1 Department of Life Science, Chung-Ang University, 06974 Seoul, Republic of Korea
2 Department of Microbiology, College of Medicine, Ewha Womans University, 07804 Seoul, Republic of Korea
Abstract
Several antiviral drugs are clinically approved to treat influenza that is a highly prevalent acute respiratory disease. However, emerging drug-resistant virus strains undermine treatment efficacy, highlighting the exigency for novel antiviral drugs to counter these drug-resistant strains. Plants and their derivates have been historically utilized as medicinal remedies, and extensive studies have evidenced the antiviral potential of phytochemicals. Notably, apigenin is a predominant flavonoid with minimal toxicity and substantial therapeutic effects in various disease models. Despite its many anti-inflammatory, anti-oxidant, anti-cancer, anti-bacterial, and other beneficial bioactivities, existing reviews have yet to focus on apigenin’s antiviral effects. Therefore, this review elucidates apigenin’s therapeutic and antiviral properties in vitro and in vivo, discussing its mode of action and future prospects. Apigenin’s remarkable inhibition by modulating multiple mechanisms against viruses has promising potential for novel plant-derived antiviral drugs and further clinical study developments.
Keywords
- flavonoids
- 4′,5,7-trihydroxyflavone
- antiviral drugs
- influenza
- phytochemicals
Influenza is a viral acute respiratory disease, and a prominent recurrent threat to human health. Several influenza pandemics have transpired in recent history, such as the Spanish flu in 1918, the Hong Kong flu in 1968, and the Swine flu in 2009 [1]. Approximately 500 million people were infected, and 50 million died during the most severe 1918 Spanish pandemic [2]. Although anti-influenza drugs and vaccines have significantly prevented and treated influenza viral infection, they remain limited due to the virus’ high mutation rate and subsequent subtype generation [3]. Thus, influenza vaccines must be updated annually to stay ahead of rapidly mutating viruses [4]. Discordance between influenza vaccine strains and circulating viruses may engender a more severe endemic or pandemic due to reduced vaccine efficacy [5]. Furthermore, emerging drug-resistant influenza strains threaten current antiviral drug applications [6], emphasizing the urgency for new antiviral drugs.
Nearly 90 drugs have been approved for nine human viral disease treatments [7]. Plants have been used as traditional remedies for thousands of years, and many studies have revealed plant derivative and metabolite therapeutic potentials against diverse diseases. An estimated 34% of approved small molecule drugs in the past four decades are natural products or their derivatives, and 25% are from plants exclusively [8, 9]. Due to recent recurrent viral pandemics, phytochemicals have garnered attention as promising antiviral drug agents [10]. Plant derivatives or phytochemicals maintain significant structural complexity and can target viral and host proteins in various ways [11].
Apigenin is a natural plant flavonoid found in fruits, herbs, and various
plants. As part of the flavone class, apigenin maintains a flavone structure with
hydroxyl group substitutions at positions 4
Apigenin’s potential as a therapeutic drug was discovered in 1960 when it
inhibited basophil’s histamine release. Apigenin significantly reduced production
of IgE, IgG2a, IgG1, histamine, and hexosaminidase in a mouse allergic rhinitis
model by inhibiting the TLR4/MyD88/NF-
In addition, apigenin ameliorated streptozotocin-induced diabetic nephropathy in
rats. Apigenin treatment mitigated renal dysfunction, fibrosis, and inflammation
in diabetic rats by significantly reducing MAPK activation, which prevented
apoptosis and production of Tumor-necrosis factor
| Disease | Therapeutic effects | Mechanism | Ref |
| Allergy | Production of IgE, IgG2a, IgG1, histamine and hexosaminidase |
Suppress NF- |
[19, 20] |
| Cancer | Apoptosis, autophagy, cell cycle arrest |
