1 Faculty of Chinese Medicine and State Key Laboratory of Quality Research in Chinese Medicine, Macau University of Science and Technology, 999078 Macao, China
2 College of Pharmacy, Hangzhou Normal University, 311121 Hangzhou, Zhejiang, China
3 Department of Medical Oncology, The Affiliated Hospital of Hangzhou Normal University, Hangzhou Normal University, 310015 Hangzhou, Zhejiang, China
4 The First Clinical Medical College of Nanjing University of Chinese Medicine, Jiangsu Collaborative Innovation Center of Traditional Chinese Medicine Prevention and Treatment of Tumor, 210023 Nanjing, Jiangsu, China
5 Center for Medical Research and Innovation, the First Hospital of Hunan University of Chinese Medicine, 410000 Changsha, Hunan, China
6 Key Laboratory of Elemene Class Anti-Cancer Chinese Medicines, 311121 Hangzhou, Zhejiang, China
7 Engineering Laboratory of Development and Application of Traditional Chinese Medicine from Zhejiang Province, 311121 Hangzhou, Zhejiang, China
8 Guangdong-Hong Kong-Macao Joint Laboratory for Contaminants Exposure and Health, 510000 Guangzhou, Guangdong, China
9 Zhuhai MUST Science and Technology Research Institute, 519000 Zhuhai, Guangdong, China
10 Zhuhai Hospital of Integrated Traditional Chinese and Western Medicine, 519020 Zhuhai, Guangdong, China
†These authors contributed equally.
Academic Editors: Antonio Barbieri and Francesca Bruzzese
Abstract
Cancer progression and metastases are the leading causes of poor outcomes in patients with colon cancer. Colon cancer metastasis is a multigene, multistep, multistage complex process in which target genes, microRNAs, epithelial-stromal transformation, tumour stem cells, the tumour microenvironment, and various cell signalling pathways are implicated in the progression and metastasis of colon cancer. Although conventional therapies have made significant advances in treating the progression and metastasis of colorectal cancer, they have failed to improve survival outcomes. Natural compounds may have more significant potential in preventing and treating colon cancer. Active natural compounds exert their antitumor effects by inducing tumour cell differentiation, promoting tumour cell apoptosis, inhibiting tumour vascular growth, and regulating immunity. Natural compounds, combined with conventional therapies, can target mutant genes and various cellular signalling pathways, inhibit epithelial-stromal transformation, and improve the tumour microenvironment to inhibit tumour progression and metastasis. The synergism of natural compounds and conventional therapeutics has the potential to become a promising therapy for treating colorectal cancer progression and metastases.
Keywords
- natural compounds
- conventional therapeutics
- colorectal cancer
- progression and metastasis
- drug combination
Colorectal cancer is the third most prevalent malignancy in the world after breast and lung cancer and has the second highest mortality rate of all malignancies. It results in more than a million fatalities each year, accounting for one-tenth of cancer diagnoses and deaths, and the incidence is annually increasing [1, 2, 3]. Approximately twenty percent of colorectal cancer patients present with metastases, and another twenty-five percent progress and develop metastases following treatment [4]. Colorectal cancer can be distinguished by three pathogenic mechanisms: chromosomal instability (CIN), microsatellite instability (MSI), and CpG island methylation phenotype (CIMP). There is a strong correlation between age, inflammatory bowel disease, and poor lifestyle choices and the development of colon cancer. Poor eating habits increase the risk of colon cancer by 70% [5]. Mesenchymal cells in tumours enhance the formation and progression of colon cancer by regulating intestinal inflammation, epithelial cell proliferation, stem cell maintenance, angiogenesis, and the extracellular matrix [6]. Colon cancer progression and metastasis often show extensive reprogramming of gene expression [7]. Identification of the major regulators driving pathological gene expression is the key to the treatment of colorectal cancer. Current studies have identified alterations in the KRAS, BRAF, PI3K and p53 genes that contribute to the development, progression and metastasis of colon cancer [8]. Genetic changes in metastatic colon cancer are the subject of drug research, clinical trials, and targeted chemotherapy protocols [9]. Alterations in cancer metabolism lead to colorectal cancer progression and metastasis [10], and supplies the energy for tumour growth, the replenishment of precursors, and the reduction of equivalents. Zheng, X. et al. [11] discovered that circPPP1R12A is essential for the proliferation, migration, and invasion of colorectal cancer cells. In addition, several cellular signalling pathways have been demonstrated to be dysregulated, resulting in the growth and metastasis of colon cancer. These include Wnt/-linked protein, p53, TGF-/SMAD, NF-kB, Notch, VEGF, and JAKs/STAT3, as well as methylation associated with the cell cycle, transcription, apoptosis, and angiogenesis (Fig. 1), and signalling pathways associated with invasion and metastasis [12]. Conventional therapies for colon cancer progression and metastasis include surgery, chemotherapy, radiotherapy, interventional therapy, targeted therapy, cell therapy and immunotherapy, either alone or in combination, depending on the patient’s condition and disease stage [13, 14]. However, the survival outcomes of patients remain poor, and conventional therapies may lead to serious side effects and tumour resistance. There is an urgent need to identify more optimal treatments for patients with colorectal cancer.
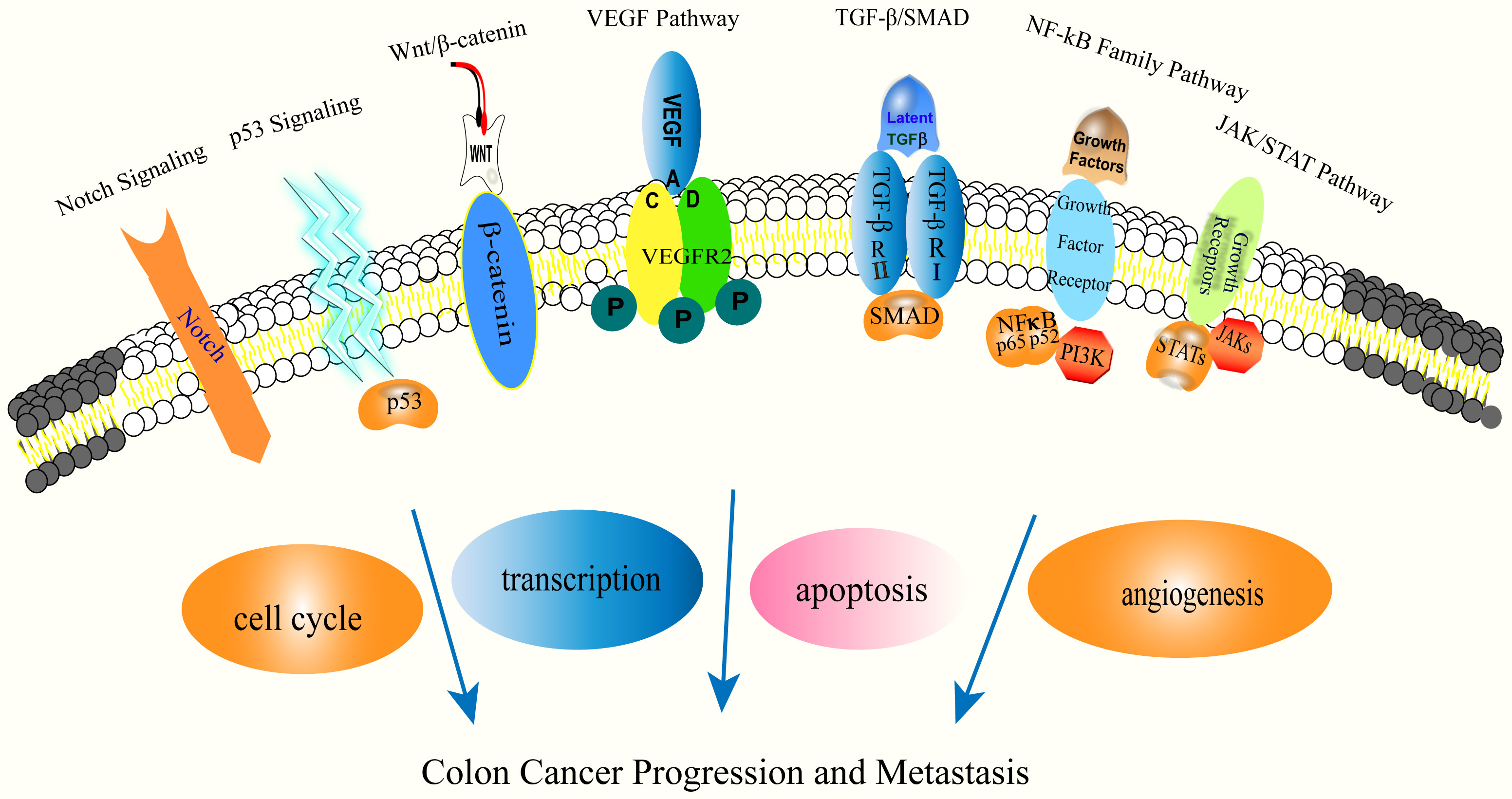 Fig. 1.
