB-57 with all conditions strongly stimulating migration in the presence of PD98059 (Fig. 7F).
To exclude cell proliferation as a cofounding factor in wound healing assays, C2C12 cells were subjected to scratch assays in the absence or presence of Mitomycin C (30 nM), a DNA synthesis inhibitor, and exposed to BMP2 (50 ng/mL) or 1 µM SY-LB compounds for 18 hours. Treatment with Mitomycin C (MC) alone had no effect on C2C12 cell migration compared with control, untreated cultures (Supplementary Fig. 6A). Importantly, the increases in C2C12 Invasion rates in response to BMP2, SY-LB-35 or SY-LB-57 were not significantly different whether in the absence or presence of Mitomycin C (Supplementary Fig. 6A). Representative phase contrast images are shown in Supplementary Fig. 6B.
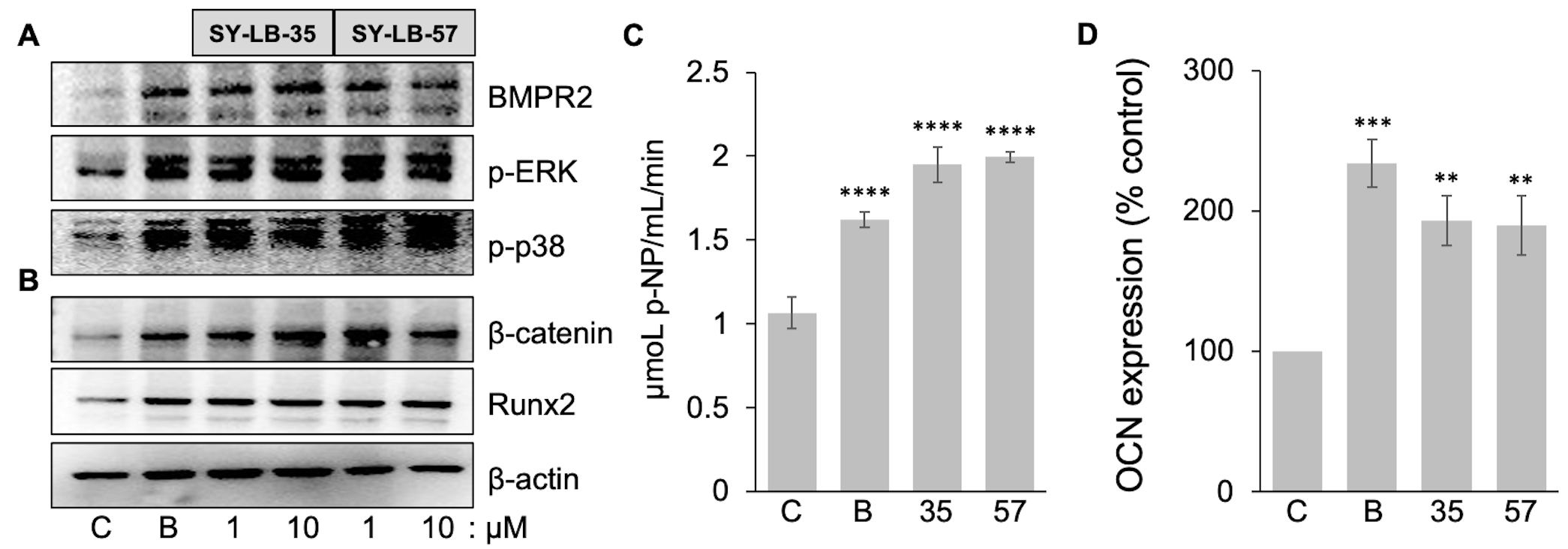
Exposure to BMP2 causes C2C12 cells to differentiate into an osteoblast lineage
accompanied by the expected expression markers for bone formation [32, 44]. To
examine the osteogenic potential of SY-LB-35 and SY-LB-57, the expression of
markers related to bone differentiation was examined in C2C12 cells that were
serum-starved and incubated with 50 ng/mL BMP2 or SY-LB compounds (1
µM and 10 µM) for 4 hours. Western blot analysis
revealed an increase in the expression of early osteogenic markers such as Runx2,
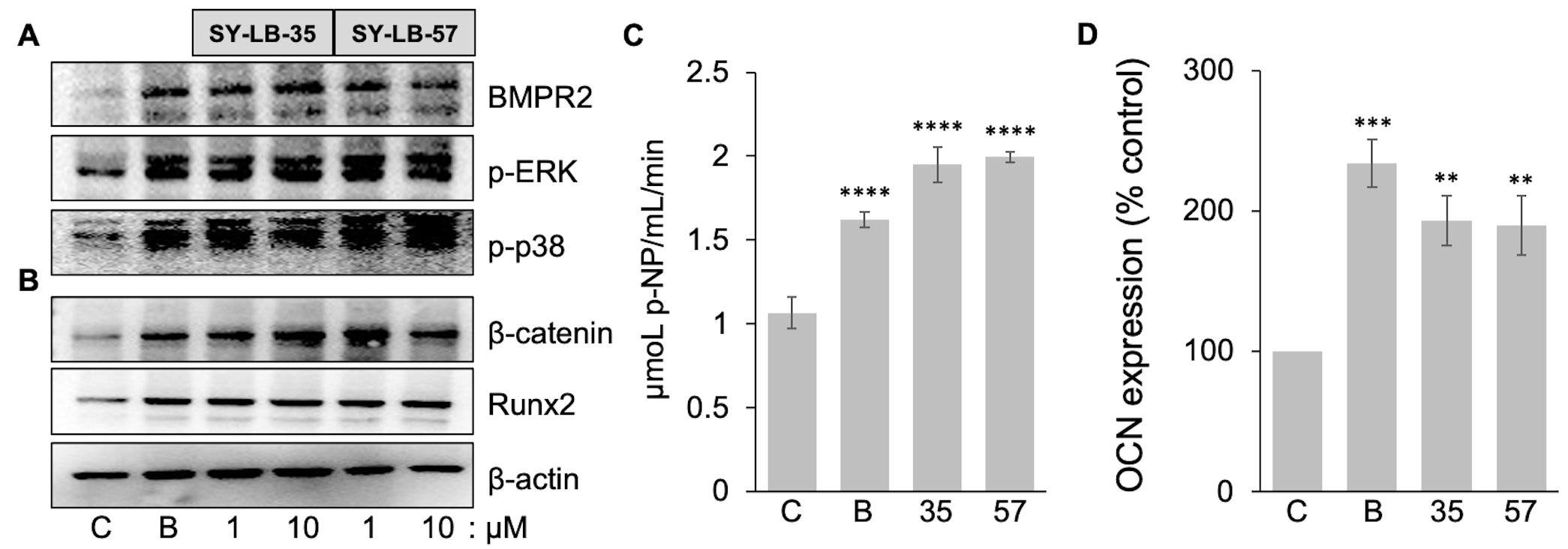 Fig. 8.