Modulate PI3K, AKT, MAPK, ERK, JAK, STAT, NF- |
[21, 22, 23, 24, 25] |
| Proliferation, migration and invasion |
|||
| Immune response |
|||
| Alzheimer’s disease | Neuronal cell viability |
Inhibiting caspase-mediated apoptosis signaling | [26] |
| Proinflammatory cytokine and nitric oxide |
|||
| Diabetes | Renal dysfunction, fibrosis, and inflammation |
Suppress of MAPK activity | [27] |
| Proinflammatory cytokine (TNF- |
|||
| Skin inflammation | UV-induced skin inflammation |
Suppress MAPK/AP-1 signaling pathways | [28, 29] |
| UV-generated pyrimidine dimer, COX2 expression |
Ig, Immunoglobulin; TNF, Tumor-necrosis factor; IL, Interleukin; UV, Ultra-violet; COX, Cyclooxygenase-2; NF-
Apigenin has exerted remarkable antiviral activity against diverse viruses such as herpes simplex virus (HSV) [30, 31, 32, 33, 34], enterovirus 71 (EV71) [35, 36, 37, 38, 39], hepatitis C virus (HCV) [40, 41, 42, 43], dengue virus (DENV) [44, 45, 46, 47], severe acute respiratory syndrome coronavirus (SARS-CoV) [48, 49, 50, 51, 52, 53], and influenza virus [54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68]. Its antiviral activity is theorized to be mediated by multiple mechanisms, such as inhibiting viral replication, suppressing viral gene expression, and modulating host immune responses.
Asteraceae plant extracts, from which apigenin is abundantly isolated, were
found to have an antiviral effect against HSV-1 in a previous report [30].
Similarly, chamomile extract is one of the richest natural apigenin sources,
exhibiting anti-HSV-1 and -HSV-2 activity in vitro. Moreover, chamomile
extract inhibits HSV-1 and HSV-2 during or after viral absorption; the most
effective viral inhibition stage was when the extract was provided during viral
absorption. Chamomile extract also presented the most substantial virucidal
activity on both HSV-1 and HSV-2 particles than other plant extracts, completely
inactivating viral particles within 3 and 1 hours against HSV-1 and HSV-2,
respectively, indicating apigenin’s potent antiviral activity against HSV [31].
Also, Rittà et al. [32] discovered that Arisaema tortuosum
leaf extract exhibited anti-HSV-2 activity even against
acyclovir-resistant HSV-1 and HSV-2. Among extract components, apigenin produced
the most inhibitory activity against HSV-1 (EC
Apigenin’s antiviral properties were also effective against Enterovirus
71 (EV71). Apigenin from dried Paulownia tomentosa flowers inhibited EV71
infection with an 11.0 µM EC
Similarly, several studies have observed apigenin’s effect on HCV. As an
Eclipta alba component, apigenin substantially interfered with HCV
non-structure protein (NS) 5B’s RNA-dependent-RNA polymerase (RdRp) activity,
exhibiting a 175.5 µM IC
Apigenin is also known to inhibit the DENV protein NS5. In a cell culture system
antagonized by DENV’s NS5 during infection [44], apigenin obstructed DENV2
replication by restoring STAT2 phosphorylation. Notably, apigenin stimulated
STAT2 even without infection by promoting STAT2 Tyr 689 phosphorylation and
activation, indicating a low probability for viral escape mutations affecting
apigenin [45]. Concomitant to the inhibitory activity of apigenin against DENV
protein, apigenin treatment reduced DENV titer in vitro. Apigenin
inhibited DENV-3 infection in macrophage (U937-DC-SIGN) up to 50% at 40 µM
[46]. Also, apigenin inhibited DENV2 infectivity with EC
SARS-CoV has recently emerged as a predominantly threatening virus, prompting
researchers to seek novel antiviral agents. Therefore, as a prominent
plant-derived antiviral material, apigenin’s antiviral effect against SARS-CoVs
has been increasingly investigated. Chaves and colleagues revealed apigenin’s
antiviral activity against SARS-CoV-2 B.1 lineage in Calu-3 cells with a 5.11
These findings substantiate that apigenin has immense potential for antiviral therapeutics against diverse viruses such as HSV, EV71, HCV, and DNV (Table 2, Ref. [30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53]).