Fig. 1.Molecular mechanisms of colon cancer progression and metastasis.
Natural compounds are macromolecular compounds formed in nature or in minerals through biochemical actions or photosynthesis. They are found in animals, plants or minerals and are secondary metabolites produced in response to external stimuli [15]. Numerous studies have confirmed the capacity of a variety of natural compounds to suppress cancer. Natural compounds exhibit anticancer action via distinct in vivo and ex vivo mechanisms and pathways, such as inducing tumour cell differentiation, triggering cell cycle arrest, promoting tumour cell apoptosis, inhibiting tumour vascular growth, and regulating body immunity, making them essential adjuncts to clinical cancer treatment [16]. Caspase-3 is an important apoptosis marker induced by cytotoxic medicines, radiation, and immunotherapy. Caspases-3-targeted therapy reduces the invasion and metastasis of cancer cells and has been shown to inhibit tumour progression and metastasis [17]. Polyphenols (flavonoids, catechin, hesperetin, flavones, quercetin, phenolic acids, ellagic acid, lignans, stilbenes, and others) are a diverse group of natural substances used to prevent and treat cancer [18]. Natural polyphenols are cytotoxic to colon cancer cells and induce increased sensitivity to chemo/radiotherapy. These benefits are most likely connected to the immunomodulatory capabilities of polyphenols, which influence cytokine and chemokine production as well as immune cell activation. Polyphenol-based combination therapy offers a unique immunomodulatory technique for inhibiting colon cancer growth. Research on the combined application of natural substances and conventional therapies has obtained promising results [19]. Some natural chemicals show a synergistic effect with conventional therapies; and can improve the sensitivity of cancer cells to conventional therapy, promote drug utilization, and lessen the side effects caused by conventional therapies [20]. Even at high concentrations, these natural substances are well tolerated by patients and have no harmful side effects [21]. Natural compounds combined with conventional therapies can target mutant genes and various cellular signalling pathways, inhibit epithelial-mesenchymal transition (EMT), and improve the tumour microenvironment to inhibit tumour progression and metastasis. In addition, they can be used in different combinations to target multiple signalling pathways to prevent tumour progression and metastasis.
In this review, we searched the PubMed Database, Web of Science, and the Chinese databases CNKI, SinoMed, and Wanfang Data Knowledge Service Platform using the keywords “colon cancer” or “colorectal cancer” and “natural products” or “natural compounds” for articles published since January 2017, on the synergistic effect of natural substances and conventional therapies on the progression and metastasis of colon cancer in both Chinese and English.
We comprehensively analysed and summarized the literature on the pharmacological effects and molecular mechanisms of these natural compounds and conventional therapies to inhibit the progression and metastasis of colon cancer, to determine the role of natural compounds in preventing and treating colon cancer.
We searched English and Chinese databases, including PubMed, Web of Science, CNKI Database, and SinoMed, and screened relevant literature published in China and abroad. The databases were searched using the following terms: “bioactive compounds” OR “Natural compound” OR “natural product” OR “traditional Chinese medicine” OR “herb-medicine” AND “colorectal neoplasms” OR “colon cancer” OR “colorectal cancer”. The publication dates were from January 2017 to May 2022.
Subjects were comprehensively searched in combination with keywords, topics, abstracts, and free words to ensure the systematization and integrity of the literature retrieval.
We searched all basic and clinical studies on the synergistic antitumor mechanism of natural compounds and conventional therapies and collected all confirmed targets. To ensure the authenticity and systematic nature of the results, we included all cell and animal samples in relevant studies.
A total of 9880 articles were retrieved. After excluding review articles, studies on TCM compounds, studies on pure natural compounds, and other articles unrelated to single drugs, our study included 411 single drug articles involving 46 natural compounds. There were nine natural compounds combined with radiotherapy, 32 natural compounds combined with chemotherapy, four natural compounds combined with targeted therapy, two natural compounds combined with immunotherapy (Fig. 2), and five clinical experimental studies. We found that natural compounds in combination with conventional therapies may play an important role in limiting the progression of metastasis of colon cancer.
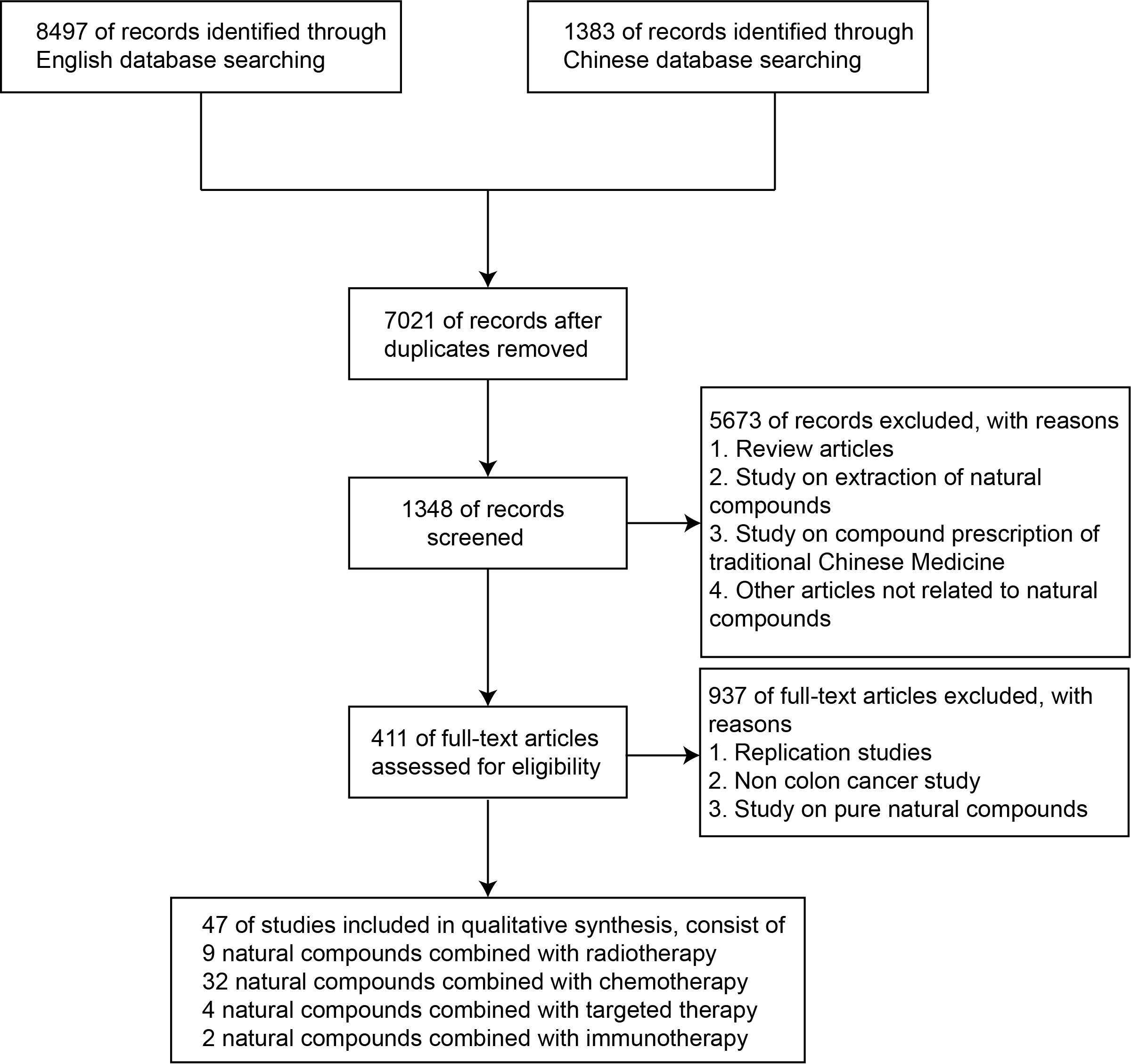 Fig. 2.