Fig. 8.Early-, mid- and late-stage markers of bone differentiation are
upregulated by SY-LB-35 and SY-LB-57. (A,B) C2C12 cultures were serum-starved
and incubated for 4 hours with BMP2 (50 ng/mL) and SY-LB-35 or SY-LB-57 at 1
µM and 10 µM. Whole cell lysates of the cultures were
analyzed by Western blot using antibodies that recognize (A) BMPR2 or (B)
Alkaline phosphatase (ALP) is widely regarded as an early pre-osteoblast marker, while proteins such as osteocalcin (OCN) are considered to be markers of differentiated osteoblasts [45]. To explore the activation of ALP enzymatic activity by SY-LB compounds, confluent C2C12 cultures were stimulated with a single treatment of BMP2 (200 ng/mL) or 10 µM SY-LB compounds. The culture media was assessed at 24, 48 and 72 hours for secreted, active ALP in the presence of the soluble p-nitrophenol phosphate (p-NPP) substrate, which is converted to p-nitrophenol (p-NP) by the ALP enzyme. ALP activity in the whole cell lysates of C2C12 cultures was measured after 72 hours of the treatment. The whole cell lysates were assessed for ALP activity by incubation with p-NPP substrate and demonstrated robust increases in ALP activity following exposure to BMP2, SY-LB-35 and SY-LB-57 compared with control lysates (Fig. 8C). A time course of ALP activity in the culture media is presented in Supplementary Fig. 7A and a standard curve for the ALP assay is shown in Supplementary Fig. 7B.