| Virus | Antiviral effects | Mechanism | Ref |
| HSV | EC |
Exert virucidal activity, Interfere with viral absorption, Inhibit the post-entry step of the virus replication | [30, 31, 32, 33, 34] |
| EV71 | EC |
Inhibit interaction between internal ribosome entry site of EV71 and hnRNP A1 and A2 | [35, 36, 37, 38, 39] |
| HCV | Reduced RNA copy number by 0.1 µM of apigenin, Inhibition of HCV replication by 5 µM of apigenin | Rapidly bind to NS5B and inhibit RdRp activity, Decrease miR122 expression levels, Suppress the phosphorylation of TRBP | [40, 41, 42, 43] |
| DENV | EC |
Restore STAT2 Tyr 689 phosphorylation and activation, Colocalized with a DENV protein in the early phase of DENV | [44, 45, 46, 47] |
| SARS-CoV | EC |
Reduce the production of proinflammatory cytokine in response to virus, Interact with viral protein (Mpro) and host factor (ACE-2 receptor, TMPRSS2) | [48, 49, 50, 51, 52, 53] |
HSV, Herpes simplex virus; EV, Enterovirus; HCV, Hepatitis C virus; DENV, Dengue virus; SARS-CoV, Severe acute respiratory syndrome coronavirus; EC, Effective concentration; NS, Non-structure protein; hnRNP, Heterogenous nuclear ribonucleoprotein; RdRp, RNA-dependent-RNA polymerase; TRBP, TAR RNA binding protein; STAT, Signal transducer and activator of transcription; Mpro, main protease; ACE, Angiotensin-converting enzyme; TMPRSS2, Transmembrane protease serine subtype 2.
Several studies have explored apigenin’s antiviral effect on influenza (Table 3,
Ref. [54, 55, 56, 59, 60, 61, 62]). Apigenin inhibited replication of influenza A virus (IAV) H1N1 strains
through diverse mechanisms such as inhibition of NA, viral attachment/entry, mRNA
expression, RdBP activity, viral particle production, nucleoprotein production,
lessening cytopathic effects caused by the virus infection [54, 56, 59, 60, 61, 62].
Similarly, apigenin inhibited the replication of the avian influenza H5N1 virus
strain A/Thailand/Kan-1/04 (IC
| Type | Subtype | Strain | EC |
Mechanism | Ref |
| A | H1N1 | California/07/2009 | EC |
NA inhibition, Viral attachment and entry inhibition, Inhibition of viral mRNA expression, Inhibition of IAV RdRP activity, Reduction of viral particle production, Nucleoprotein reduction | [54, 56, 59, 60, 61, 62] |
| PR/8/34 | IC |
||||
| EC |
|||||
| Toyama/129/2011 | EC |
||||
| Toyama/26/2011 | EC |
||||
| Osaka2024/2009 | IC |
||||
| Osaka71/2011 | IC |
||||
| H3N2 | Jinan/15/90 | IC |
NA inhibition (IC |
[56] | |
| H5N1 | Thailand/1(Kan-1)/04 | - | Nucleoprotein reduction (IC |
[55] | |
| B | Jiangsu/10/2003 | - | NA inhibition (IC |
[56] |
PR, Puerto Rico; EC, Effective concentration; IC, Inhibitory concentration; CC, Cytotoxic concentration; CPE, Cytopathic effect; NA, Neuriminidase; IAV, Influenza A virus.