Fig. 2.Study flow diagram.
Neoadjuvant radiotherapy (NACRT) has been established as the standard of care
for the progression and metastasis of colon cancer and for the treatment of liver
and lung metastases and has been found to reduce local recurrence [22]. Radiation
therapy for liver and lung metastases of colon cancer is now recommended in
treatment guidelines and can improve local control of metastases and prolong
survival. However, colon cancer exhibits therapeutic resistance to ionizing
radiation (IR), resulting in increased doses for clinical treatment, which can
cause damage to adjacent normal tissues and organs [23]. The presence of
alkaloids, resins, volatile oils, and tannins in the chemical structure of
natural substances confers radioprotection [24]. Some contain natural compounds,
such as curcumin, resveratrol (RV), and emodin, which have been shown to promote
the mitigation of side effects caused by chemotherapy and radiotherapy [25].
Starfish is a marine anuran that contains sterols [26], polar steroids [27] and
sphingolipids [28]. Sea stars are rich in various low molecular weight
metabolites with various biological activities, such as antiviral, anticancer and
neuroprotective activities [29]. Pectin aeruginosa is an antibacterial and
anticancer peptide isolated from the epithelial extract of the body cavity of
starfish [30]. Malyarenko et al. [27] discovered that Asterosaponin P1,
the polar steroidal active component of the starfish Patiria (=Asterina)
pectinifera, increases the efficacy of radiation therapy by modulating anti- and
proapoptotic protein production, caspase protein activation, and DNA degradation.
D-Limonene is a citrus oil extract with anticancer potential [31]. It inhibits
tumorigenesis, growth and angiogenesis [32] and increases the expression of Bax,
activates cysteine aspartase and induces cellular regulation. Vukmirovic
et al. [33] found that d-Limonene improved the radiosensitivity of
HCT116 p53(+/+) cells. It can be used as a sensitizer for radiotherapy. Piperine
(1-piperonylpiperidine), the primary extract of Piper longum and Piper nigrum,
comprises long tissue-structured alkaloids that have been shown to have
antiproliferative, antitumor, antiangiogenic, and antioxidant properties
in vitro and in vivo [34]. Piperine has been demonstrated to
decrease cancer cell proliferation and migration by modulating cell cycle
progression and triggering apoptosis [35]. Shaheer et al. [36] reported
that piperine combined with radiation therapy increased cell proliferation by
interfering with cell proliferation, preventing G2/M phase cells, DNA damage, and
death of a colon cancer cell line.
| Tested molecule | In combination with | Experimental model | Main result | Proposed mechanism | References |
| Asterosaponin P1 | X-ray | In vitro: DLD-1, HCT 116, HT29 | Increased radiosensitivity | Upregulation of cleaved caspase-3, Bax; Downregulation of Bcl-XL, caspase-3, caspase-9 | [27] |
| D-limonene | In vitro: HCT116 p53+/+ | Increased radiosensitivity | - | [33] | |
| Piperine | In vitro: HT29 | Increased radiosensitivity | G2/M cell cycle arrest, Upregulation of c-caspase-3, c-PARP-1, Bax; Downregulation of Bcl-2 | [36] | |
| APP | IR | In vitro: HCT116, DLD-1, SW480, COLO320DM; In vivo: HCT116 cells/mouse | Increased radiosensitivity | Upregulation of c-caspase-3, c-PARP, c-caspase-9, ROS, |
[38] |
| TET | IR | In vivo: CT26/tk-luc cells/mouse | Increased radiosensitivity | Upregulation of c-caspase-3 | [41] |
| Quercetin | IR | In Vitro: HT29, DLD-1; In vivo: HT29 cells/mouse | Increased radiosensitivity | Upregulation of c-caspase-3, c- caspase-7, c-PARP-1, Downregulation of Notch-1, Hes-1 | [44] |
| PD | IR | In vitro: CT26, HCT116; In vivo: C57BL/6 CRC mouse model by AOM/DSS | Increased radiosensitivity | Upregulation of c-caspase-3, Notch-1 | [47] |
| Shikonin | In vitro: SNU-C5RR | Reversal of radiation resistance | Upregulation of cleaved caspase-3, cleaved caspase-9, Bax, E-cadherin; Downregulation of Bcl-2, ROS, N-cadherin | [51] | |
| Phenylacetate and tauroursodeoxycholate | In vitro: HCT116 p53 wild-type | Radioprotector | - | [54] | |
| Genistein | In vitro: HCT116 | Increased radiosensitivity | Inhibited EGFR phosphorylation | [56] | |
| APP, | |||||
Chemotherapy is an essential treatment for preventing colon cancer progression and metastases. 5-Fluorouracil (5-FU), capecitabine, oxaliplatin, doxorubicin and irinotecan are commonly utilized agents. However, medication resistance and severe toxicity might develop over time. Numerous studies have demonstrated that chemotherapy in combination with natural compounds can exert synergistic effects through various cell cycle pathways, as well as those associated with drug-resistant phenotypes: transcription factors, membrane receptors, adhesion and structural molecules, cell cycle blockade, and apoptosis [57]. It can inhibit tumour progression and metastasis, reduce the dose of conventional chemotherapeutic drugs, produce the same or higher efficacy, and reduce treatment resistance [58]. The combined treatment of chemotherapy and natural compounds has three main functions: enhancing the effectiveness of chemotherapy drugs, reducing treatment resistance and reducing the toxicity and side effects associated with chemotherapy.
Chemotherapy with 5-fluorouracil (5-FU) is the standard chemotherapeutic agent
for the treatment of colon cancer. Resistance to 5-FU is a significant barrier to
the successful treatment of colon cancer. 5-FU-resistant colon cancer cells have
enhanced EMT and antiapoptotic capacity. Drug-resistant cells generally exhibit
accelerated proliferation and distant metastases [59]. The combination of natural
compounds with 5-FU treatment can inhibit colon cancer progression and metastases
by inhibiting epithelial-stromal transformation and promoting apoptosis. Vine
pruning residue (VPE), which has anticolon cancer potential, is a polyphenol-rich
extract generated by electrifying and heating vine pruning residue. Jesus
et al. [60] found that VPE combined with 5-FU inhibits human colon
cancer cell
proliferation, through DNA modulation and cell cycle regulation, and
improves cell sensitivity to 5-FU. Sulforaphane is abundant in Brassica juncea,
has antioxidant and anticancer properties, and activates the transcription factor
Nrf2 [61] to maintain intracellular homeostasis. Milczarek et al. [62]
found that combined treatment with 5-FU and lysostaphin showed higher efficacy by
synergistically blocking the cell cycle and downregulating related proteins
involved in the apoptotic process in HT29 cells, such as caspase-3, caspase-8,
and caspase-9, which significantly promoted apoptosis in colon cancer HT29 cells.