To determine if the SY-LB compounds increase the expression of OCN, fully confluent C2C12 cells were exposed to BMP2 (200 ng/mL), SY-LB-35 (10 µM) or SY-LB-57 (5 µM). After day 7, the cells were collected, and the resulting cell lysate was used in the OCN ELISA. The protein level of osteocalcin in the samples was obtained from concentration of standard solutions of OCN plotted versus absorbance at 450 nm (Supplementary Fig. 7C). Quantitative analysis of OCN expression revealed that BMP2, SY-LB-35 and SY-LB-57 caused a significant increase in the level of OCN expression compared with control, untreated C2C12 cell cultures (Fig. 8D).
To examine the effects of SY-LB-35 on differentiation of C2C12 cells into
osteoblasts following long-term treatment, C2C12 cell cultures were grown to a
fully confluent state and exposed to BMP2 (200 ng/mL) or SY-LB-35 (10
µM) every 24 hours for 72 hours. Treatments were prepared in Low
Serum media (2% FBS/DMEM/1
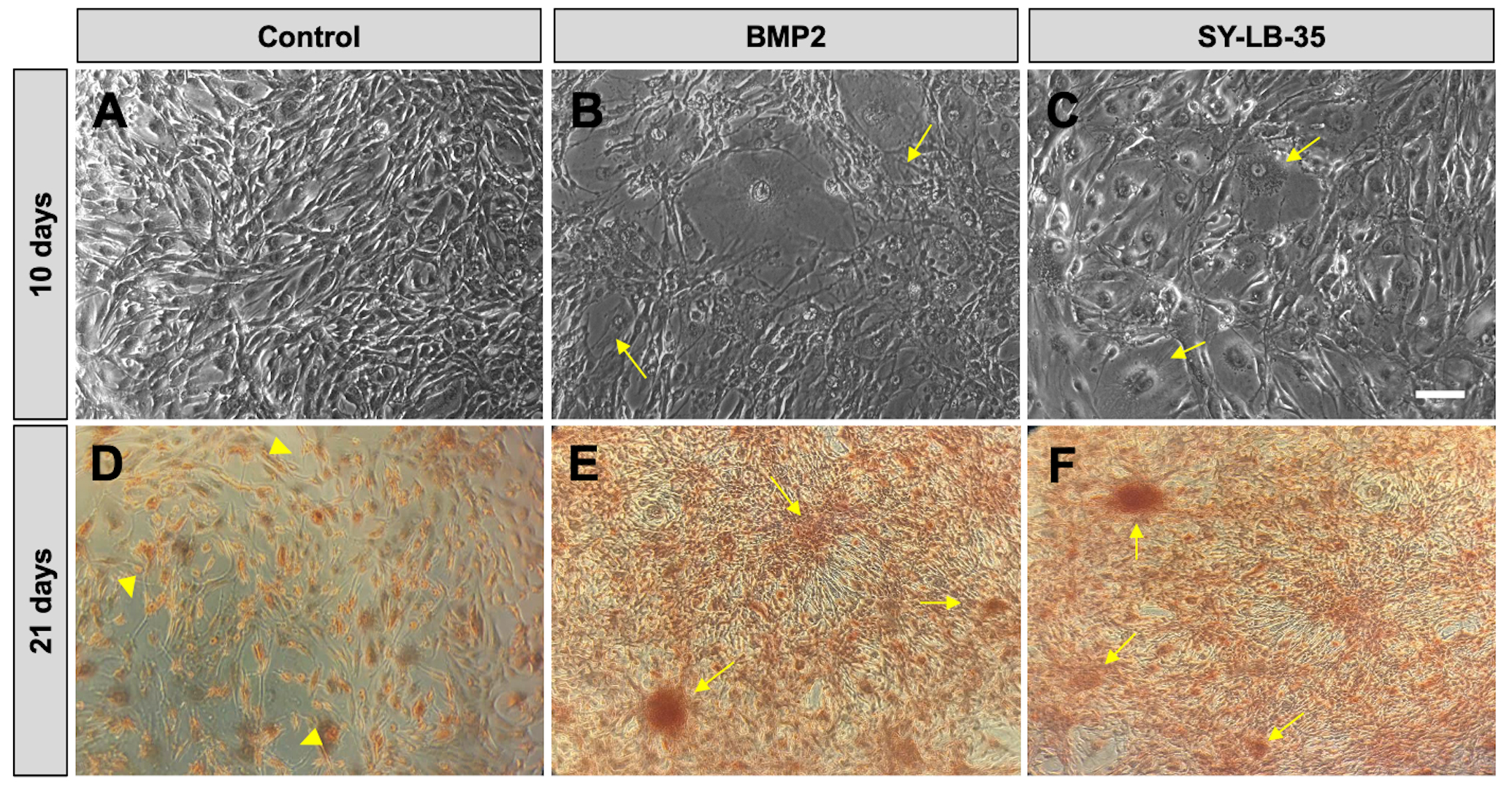
In longer-term cultures, fully confluent C2C12 cultures were serum-starved for 16 hours followed by treatment with BMP2 (100 ng/mL) or SY-LB-35 (10 µM) every other day for 10 days in complete growth medium containing 10% FBS. Unstimulated cells develop a radial branching