Upon replication onset (0–4 hours post-infection, hpi), influenza viruses attach to host cells and enter by endocytosis [57, 58]. Apigenin treatment before viral infection (cell protection assay) did not significantly reduce IAV mRNA expression [54] or cytopathic effect [59, 60], whereas apigenin pre-incubation with IAV did [54, 60]. These results demonstrate apigenin’s disruptive activity against IAV and provide insight into IAV particle and apigenin interactions. Moreover, apigenin inhibited replication when cells were simultaneously treated with apigenin and the influenza virus. Similarly, apigenin post-treatment (2 and 4 hpi) effectively reduced virus-induced cytopathic effects and IAV mRNA expression in host cells. Therefore, these results following apigenin administration 0–4 hours post-infection (hpi) indicate interference with the influenza A virus’ attachment and penetration. Apigenin’s anti-influenza viral activity during early-stage infection was also observed for other IAV strains, including H1N1 (A/California/07/2009, A/PR/8/34) and H3N2 (A/Jinan/15/90, A/Minfang/151/2000) [54, 56, 59, 60, 61, 62]. Collectively, these results prove that apigenin can inhibit early-stage IAV replication (Fig. 1).
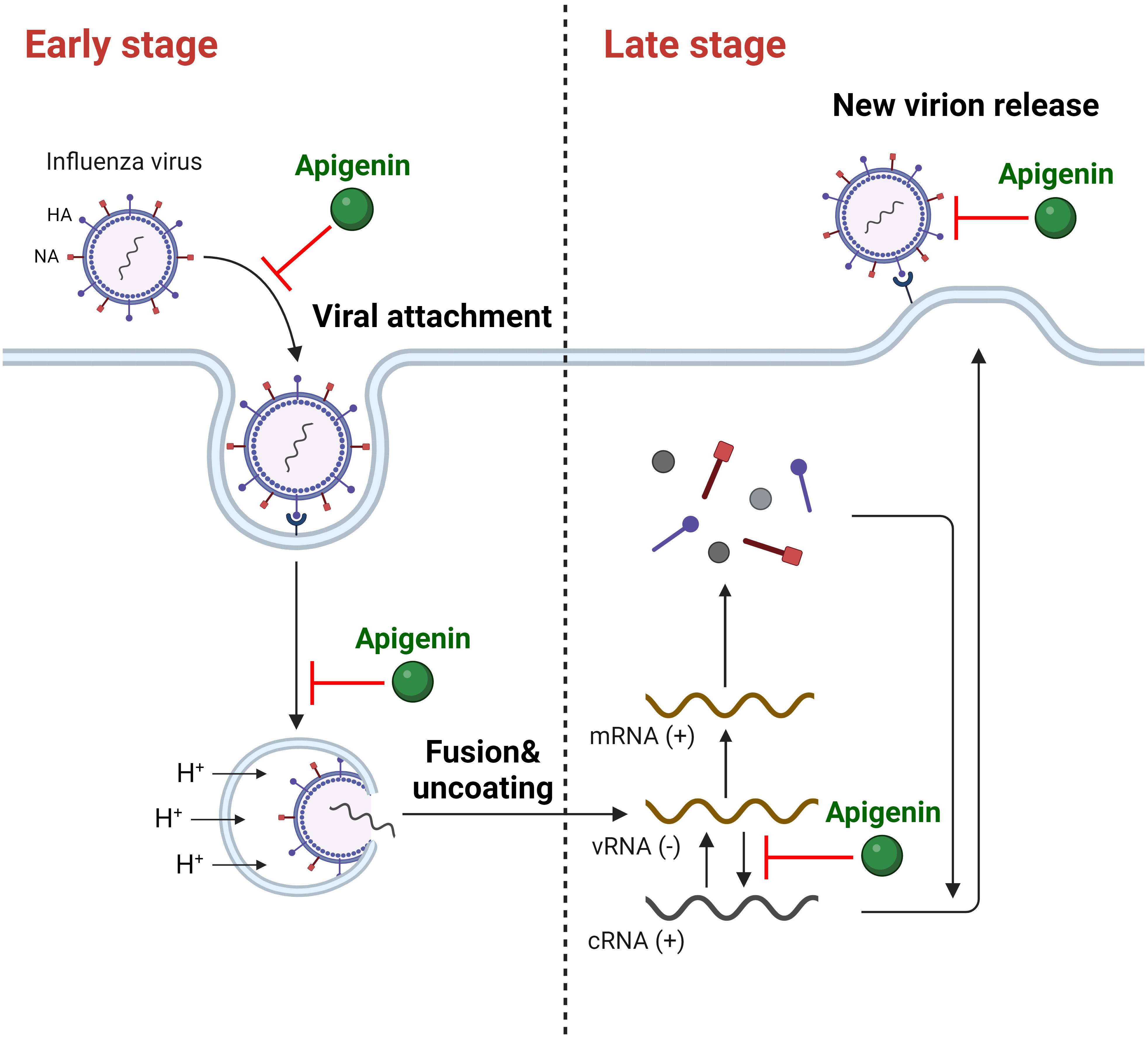 Fig. 1.