Ganoderma lucidum (GLC) is a medicinal mushroom. Its main bioactive compounds are
polysaccharides and triterpenoids, which show antitumor and immunomodulatory
activities [63]. Opattova et al. [64] demonstrated that Ganoderma
lucidum selectively induced oxidative DNA damage in colon cancer cell lines and
that accumulation of DNA damage led to sensitization of cancer cells to 5-FU.
In vivo experiments revealed that GLC combined with 5-FU reduced the
effective therapeutic dose of anticancer drugs, increased survival and reduced
tumour volume in mice [65]. By decreasing STAT3 phosphorylation and binding to
the human telomerase reverse transcriptase (hTERT) promoter area, combination
therapy with resveratrol and 5-FU induces apoptosis in colon cancer cells and
re-sensitizes tumours to chemotherapy. Curcumin suppressed the expression of NNMT
and p-STAT3 in 5-FU-resistant colorectal cancer cells (HT29 and SW480) [66].
Inhibition of cell growth, arrest in the G2/M phase of the cell cycle, and
generation of reactive oxygen species (ROS) reduced treatment resistance.
Autophagy is a crucial mechanism of cellular chemoresistance. Curcumin
significantly increased the killing impact of 5-FU on HCT116 and HT29 cells [67].
Attia et al. [68] found that Verbascoside is sensitive to 5-FU in an
in-vitro model [66]. It lowered 5-FU resistance in colorectal cancer
cells by targeting the PI3K/Akt pathway and triggered apoptosis primarily through
overexpression of Bax and downregulation of BCL-2. Vanillin
(4-hydroxy-3-methoxybenzaldehyde) is a natural component extracted from vanilla
bean that possesses antioxidant, anti-inflammatory, and antitumor properties and
protects against kidney damage induced by chemotherapy [69]. Kong et al.
[70] showed that vanillin suppressed the mRNA and protein expression of NNMT in
colon cancer cells by upregulating p53, c-PARP, c-caspase-3, and c-caspase-9 and
activating the ASK1-p38 MAPK pathway to enhance apoptosis and reduce 5-FU
resistance. S-adenosylmethionine (AdoMet) is an antiproliferative, proapoptotic,
and drug-resistant agent with several targets in colon cancer cells. Mosca
et al. [71, 72] demonstrated that simultaneous treatment with AdoMet and
5-FU in HCT116p53-/-, HCT 116p53+/+, and LoVo cell lines inhibits autophagy and
increases apoptosis, thereby boosting the death of tumour cells and overcoming
5-FU resistance. PD is an extract of the PD plant that possesses antioxidant,
anti-inflammatory, and anticancer properties and enhances the sensitivity of
radiation and chemotherapy against cancer cells. Bae et al. [73] found
that the combination of PD and 5-FU had a synergistic anticancer impact on HCT116
and HT-29 cells. Oxidative stress and the loss of mitochondrial membrane
potential promote mitochondrial malfunction. Alterations in calcium regulation
elevate the expression of apoptosis and its associated proteins. PD inhibits the
MAPK and PI3K/AKT signalling pathways and counteracts drug resistance in
5-FU-resistant cells. Gallocatechin gallate (EGCG), an active catechin in green
tea, inhibited tumor growth and enhanced the sensitivity of colon cancer cells to
5-FU. EGCG in combination with 5-FU significantly reduced the IC50 of HCT116 and
DLD1 cells and promoted apoptosis and DNA damage in cancer cells. Further
mechanistic studies showed that EGCG activated NF-
Oxaliplatin (OXA) is a third-generation platinum anticancer drug and a platinum compound of dicyclohexane. It has the same effect as other platinum drugs, in that they all target DNA as the site of action, and the platinum atoms form a cross-association with DNA, antagonizing its replication and transcription. The combination of natural compounds with oxaliplatin treatment can inhibit colon cancer progression and metastases by regulating signalling pathways and promoting apoptosis. Resveratrol is a naturally occurring stilbene and nonflavonoid polyphenol found in grapes, mulberries, peanuts, rhubarb, and other plants [56] that has antioxidation, heart protection and anticancer characteristics. Studies indicate that resveratrol when combined with chemotherapy can boost the sensitivity of cancer cells to conventional chemotherapy drugs. Wang et al. [76] discovered that the combination of oxaliplatin and resveratrol nanoparticles greatly decreased the levels of SMA and CUGBP1 in tumours and significantly increased the cytotoxicity of SW480 and CT26 cells in vitro. In vivo experiments demonstrated that this combination decreased the number of mesenchymal stem cells in tumour-bearing mouse bone marrow, decreased tumour immune evasion, and considerably boosted its anticancer effects. Anthocyanins and polyphenols found in blueberry extract (BE) have antioxidant and anticancer properties [77]. Lin et al. [78] found that the combination of blueberry extract with oxaliplatin for the treatment of HCT-116 cells induced G0/G1 cell cycle arrest and apoptosis, which had a synergistic anti-colon cancer impact and reduced the toxicity of chemotherapy agents. Nobiletin is an extract from citrus peel with anticancer properties. Nobiletin increases the inhibitory effect of oxaliplatin on colon cancer cell proliferation, promotes apoptosis, upregulates Bax and cleaved-caspase3 protein expression, and downregulates Bcl-2 protein expression to enhance the sensitivity of colon cancer to the chemotherapeutic drug oxaliplatin [79]. Hypericin is a photosensitizer localized in the ER, which in modest quantities of hypericin can preferentially destroy tumour cells [80]. Macejová et al. [81] found that the combination of chrysin and oxaliplatin treatment synergistically decreased cell viability, inhibited cell proliferation, downregulated IAP protein levels, triggered apoptosis, promoted autophagy, and restored oxaliplatin chemosensitivity. Forsythia viridissima fruits (EFVF) are one of the fruits of Forsythia (FF) that have antioxidant and antitumor activity [82]. Yi et al. [83] demonstrated that Forsythia viridissima significantly reduced oxaliplatin-induced mechanical sensitivity. In the pretreatment and combined treatment of oxaliplatin and EFVF, EFVF can also prevent mechanical hyperalgesia generated by oxaliplatin and prevent the neurotoxicity caused by oxaliplatin. Hypericum perforatum L. is a perennial flowering plant that has been used for centuries as a natural remedy for a variety of disorders. Cinci et al. [84] found that the hydrophilic active components of Hypericum perforatum L. had a strong antioxidant effect, which could reduce oxaliplatin-induced neurotoxicity by reducing caspase-3 activity but would not reduce the cytotoxicity of low oxaliplatin on HT-29 cells. Hypericin is a naturally occurring polycyclic aromatic naphthacenone. Evidence suggests that hypericin possesses significant antiproliferative effects on various tumour cells in photodynamic therapy. The anticancer effects modulated by pharmacokinetic therapy with hypericin are mainly mediated by the p38 mitochondrial-activated protein kinase enhancer-binding protein homologous protein receptor and mitochondrial and exogenous signalling pathways [85]. Lin et al. [86] determined that autophagy was responsible for the sensitization and antitumor synergy of hypericin-PDT/L-OHP. High-dose Hy-PDT produces autophagic cell death; low-dose Hy-PDT predominantly induces protective autophagy and promotes cell growth. Low-dose Hy-PDT can lower the cytotoxicity of L-OHP on colon cancer cells resistant to oxaliplatin. Dihydromyricetin (DMY) is an antioxidant, anti-inflammatory, anticancer, and neuroprotective flavonoid derived from Dahlia poplar [87]. Wang et al. [88] reported that the combination of OXA and DMY exhibited synergistic antitumor effects. By reducing MRP2 expression and its promoter activity, DMY restored chemosensitivity (OXA and VCR) in HCT116/OXA and HCT8/VCR cell lines. In addition, DMY suppressed NF-B/p65 expression and reduced NF-B nuclear translocation, silencing Nrf2 signalling essential for MRP2 expression and targeting NF-B to limit Nrf2 transcription in colon cancer to prevent and reverse multidrug resistance.