morphology consisting of long fibers extending in many directions (Fig. 9A). While long-term stimulation of C2C12 cells with SY-LB-35 (Fig. 9C) exhibited morphological changes, differentiating into large, round cells and losing the branching morphology, distinct from the control group but similar to BMP2-treated cultures (Fig. 9B).
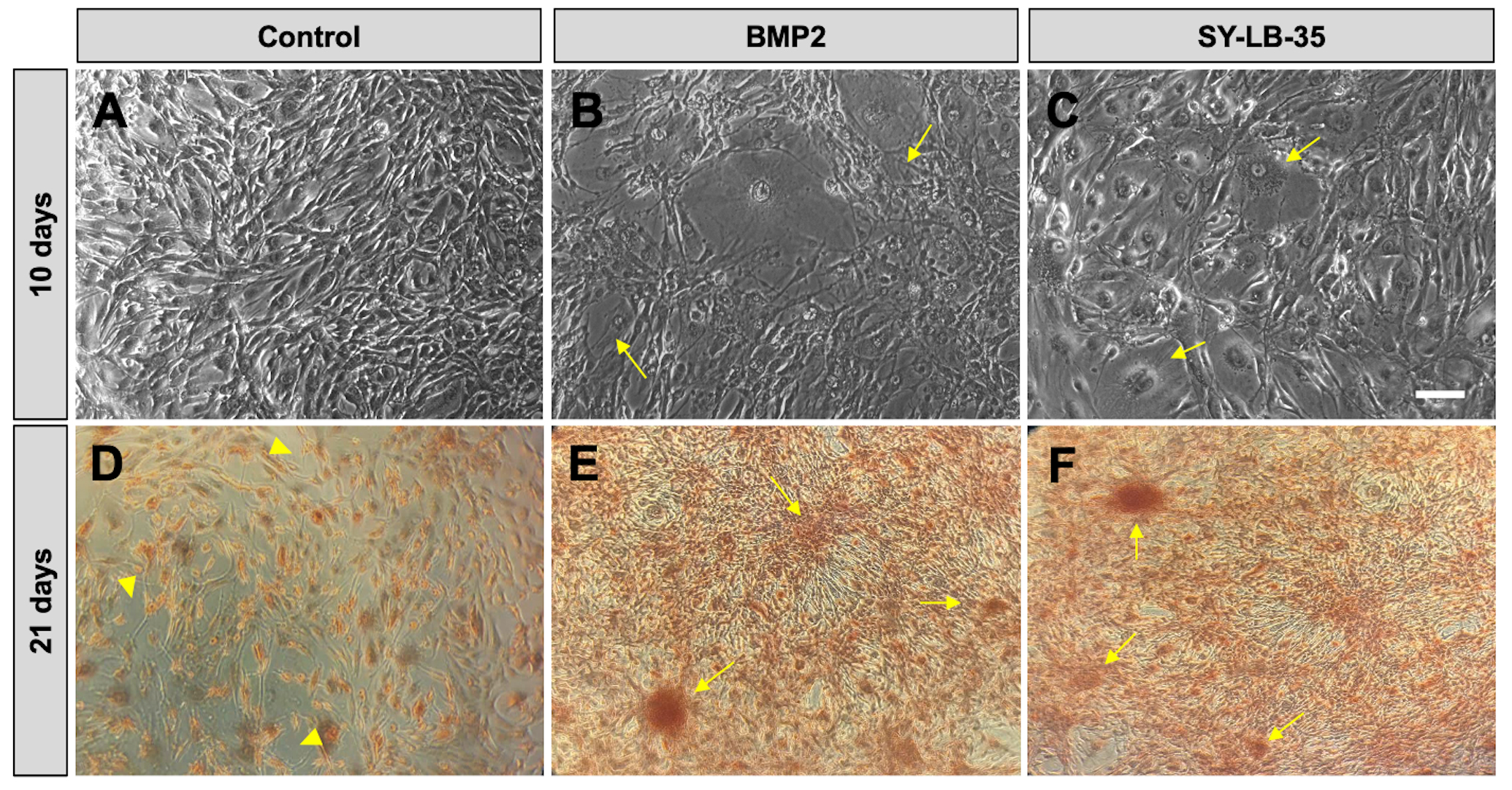 Fig. 9.
Fig. 9.Morphological changes and evidence of calcium deposition in
long-term C2C12 cell cultures treated with SY-LB-35. (A–C) Representative
20
To determine whether evidence of calcium deposition could be detected in cultures of differentiating C2C12 cells, Alizarin Red S staining was carried out, which indicates mineralization in the cultures by binding to calcium deposits in the extracellular matrix. To this end, fully confluent C2C12 cell cultures were incubated with BMP2 (200 ng/mL) or SY-LB-35 (10 µM) for 21 days in complete growth medium followed by staining with 2% Alizarin Red S solution. Alizarin Red S staining in control, untreated C2C12 cells showed diffuse, light red staining (Fig. 9D). In contrast, cultures treated with BMP2, or SY-LB-35 showed strong calcium depositions with large, intensely staining clusters of cells (Fig. 9E,F, respectively).
The initial characterization of SY-LB-35 and SY-LB-57 was carried out in the pluripotent C2C12 cell line and demonstrated that these novel, small molecule heterocyclic compounds strongly stimulated canonical Smad signaling through a type I BMP receptor-dependent mechanism [30]. Prior to our study, several small molecules were reported to stimulate BMP receptor signaling but exhibited weak responses or acted through a BMP receptor-independent mechanism or both [46, 47, 48, 49, 50]. In our previous study, the substantial SY-LB-induced amplification of Smad phosphorylation and cell viability was blocked by non-selective inhibition of type I BMP receptor activity [30]. The dependence on type I BMP receptor activity set SY-LB-35 and SY-LB-57 apart from previously reported small molecule activators of BMP signaling and suggested that the SY-LB compounds may act at the BMP receptor complex to regulate activity [30, 46, 47, 48, 49, 50]. The SY-LB compounds also exhibited rapid activation of signaling via non-canonical BMP receptor pathways through PI3K/Akt-, p38- and ERK-mediated signaling [30]. These findings suggested that SY-LB-35 and SY-LB-57 function as true BMP receptor agonists with the ability to strongly activate Smad-dependent and Smad-independent signaling.