Fig. 1.The inhibitory activity of apigenin against IAV on multiple stages of infection. (+), positive strand; (-), negative strand. Created with https://www.biorender.com/.
Viral mRNA translation, protein packaging, egress, and other critical propagation processes occur during late-stage viral replication (4–8 hpi). The influenza virus forms a complete structure by assembling its progeny particles and releasing them from the host cell for propagation [57, 58]. Notably, apigenin also inhibited late-stage IAV replication. When cells were treated with apigenin 5 hpi, (+)-strand viral RNA synthesis was attenuated, supporting apigenin’s potential for IAV RNA-dependent-RNA polymerase (RdRP) inhibition [54]. Similarly, viral particle production was considerably reduced when apigenin was administered 4–8 hpi [59, 60, 61]. Apigenin treatment after virus infection decreased the level of viral NP after 8 hpi with inhibited NA activity, indicating the inhibitory role of apigenin in virus assembly and release stages [41]. Thus, apigenin suppresses IAV replication by obstructing multiple IAV replication stages (Fig. 1).
Apigenin’s antiviral effect on drug-resistant IAV strains was also investigated.
Morimoto et al. [61] noted that apigenin exhibited antiviral effects on
oseltamivir-resistant strains (Osaka/2024/2009 and Osaka/71/2011) with 23.3
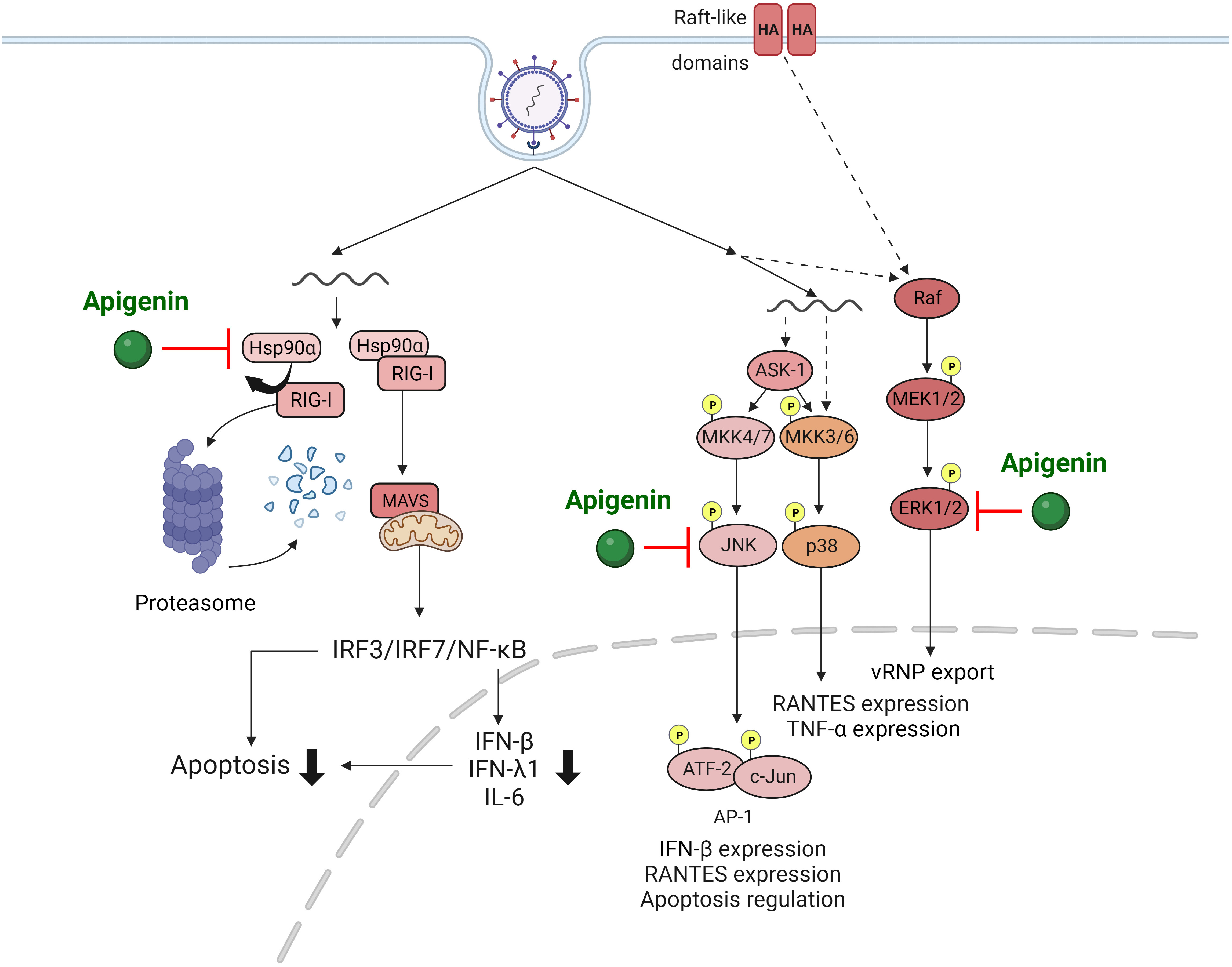