Doxorubicin is an antitumor antibiotic that can inhibit the synthesis of RNA and DNA, and has an effect on a variety of tumours. It is a cycle-nonspecific drug that kills tumour cells in various growth cycles. The combination of natural compounds and doxorubicin can inhibit colon cancer progression and metastases through cell cycle arrest and by promoting apoptosis. Oxymatrine (OMT) is a quinoline alkaloid produced from the roots of the Sophora japonica plant. It has cancer-fighting, anti-inflammatory, and neuroprotective properties [89]. It can cause apoptosis, suppress tumour cell proliferation, diminish tumour growth in various in vivo models, and augment the anticancer effect of existing chemotherapeutic agents on tumour cells. Pan et al. [90] reported that the combined impact of OMT + ADM significantly reduced the growth of HT-29 and SW620 cells, and that this combination induced cellular regulation by upregulating the ratio of cleaved caspase-3, cleaved caspase-9, and Bax/Bcl-2. FHL-2 downregulation and cleaved SPTAN1 upregulation were validated at both the mRNA and protein levels in SW620 and HT-29 cells. Extract of Scabiosa atropurpurea has antioxidant and anticancer properties [91]. Toumia et al. [92] discovered that Scabiosa atropurpurea extract increased the cytotoxic effect on adriamycin-resistant Caco-2 tumour cells. RT–qPCR demonstrated that the combination enhanced the mRNA expression of Bax, caspase-3, and p21 while decreasing Bcl-2. It reverses P-glycoprotein or multidrug resistance-associated protein in Caco-2 cells while reducing chemotherapeutic resistance. The newly synthesized chalcone derivative (1C) is a chalcone derivative. It was discovered that 1C induces apoptosis and DNA damage repair in HCT116 cancer cells [93]. Čižmáriková et al. [94] discovered in vitro synergistic antiproliferative and cytotoxic actions of 1C and adriamycin. It can be used to sensitize drug-resistant colon cancer to chemotherapy. Isothiocyanates (ITCs) that occur naturally are bioactive hydrolysis products of thioglucosides from cruciferous vegetables (CVs) with antioxidant, anti-inflammatory, and anticancer properties [95]. In an antiproliferation experiment, Psurski et al. [96] detected significantly increased caspase-3 activity in dMBITC-pretreated LoVoDX cells but no significant change in caspase-3 activity in dMBITC-pretreated LoVo-sensitive cells. dMBITC boosted the intracellular retention of adriamycin and lowered glutathione, the formation of reactive oxygen species, and the apoptotic rate, lowering in vivo toxicity and drug resistance. Saffron extract (TMPE) possesses many biological actions, such as antioxidant, anticancer, and antidrug-modifying effects. In a study by Środa-Pomianek et al. [97], the anticancer effects of TMPE, a newly synthesized monoterpene derivative of cyclic citral, were examined, and molecular simulations revealed that TMPE was more potent than the parent molecule, cyclic citral. TMPE was identified as a potent MDR modulator in adriamycin-resistant cancer cells and was proven to have a selective cytotoxic effect against adriamycin-resistant colon cancer cells.
Irinotecan (IRT) and etoposide can form complexes with topoisomerase and DNA that can lead to single-stranded DNA breaks, prevent DNA replication and inhibit RNA synthesis. It is specific to the S phase of the cell cycle. Cisplatin can bind to DNA and cause cross-association, thereby disrupting DNA function and inhibiting cell mitosis as a cell-nonspecific drug. It is a commonly used chemotherapeutic agent for colon cancer progression and metastasis. Dendropanax morbifera (DM), an aqueous extract of Acanthopanax senticosus, possesses anticancer and antioxidant properties [98]. The combination of DM and irinotecan has the potential to be developed as a new anticancer medication and chemosensitizer [99]. Arctigenin is a natural lignan chemical isolated from burdock seeds that inhibits the growth of numerous cancer cells in the stomach, lungs, liver, and colon [100]. Under normal growth conditions, Yoon and Park [101] discovered that arctigenin had little inhibitory effect on HT29 cells. Arctigenin suppressed the degradation of topoisomerase II, lowered GRP78 expression, and reversed etoposide resistance in the microenvironment of stress-induced drug resistance in colon cancer cells. Wang et al. [102] reported that arctigenin increased apoptosis in cisplatin-treated r-sw480 and r-sw620 cells and upregulated the expression of the proapoptotic proteins c-caspase-3 and caspase-9. It also triggered autophagy and promoted the expression of LC3-II and p65 while inhibiting the expression of LC3-I. Inhibiting the mRNA and protein expression of MDR1 and PGP reversed cisplatin resistance. Polysaccharides (PG2) are a mixture of Astragalus polysaccharides (APS) with diverse biological actions, including immunomodulatory, anticancer, and neuroprotective properties [103]. Chang et al. [104] reported that PG2 isolated from Astragalus can inhibit the tumour cell production of indoleamine 2,3-dioxygenase 1 and PD-L1, through the Akt/mTOR/p70S6K pathway, thereby downregulating cell surface PD-L1 expression and enhances cisplatin sensitivity.
Salidroside is a naturally occurring active element derived from Rhodiola rosea L that inhibits the growth of cancer cells in vivo and in vitro. Li and Chen [105] observed that inhibition of autophagy by salidroside in combination with anticancer drugs (oxaliplatin, 5-FU, and adriamycin) could improve synergistic sensitization. Cucurbitacin E (CE) is a tetracyclic triterpene chemical primarily found in the Cucurbitaceae family of squashes. CE’s anti-inflammatory and antitumor properties can suppress the malignant evolution of cancer via a range of properties, including cell proliferation, invasion, cell cycle arrest, and death [106]. Combining CE with 5-FU or oxaliplatin significantly sensitized DLD1 and HCT-116 cells to chemotherapy [107]. CE increases the effectiveness of chemotherapeutic drugs by downregulating ABCC1 and multidrug resistance 1 (MDR1) and reducing the production of -linked proteins. Experiments in animals revealed that tumour tissue size, volume, and weight in the combination treatment group were significantly lower than those in the single-drug treatment group. These findings indicate that CE significantly increased the sensitivity of colon cancer cells to oxaliplatin and 5-FU treatment. Nobiletin is an extract from citrus peel with anticancer properties. Nobiletin and its derivatives target cancer through multiple pathways, including cell cycle arrest, inhibition of cell proliferation, induction of apoptosis, reduction of the inflammatory response, and inhibition of angiogenesis [108]. Nobiletin and its derivatives in combination with chemotherapy influence the activity of pure CR-CSC and upregulate ATG3, ATG5, ATG12, B2 M, CD40, CYLD, FAS, and GADD45A, enhancing the efficacy of chemotherapy while decreasing cancer cell survival and chemotherapeutic drug cytotoxicity [109]. Curcumin is a polyphenol derived from turmeric that possesses antioxidant and anticancer properties. Curcumin was discovered to alter the endogenous and exogenous metabolism of NAFLD mice via various pathways [110, 111]. Genovese et al. [112] examined the synergistic effect of isoprenoid curcumin (a semisynthetic derivative of curcumin) and FOLFOX (5-fluorouracil and oxaliplatin) and discovered that the combination significantly inhibited the growth of cancer cells resistant to 5-FU and oxaliplatin, and has the potential to reduce chemoresistance. Neuropathic pain is a common side effect of oxaliplatin-based chemotherapy [113]. Lemongrass is an aromatic grass widely grown in the tropics and is rich in essential oils [114]. Lemongrass (Cymbopogon citratus) extract contains several biological activities, including antibacterial, antiviral, anticancer and antioxidant properties [115]. Ruvinov et al. [116] reported that the combination of low-dose lemongrass extract with FOLFOX enhanced apoptosis and did not inhibit the cytotoxicity of other drugs. When lemongrass extract was combined with FOLFOX and paclitaxel, it decreased oxidative stress and dissipated MMP; in a xenogeneic colon cancer mouse model. Lemongrass significantly inhibited mouse tumours, enhanced the efficacy of FOLFOX, and reduced drug-related side effects. Combination effects of natural compounds and chemotherapy are shown in Table 2 (Ref. [60, 62, 64, 65, 66, 67, 68, 70, 71, 72, 73, 74, 75, 76, 78, 79, 81, 83, 84, 86, 88, 90, 92, 94, 96, 97, 99, 101, 102, 104, 105, 107, 109, 112, 116]).