There is an unmet clinical need for the development of BMP receptor agonists or for agents that can enhance BMP-regulated intracellular signaling. Not only are formulations of rBMPs being used clinically to promote bone growth following reconstructive surgery or after injury [27, 28, 29], but preclinical experiments suggest that enhancing or restoring BMP receptor-dependent intracellular signaling would be beneficial for the treatment of diseases like chronic kidney disease or pulmonary arterial hypertension [2, 38, 46, 51, 52]. While rBMPs are able to stimulate in vivo repair processes [27, 43], the use of recombinant proteins as therapeutics has significant drawbacks [29, 53, 54, 55]. Recombinant BMPs, which are dimeric ligands, require complicated bio-synthesis processes to produce active proteins, which ultimately translates to high patient costs [28, 29]. Moreover, numerous reports of adverse effects resulting from in vivo rBMP administration call into question whether the benefits outweigh the harm rBMP therapeutics offer [53, 54, 56, 57]. Small molecules can be synthesized at a fraction of the cost and can overcome obstacles regarding bioavailability or tissue penetration that often plague protein-based therapies such as rBMP2 or monoclonal antibody therapies. SY-LB-35 and SY-LB-57 are efficiently synthesized at large scale from commercially available compounds by a highly efficient one-pot synthesis method [30, 31]. Thus, a strong case can be made for the development of these novel indolyl-benzimidazole compounds as therapeutics for the treatment of injuries or disorders for which the production of new bone is needed. These compounds might also be beneficial in disorders like hereditary pulmonary arterial hypertension, where patients are typically lacking functional BMPR2 receptor subunits or other key signaling components downstream of receptor activation [52, 58, 59, 60].
The present study expands the results of the original study to include similarly robust activation by SY-LB-35 and SY-LB-57 of intracellular signaling regulated by BMPs in multiple cell types, the C2C12 mouse myoblast cell line, the WEHI 274.1 mouse monocytic cell line and primary endothelial cells isolated from mouse pulmonary arteries. SY-LB compounds stimulated highly significant increases in cell viability and wound healing that were specifically dependent upon signaling through PI3K and p38 activity, but, interestingly, not ERK activity. Moreover, these compounds exhibited specificity for type I BMP receptor, ALK2, where the substantial changes in Smad phosphorylation, cell viability and wound healing produced by SY-LB-35 and SY-LB-57 were completely eliminated by the selective ALK2 inhibitor, LDN193189. The prior study examined SY-LB-induced intracellular responses over a short time course using a field standard of 30 minutes for peak Smad phosphorylation and 15 minutes for phosphorylation events occurring through non-canonical BMP signaling pathways. Herein, the responses to SY-LB-35 and SY-LB-57 were surveyed at 24 hours in C2C12 cells and showed upregulation of Id1, BMPR2 and the inhibitory Smads, Smad6 and Smad7, and sustained phosphorylation of p38, ERK and CDK1. Moreover, increased cell viability and non-canonical BMP-regulated signaling was elicited by SY-LB-35 and SY-LB-57 at concentrations as low as 1 pM. In contrast, canonical Smad signaling is undetectable at these low concentrations.
Given the remarkable parallels between the responses in C2C12 cells to these
novel benzimidazoles and rBMP2, SY-LB-35 and SY-LB-57 were assessed for the
ability to induce the differentiation of the pluripotent C2C12 cell line into
osteoblasts. C2C12 cells are widely used to assess the osteogenic activity of
rBMPs and candidate BMP receptor activators [32, 44, 48, 61]. In C2C12 cells,
expression of Runx2,
The current study makes clear that the SY-LB compounds are potent BMP receptor agonists that stimulate robust BMP-regulated signaling through a common mechanism in multiple cell types. Moreover, rBMP-like increases in intracellular signaling through Smad, PI3K/Akt and p38 signaling pathways stimulated by the SY-LB compounds are associated with functional outcomes since inhibiting type I BMP receptor-, PI3K/Akt- and p38-dependent activity completely blocked increases in cell proliferation and wound healing in response to SY-LB-35 and SY-LB-57. The ability of SY-LB-35 and SY-LB-57 to stimulate increases in p-Akt, p-p38, p-ERK and p-CDK1 at picomolar concentrations and, importantly, significantly increased cell viability at the same very low concentrations demonstrates the potency of these novel compounds. It remains to be seen what concentrations would be required to achieve efficacy in vivo.