IAV infection activates various signaling pathways within the host cell, such as mitogen-activated protein kinase (MAPK) [63, 64] and retinoic acid-inducible gene-I (RIG-I) pathways [65]. Assessing alterations in signaling pathways up-regulated by IAV provides invaluable insights for antiviral drug developments targeting these components. Joo et al. [54] revealed that apigenin effectively suppressed IAV infection-induced MAPK signaling pathway activations that are critical for virus replication and cytokine production. Importantly, apigenin severely suppressed the elevation of extracellular signal-regulated (ERK) and stress-activated (SAPK) protein kinases phosphorylation upon IAV infection, subsequently reducing viral replication.
RIG-I is a prominent innate RNA sensor that recognizes influenza viral RNA and
activates antiviral host responses, including cytokine cascade [65]. However,
uncontrolled overproduction of cytokines such as interferons (IFNs) is a major
factor that exacerbates immunopathologic lung injury upon IAV infection [66, 67, 68].
Therefore, regulating cytokine production induced by RIG-I pathway activation is
vital for managing IAV infection outcomes. Xu et al. [59] revealed that
apigenin treatment significantly suppressed IAV-induced RIG-I activation and
proinflammatory cytokines such as IFNs. Interestingly, apigenin-mediated
suppression of RIG-I was due to ubiquitin-mediated RIG-I protein degradation,
leading to disruption of interactions between RIG-I and heat shock protein
90
Lastly, apigenin’s anti-IAV activity strongly suggests its potential as an
antiviral drug in vitro, inspiring many in vivo studies.