| Tested molecule | In combination with | Experimental model | Main result | Proposed mechanism | References |
| VPE | 5-FU | In vitro: HCT116, RKO | Increased chemosensitivity | G0/G1 cell cycle arrest | [60] |
| Sulforaphane | 5-FU | In vitro: HT-29, Caco-2 | Increased chemosensitivity | Downregulation: caspase-3, caspase-8, caspase-9 | [62] |
| Ganoderma Lucidum | 5-FU | In vitro: HCT116, HT29, NCM460 | Increased chemosensitivity | Oxidative DNA damage | [64] |
| Resveratrol | 5-FU | In vitro: DLD-1, HCT116, HT29 | Overcoming drug resistance | Inhibited epithelial-mesenchymal transition Downregulation: CD44, p-STAT3, p-AKT | [65] |
| Curcumin | 5-FU | In vitro: HT-29, SW480; In vivo: HT-29, SW480 | Overcoming drug resistance | G2/M Phase Cell Cycle Arrest; Downregulation: p-STAT3 | [66] |
| Curcumin | 5-FU | In vitro: HCT116, HT29; In vivo: xenograft mice | Overcoming drug resistance | Upregulation: p62; Downregulation: LC3II/LC3I, Beclin-1, p-AMPK, p-ULK1 | [67] |
| Verbascoside | 5-FU | In vitro: Caco-2, HCT-116 | Increased chemosensitivity | Upregulation: Bax, caspase 3, caspase 8, caspase 9; Downregulation: Bcl-2, Bcl-xL, PI3K, p-AKT | [68] |
| Vanillin | 5-FU | In vitro: HT-29, SW480; In vivo: HT-29, SW480 | Overcoming drug resistance | Upregulation: p53, c‑PARP, c‑caspase‑3, c‑caspase‑9 | [70] |
| AdoMet | 5-FU | In vitro: HCT 116p53+/+, LoVo | Overcoming drug resistance | Downregulation: PARP-1, pro-caspase 9, pro-caspase 8, pro-caspase 3, Bcl-2; Upregulation: Bax | [71] |
| AdoMet | 5-FU | In vitro: HCT 116p53−/−, uL3∆HCT 116p53−/− | Overcoming drug resistance | Downregulation: PARP-1, pro-caspase 3, 9, 8, Bcl-2 Upregulation of Bax | [72] |
| PD | 5-FU | In vitro: HCT116, HT29 | Overcoming drug resistance | Upregulation: c-caspase-3, c-caspase-9, BAK, BAX; Downregulation: PI3K, AKT | [73] |
| Epigallocatechin Gallate (EGCG) | 5-FU | In vitro: DLD-1, HCT116 | Increased chemosensitivity | Upregulation: c-caspase-3, c- PARP, BAX, miR-155-5p, NF-κB; Downregulation: Bcl-2, MDR1, GRP78 | [74] |
| Epigallocatechin Gallate (EGCG) | 5-FU | In vitro: CT26, HT29; In vivo: Mice with in situ colon cancer | Increased chemosensitivity | - | [75] |
| Resveratrol | Oxaliplatin (OXA) | In vitro: SW480 CT26; In vivo: CT26 BALB/c male mice | Increased chemosensitivity | Downregulation: |
[76] |
| BE | Oxaliplatin (OXA) | In vitro: HCT116 | Increased chemosensitivity | Downregulation: cyclin D1, CDK4, Bad, p-Bad, Bcl-2, AKT, p-AKT, caspase‑3, caspase‑9; Upregulation: c-caspase‑3, c-caspase‑9 | [78] |
| Nobiletin | Oxaliplatin (OXA) | In vitro: HT-29, SW480 | Increased chemosensitivity | Downregulation: Bcl-2, P-Akt, p-mTOR; Upregulation: Bax, c-caspase 3 | [79] |
| HY | Oxaliplatin (OXA) | In vitro: HT-29-OXR | Overcoming drug resistance | Upregulation: c-PARP; Downregulation: cIAP1, cIAP2, XIAP, caspase-3 | [81] |
| EFVF | OXA (LOHP) | OXA-induced peripheral neuropathy in two rodent animal models | effects against LOHP-induced neurotoxicity | Upregulation: ROS | [83] |
| H. perforatum | OXA | In vitro: HT-29 | Reduce neurotoxicity | Downregulation: caspase-3 | [84] |
| Hypericin | OXA (LOHP) | In vitro: HCT116, HCT8 | Overcoming drug resistance | Upregulation: ROS, GRP78, CHOP, LC3II | [86] |
| DMY | OXA, VCR | In vitro: HCT116/OXA and HCT8/VCR; In vivo: BALB/c mice | Overcoming drug resistance | Downregulation: NF-κB/p65, Nrf2, MRP2 | [88] |
| OMT | ADM | In vitro: SW620, HT29; In vivo: HT29 xenograft mice | Increased chemosensitivity | Upregulation: cleaved SPTAN1, c-caspase-3, c-caspase-9, Bax/Bcl-2; Downregulation: FHL-2 | [90] |
| Scabiosa atropurpurea | ADM | In vitro: Caco-2 | Overcoming drug resistance | Upregulation: Bax, caspase-3, p21; Downregulation: Bcl-2 | [92] |
| 1C | ADM | In vitro: ADM-sensitive (CCL-222), Colo 320/MDR1-LRP multidrug resistant | Overcoming drug resistance | - | [94] |
| dMBITC | ADM | In vitro: LoVo, LoVoDX; In vivo: female NOD/SCID mice, LoVo, LoVo/DX | Overcoming drug resistance | Upregulation: c-caspase 3, ROS | [96] |
| TMPE | ADM | In vitro: HT-29, LoVo | Increased chemosensitivity | - | [97] |
| DM | Irinotecan | In vitro: HT-29; In vivo: HT-29, SNU-C5, HCT 116, SW-480, HCT-15 | Increased chemosensitivity | - | [99] |
| Arctigenin | Etoposide | In vitro: HT-29 | Overcoming drug resistance | Downregulation: GRP78, topoisomerase II |
[101] |
| Arctigenin | Cisplatin | In vitro: R–SW480, R–SW620 | Overcoming drug resistance | Upregulation: c-caspase‑3, c-caspase‑9, LC3-II, p65; Downregulation: LC3-I | [102] |
| PG2 | cisplatin | In vitro: CT26; In vivo: CT26 | Increased chemosensitivity | Downregulation: p-Akt, p-p70S6K, p-mTOR, PD-L1 | [104] |
| Salidroside | OXA, 5-FU ADM | In vitro: HCT116 | Increased chemosensitivity | Upregulation: LC3B, Becline-1 p-AMPK; Downregulation: p-mTOR, p-NF-κB (p65), TGF |
[105] |
| CE | OXA, 5-FU | In vitro: DLD1, HCT-8, HCT-116, and FHC; In vivo: BALB/c mice HCT8 | Increased chemosensitivity | Downregulation: ABCC1, MDR1, |
[107] |
| Nobiletin and Xanthohumol | FOX | In vitro: HCT116 and RKO | Increased chemosensitivity | Upregulation: ATG3, ATG5, ATG12, B2 M, CD40, CYLD, FAS, GADD45A, CR-CSphCs | [109] |
| Curcumin | FOLFOX | In vitro: CR-HT29, HCT-116 | Overcoming drug resistance | - | [112] |
| Lemongrass extract (Cymbopogon citratus extract) | FOLFOX, Taxol | In vitro: HCT-116, HT-29; In vivo: HCT-116, HT-29APCmin/+ mice | Increased chemosensitivity Reduce toxicity | Increase in ROS | [116] |
| 1C, chalcone derivative; 5-FU, 5-fluorouracil; ADM, Doxorubicin; AdoMet, S-Adenosylmethionine; BE, Blueberry extracts; CE, cucurbitacin E; DM, Dendropanax morbifera; dMBITC, 3,4-dimethoxybenzyl isothiocyanate; DMY, Dihydromyricetin; EFVF, Forsythia viridissima fruits; FOX, 5-fluorouracil and oxaliplatin; HY, hypericin; OMT, oxymatrine; OXA or OX, oxaliplatin; PD, polydatin; PG2, membranaceus; TMPE, saffron extract; VCR, vincristine; VPE, vine pruning residue. | |||||
Research on natural compounds combined with chemotherapy in colon cancer progression and metastases has achieved outstanding results in clinical trials. As this research progresses, we anticipate there will be more significant breakthroughs in the treatment of colon cancer using these agents.