Therapies involving rBMPs in humans require potentially dangerous concentrations of rBMPs [27, 28, 29]. One reason for the need to use such high concentrations of rBMPs is due to endogenous antagonism of BMPs. BMP antagonists are families of extracellular factors that bind directly to BMPs and prevent BMP ligands from interacting with the BMP receptor complex [42]. Noggin is a BMP antagonist with a particularly high affinity for BMP2, which is precisely the BMP that is approved by the FDA for human use [27, 67]. Although SY-LB-35- and SY-LB-57-evoked p-Smad responses were blocked by pre-incubation of C2C12 cells with Noggin, the SY-LB compounds were able to overcome inhibition by Noggin in competition assays suggesting that these potent agents may be able to do so in vivo as well. Whether these compounds are sensitive to other classes of BMP antagonists is still unclear. The ability of Noggin to block SY-LB-stimulated Smad phosphorylation also indicates that these compounds might interact with the extracellular domains of the BMP receptor complex.
Previously, SY-LB-35 and SY-LB-57 were shown to stimulate Smad phosphorylation through a type I BMP receptor-dependent mechanism using the non-selective inhibitor, Dorsomorphin [30]. Here, the SY-LB compounds were shown to increase p-Smad levels, cell viability and the rate of wound healing through a specific type I BMP receptor subunit, ALK2. The selective ALK2 inhibitor, LDN193189, eliminated the responses to the SY-LB compounds in C2C12 and WEHI cells indicating a conserved mechanism involving ALK2 as part of the BMP receptor complex activated by SY-LB-35 and SY-LB-57. How and where these small molecule activators of BMP receptor signaling interact with an ALK2-containing BMP receptor complex is an area of ongoing investigation.
The activation of particular intracellular signaling pathways by the SY-LB compounds to increase cell viability and the rate of wound healing raises an enduring problem in the BMP/BMP receptor field of how the choice of downstream pathway is regulated [9, 15, 19, 68, 69, 70]. Inhibition of PI3K/Akt and p38 signaling prevented the two indolyl-benzimidazoles from increasing cell viability or Invasion rates. In contrast, inhibition of ERK signaling had no effect on these processes indicating p-ERK stimulation may not have a role in cell proliferation or motility in C2C12 cells. Thus, it appears that these small molecules selectively utilize the PI3K/Akt and p38 signaling pathways to increase cell viability and rates of wound closure. Functional consequences of robust increases in ERK phosphorylation stimulated by SY-LB compounds remains to be determined.
A less appreciated feature of BMP/BMP receptor-dependent mechanisms is the distinct concentration range at which canonical and non-canonical BMP-regulated signaling is activated. For example, the PI3K/Akt pathway can be stimulated at picomolar concentrations of BMP7 [34, 36, 71]. Moreover, the peak of BMP7-stimulated WEHI monocyte chemotaxis was at 1 pg/mL BMP7 and found to be dependent upon the PI3K/Akt pathway [36]. Here, Smad phosphorylation was not detected in response to SY-LB-35 or SY-LB-57 at 0.1 nM and 1 nM, which is 100–1000 times the concentrations needed to activate non-canonical signaling pathways, given that p-Akt, p-p38, p-ERK, p-CDK1 and increases in cell viability were all stimulated by concentrations as low as 0.001 nM or 1 pM. Collectively, these results indicate that regulation of BMP receptor subunit activity by indolyl-benzimidazoles at low concentration is selective for non-canonical BMP-regulated signaling.