Intranasally administered apigenin significantly reduced viral loads in mouse
lungs infected with the IAV H1N1 strain (A/California/07/2009), corroborating
apigenin’s IAV suppression in vitro. Furthermore, oral administration of
an aqueous Agrimonia pilosa and Galla rhois extracts mixture
(APRG64), where apigenin is the main antiviral component, considerably curtailed
IAV-infected mice morbidity and mortality. In addition, proinflammatory cytokine
expressions, such as IFN-
Influenza has continuously threatened human health, invoking unpredictable and recurrent pandemics; four influenza A pandemics have occurred within the last century. Although most influenza A and B strains are susceptible to oseltamivir or zanamivir (neuraminidase inhibitors), resistant strains were observed frequently since the 2009 H1N1 influenza A pandemic. Therefore, developing novel anti-influenza drugs that effectively target current drug-resistant strains is urgently needed. Apigenin is a plant flavonoid component with substantial antiviral effects against various viruses, including influenza virus, evidenced through numerous in vitro and in vivo experiments. As summarized in the present review, apigenin exerts anti-viral activity against HSV, HCV, EV71, DENV, SARS-CoV, and influenza by disrupting virus particles, interfering with multiple viral replication stages by inhibiting viral proteins, regulating virus-induced signaling pathways, and interrupting virus interactions with host factors, such as RdRP, the ACE-2 receptor, and TMPRSS2.
Apigenin directly inhibits influenza virus and host factor interactions while alleviating pathogenic inflammatory responses caused by infection, similar to clinically used anti-IAV drugs. Furthermore, apigenin has also proven effective against drug-resistant influenza strains. These results indicated that apigenin is a promising candidate for anti-influenza therapy, as it can directly inhibit influenza infectivity and replication while reducing clinical symptoms associated with robust inflammatory responses. However, despite its potential as an anti-influenza drug, further studies using in vivo and nonhuman primate models are needed to reveal apigenin’s intricate anti-influenza mechanisms for clinical use, as current findings are limited to in vitro and in vivo mouse experiments.
Various studies have studied apigenin’s pharmacological features, reporting its nontoxic and nonmutagenic nature with various biological benefits, including anti-inflammatory, anti-cancer, anti-oxidant, anti-bacterial, and anti-depressant. However, high apigenin doses may induce slight side effects [71, 72, 73]. Apigenin exerts biological effects through numerous signaling molecule interactions, including ERK, c-Jun N-terminal kinase (JNK), p38, enzymes like cytochrome P450s, and the benzodiazepine receptor [73, 74, 75]. Based on their remarkable beneficial effects, apigenin’s pharmacokinetics has been investigated. Researchers found that oral apigenin administration in a rat model was absorbed, resulting in deficient blood levels [76, 77]. Apigenin absorption primarily undergoes Phase I to produce luteolin, scutellarein, and iso-scutellarein by utilizing Phase I enzymes and Phase II metabolism through glucuronidation and sulfation of apigenin in enteric and enterohepatic cycling [72, 77, 78, 79, 80]. Up to 63% of apigenin was excreted through urinary and fecal routes, as corroborated in clinical trials [77, 81]. Also, apigenin elimination was reported as considerably slow; 24.8% of radiolabeled apigenin was observed in the body ten days post-administration [77]. Apigenin’s higher absorption and accumulation extent than other dietary flavonoids, such as luteolin and quercetin, indicate better oral bioavailability and pharmacokinetics [77, 79, 82, 83]. However, apigenin’s clinical developments are severely limited due to its low solubility in water and organic solvents. Therefore, new technologies or formulations must be developed to improve apigenin’s bioavailability.
Similar to other plant extracts, apigenin’s slow production rate or inefficient processing techniques could hamper its clinical applications, particularly during an influenza pandemic. Therefore, a systemic mass-production manufacturing system must be developed.
Ig, Immunoglobulin; TLR, Toll-like receptor; MyD, Myeloid differentiation
primary response protein; NF-
Conceptualization: YS, SH, IL, JL; writing/original draft preparation: IL, JL, YS, SH; writing-review and editing; YS, SH. All authors read and approved the final version of the manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
The figures were created with Biorender.com.
This work was supported by the Chung-Ang University Research Scholarship Grants in 2023 and by the National Research Foundation of Korea (NRF) grant funded by the Korean government [grant number: NRF-2018R1A5A1025077].
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.