There is growing clinical evidence that targeted therapies have achieved
significant efficacy in patients with specific genotypes and the development of
targeted drugs for driver mutations in Rideau has the potential to improve
survival in patients with colorectal cancer. Most patients with colon cancer die
due to disease progression and metastases to other organs. Targeted therapy is a
specific treatment method that directly or indirectly acts on cancer cell
receptors, regulatory molecules and related signalling pathways by giving
targeted drugs to eliminate tumour cells. Targeted therapy for patients with
specific genomic changes can significantly improve overall survival and reduce
adverse reactions to cancer treatment. Mutations in KRAS, p53, Smad4 and BRAF
play an essential role in CRC metastasis and may be potential biomarkers of CRC
metastasis and therapeutic targets [117]. Mutations in KRAS, NRAS or BRAF and
possible amplification in Her2 should be used to guide the use of
anti-endothelial growth factor receptor therapy in patients with metastatic colon
cancer [9]. Commonly used drugs include targeting vascular endothelial growth
factor (VEGF) to inhibit angiogenesis (bevacizumab, ramucirumab and
ziv-atriptanib) and drugs inhibiting the epidermal growth factor receptor (EGFR)
signalling pathway (cetuximab and panitumumab). Patients receiving matched
targeted therapy showed significantly improved overall survival (OS) and
progression-free survival (PFS) [118]. However, targeting also has significant
side effects, such as allergic reactions, skin toxicity, gastrointestinal
toxicity, cardiotoxicity, pulmonary toxicity, etc. Most patients with advanced
cancer die because their cancer develops resistance to existing therapies.
Reactivation of pathways and reduction of therapeutic resistance are the keys to
prolonging survival in these patients [119]. Combining natural substances with
targeted medicine increases clinical efficacy and decreases adverse effects.
Brassin (BSN), a plant antitoxin precursor isolated from Chinese cabbage, is
cytotoxic and reduced cell proliferation in colon cancer cells [120]. The
brassin-imatinib combination was found to dramatically boost cytotoxicity and
block the cell cycle in the G0/G1 phase [121]. In addition, the
brassinin-imatinib combination significantly decreased MMP-9 activity and
relative MMP-9 gene expression. Ryegrass seeds contain the bioactive component
thymoquinone (TQ). By reducing inflammation and oxidative stress [122], it has
anticancer and chemical sensitization properties [118]. The anticancer properties
of TQ include promoting apoptosis, cell cycle arrest and ROS production. In
addition, it can strengthen the immune system and reduce the side effects
associated with anticancer treatments [123]. Thabet et al. [124] found
that TQ significantly enhanced the cellular uptake of IM in HCT116 cells in a
time- and concentration-dependent manner.
| Tested molecule | In combination with | Experimental model | Main result | Proposed mechanism | References |
| BSN | Imatinib | In vitro: SW480 | Enhanced sensitivity to targeted therapy | Downregulation of MMP‐9 | [121] |
| TQ | Imatinib | In vitro: HCT116 | Enhanced efficacy of targeted therapies | Downregulation ABCG2, hOCT1 | [124] |
| Cetuximab | In vitro: HCT116, Lovo, CaCO2 | Enhanced sensitivity to targeted therapy | Upregulation of transferrin, HO-1; Downregulation GPX4, SLC7A11, FTH1, glutaminase, SLC40A1, MMP‐9 | [126] | |
| Curcumin | Erlotinib | In vitro: SW480 | Overcoming drug resistance | Upregulation of PDK4 gene; Downregulation |
[128] |
| BSN, Brassinin; TQ, thymoquinone. | |||||
Immunotherapy offers significant therapeutic benefits in the progression and
spread of colon cancer by increasing the antitumor immune response and,
inhibiting suppressor mechanisms that support tumour growth. Immunotherapy that
activates and promotes an optimum immune status in colorectal cancer patients has
the potential to increase patient survival [129]. Immunomodulatory strategies,
such as immunization, pericyte treatment, and checkpoint inhibition, have
demonstrated various therapeutic effects, most of which are represented in
checkpoint inhibition [130]. Enhanced TGF
| Tested molecule | In combination with | Experimental model | Main result | Proposed mechanism | References |
| Pectin | Anti-PD-1 mAb | In vitro: MC38; In vivo: MC38 C57BL/6 mice | Enhanced efficacy of targeted therapies | Upregulation of CD4+ T cell, CD8+ T cell, INF- |
[133] |
| DHA | OxPt+ |
In vitro: MC38 and CT26; In vivo: BALB/c, C57BL/6, Rag2−/−, SD/CD female mice | Increased chemosensitivity | Upregulation: ROS; Downregulation: GSH | [135] |
| DHA, dihydroartemisinin; OxPt, oxaliplatin. | |||||
Some natural compounds have already been used in clinical practice and have demonstrated superior antitumor effects. Clinical investigations of natural compounds and conventional medicines have also produced encouraging findings. Postoperative colon cancer frequently causes alterations to the intestinal flora. Ganoderma lucidum extract possesses gut microbiota modifying and anti-inflammatory properties. Clinical experiments have demonstrated that the Ganoderma lucidum extract nutraceutical MICODIGEST 2.0 can be utilized to reduce the disruption of gut flora (NCT04821258). Phase I of the resveratrol SRT501 safety, pharmacokinetic, and pharmacodynamic trial in patients with colon cancer and liver metastases has begun (NCT00920803). However, the low bioavailability and poor selectivity of some natural compounds seriously affect the clinical use of the drugs. The development of drug delivery systems that enhance the pharmacokinetics, cellular uptake, and targeting of the anticancer active ingredients of natural compounds is the key to address the clinical translation of anticancer natural compounds [136]. The common methods used to improve the bioavailability of natural compounds are: chemical and physical modifications [137], new solvents [138], cyclodextrins (CD) [139], nanocarriers [140], etc. (Fig. 3). Taking curcumin as an example, curcumin is poorly water soluble and can be rapidly metabolized through the intestinal tract, resulting in low bioavailability for both oral or intravenous administration. Currently, in order to improve the oral bioavailability of curcumin, the solubility of curcumin can be increased, the intestinal stability of curcumin can be improved, and the absorption pathway of curcumin can be changed by using including coupling compounds, nanoparticles, polycrystalline forms, polymer capsules, cyclodextrins, nanosuspensions, and lipid nanocarriers [141, 142]. Exosomes improve the stability, solubility, and bioavailability of curcumin, according to a recently published phase I clinical trial study conducted at the James Graham Brown Cancer Center (NCT01294072). Several investigations of curcumin in conjunction with conventional outpatient therapy (including 5-FU, Avastin/FOLFIRI, FOLFOX, and irinotecan) have entered the clinical phase (Table 5). It is thought that natural chemicals will play a more significant role in the future treatment of colon cancer when combined with conventional medicines.