BMP2 prevents the myogenic differentiation of C2C12 cells and directs the
differentiation of these cells into an osteoblast lineage [32]. Id1 is a negative
regulatory protein that is upregulated during C2C12 osteoblast differentiation
and is the most significantly upregulated gene in response to BMP2, BMP6 and BMP9
in various human and murine cell types [39, 44]. BMP2 induces osteogenic
differentiation in C2C12 cells by upregulating the expression of Id1 and the
activity of ALP, an early marker of osteogenesis [45, 65]. Osteoblast-specific
markers include transcription factors such as Runx2, DLX5 and Osterix, while
markers that are related to matrix formation include OCN and fibromodulin [25, 26, 72]. Moreover, in vitro and in vivo studies suggest a
relevant role for p38 and ERK throughout the osteoblastic commitment process,
from a mesenchymal progenitor into a fully functional anabolic bone cell [24, 26, 63, 64]. Furthermore, it is clear that Wnt-dependent activation of
Exposure of C2C12 cell cultures to SY-LB-35 and SY-LB-57 caused upregulation in
the expression of Id1 and BMPR2, as would expected of any BMP-like ligand. The
SY-LB compounds also stimulated the expression of protein markers specific to
osteogenesis such as Runx2,
SY-LB-35 and SY-LB-57 mimicked rBMP2-induced responses in numerous in vitro assays but did not behave identically. SY-LB-57 consistently induced responses that were significantly greater in magnitude than responses to SY-LB-35 demonstrating greater efficacy of SY-LB-57 for stimulating BMP-regulated processes. Given that the two compounds differ by a single substitution, a hydroxyl group on SY-LB-35 or a methoxy group in the equivalent position on SY-LB-57, it will be of interest to vary the substitutions and/or the position of the substitutions to evaluate how such changes might change the activity of the benzimidazole compounds.
SY-LB-35 and SY-LB-57 are heterocyclic, indolyl-benzimidazole small molecules that faithfully and robustly reproduce BMP-stimulated in vitro responses in multiple cell types. Interestingly, the SY-LB compounds activate non-canonical BMP-regulated signaling pathways and stimulate increases in cell viability at concentrations at which canonical Smad phosphorylation is not detected demonstrating differential regulation of downstream signaling by SY-LB-35 and SY-LB-57. The functional consequences of exposure to the SY-LB compounds include substantially increasing cell viability and the rate of wound closure. In addition to promoting cellular proliferation or migration, the indolyl-benzimidazole compounds exhibit clear evidence of promoting osteogensis. Markers of early-, mid- and late-stage osteoblast differentiation are all upregulated in C2C12 cells exposed to these small molecules. Taken together, the evidence presented confirms that these SY-LB compounds are potent BMP receptor agonists with osteogenic activity. Future efforts to explore the osteogenic potential of the compounds include rodent models of subcutaneous ectopic bone growth to first screen for responses to in vivo exposure to SY-LB-35 or SY-LB-57 [74]. Next, bone defect and ovariectomy-induced postmenopausal osteoporosis are attractive models that could be used to test the in vivo efficacy of the indolyl-benzimidazoles [55, 75, 76]. Moreover, inducible models of PAH or chronic kidney disease are also appealing avenues for pre-clinical investigation of the in vivo efficacy of these small molecules [2, 77, 78]. With the advantages associated with small molecules, the SY-LB compounds could potentially be cost-effective replacements for rBMP-based therapies targeting a number of organ systems.
ALK2, activin-like kinase 2; ALP, alkaline phosphatase; BMP, bone morphogenetic protein; BMPR2, BMP receptor type 2; OCN, osteocalcin.
All data and materials obtained from this study are reported in the submitted article.
SN and JCP designed the research study. SY designed the synthesis method for the indolyl-benzimidazoles. LB carried out the chemical synthesis, purification, and characterization of the indolyl-benzimidazoles. SN, JMJF and JHA performed the research. SN, JHA and JCP analyzed the data. SN and LB wrote the original manuscript. SN, JCP, LB, SY, JHA and JMJF contributed to revisions of the manuscript. All authors have read and agreed to the published version of the manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
We would like to thank the Department of Pharmaceutical Sciences and the College of Pharmacy and Health Sciences at St. John’s University that provided generous financial support for undergraduate and graduate student projects and publications. Thank you to Maleka Stewart for assistance with this study and to members of the Perron Lab who provided comments on the project and manuscript. Special thanks to the staff at Science Supply for managing our supplies.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.