| Natural product | Anticancer treatment | Clinical phase | Status | Enrolled patients | ClinicalTrials.gov Identifier |
| Curcumin | 5-FU | Early Phase 1 | Active, not recruiting | 13 patients with 5-FU-resistant metastatic colon cancer | NCT02724202 |
| Curcumin | Avastin/FOLFIRI | Phase 2 | Completed | 50 colorectal cancer patients with unresectable metastasis | NCT02439385 |
| Curcumin | FOLFOX | Phase 1/Phase 2 | Completed | 41 patients with inoperable colorectal metastases | NCT01490996 |
| Curcumin | Irinotecan | Phase 1 | Completed | 23 patients with metastatic colorectal cancer | NCT01859858 |
| Curcumin | Capecitabine | Phase 2 | Active, not recruiting | 45 patients with advanced rectal cancer | NCT00745134 |
| FOLFIRI, Irinotecan and 5-fluorouracil and leucovorin; FOLFOX, 5-fluorouracil and oxaliplatin. | |||||
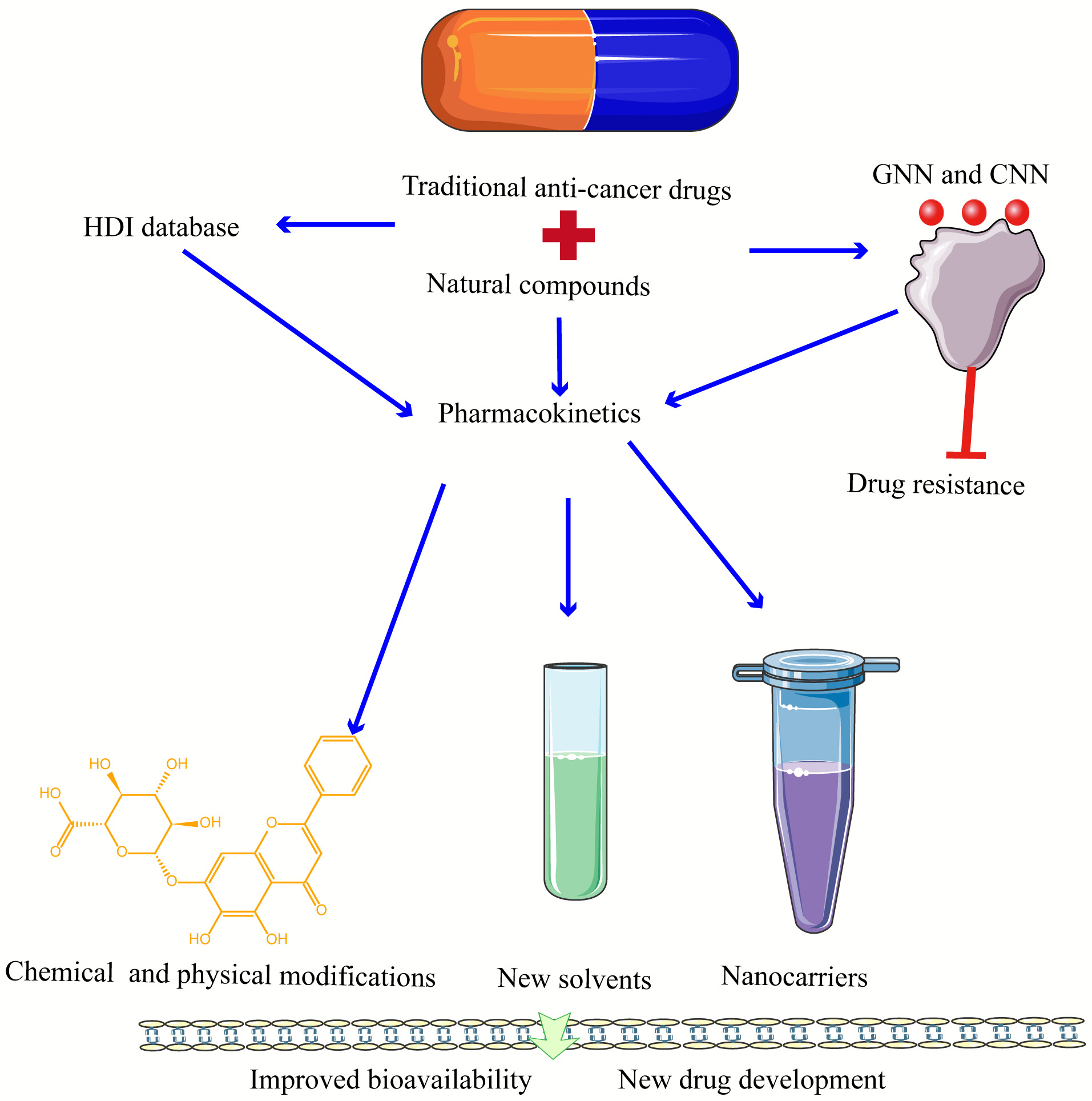 Fig. 3.
Fig. 3.Natural compounds interacting with traditional drugs, pharmacokinetics on drug to drug bioavailability and application in new drug development. Blue arrows represent research directions and red represents inhibition. GNN, graph neural network; CNN, convolutional neural network; HDI, HERB-Drug Interaction.
Metastasis is a characteristic of colon cancer deterioration, which results in the invasion of colon cancer tumour cells from the primary site into the lymphatic vessels, blood vessels, or other channels to continuously grow in other areas of the body. The most common treatments for metastatic colon cancer include systemic chemotherapy, targeted therapy, and immunotherapy, individually or in combination. Surgical resection may be performed when the initial and metastatic lesions meet the criteria for operative resection. Alternatively, radiation therapy can be administered initially, followed by surgical resection after the metastatic lesions have shrunk to the requirements for operative excision. The combination of natural substances with conventional therapies can target caspase-3 to increase colon cancer cell sensitivity to chemotherapy, radiation, targeted therapy, and immunotherapy, as well as limit the invasion and metastasis of cancer cells. Alternatively, by suppressing autophagy, some TCM treatments can diminish drug resistance. It can target common gene alterations, such as KRAS, BRAF, PI3K, and p53, to increase the efficacy of conventional therapies and lessen side effects (Fig. 4). In the treatment of colon cancer progression and metastasis, most natural substances and traditional medicines are still in the experimental phase, and the mechanism of their combined application must be further investigated. The bioavailability of natural compounds in humans needs to be addressed, including bioaccessibility, uptake and translation of bioactive compounds and bioactive-loaded nanocarriers. In particular, the mechanisms involved in cellular uptake of bioactive nanocarriers include trans-cellular transport, as well as active transport of bioactive compounds in the presence of membrane transport proteins [143]. Improvements have been obtained through the development of new drug delivery technologies, including structural modifications, colloidal systems and nanotechnology [144]. Alternatively, deciphering the network of nutritional genetic links associated with cancer through genetic techniques, studying the relationship between ingested phytochemicals and chemoprevention or chemotherapy, understanding precisely how natural compounds interact with conventional therapies, and using genomic approaches to achieve the ability to suppress drug resistance [145]. With the use and investigation of new technologies and methods, it is anticipated that natural substances and conventional medicines will make significant strides in halting the progression and spread of colon cancer.
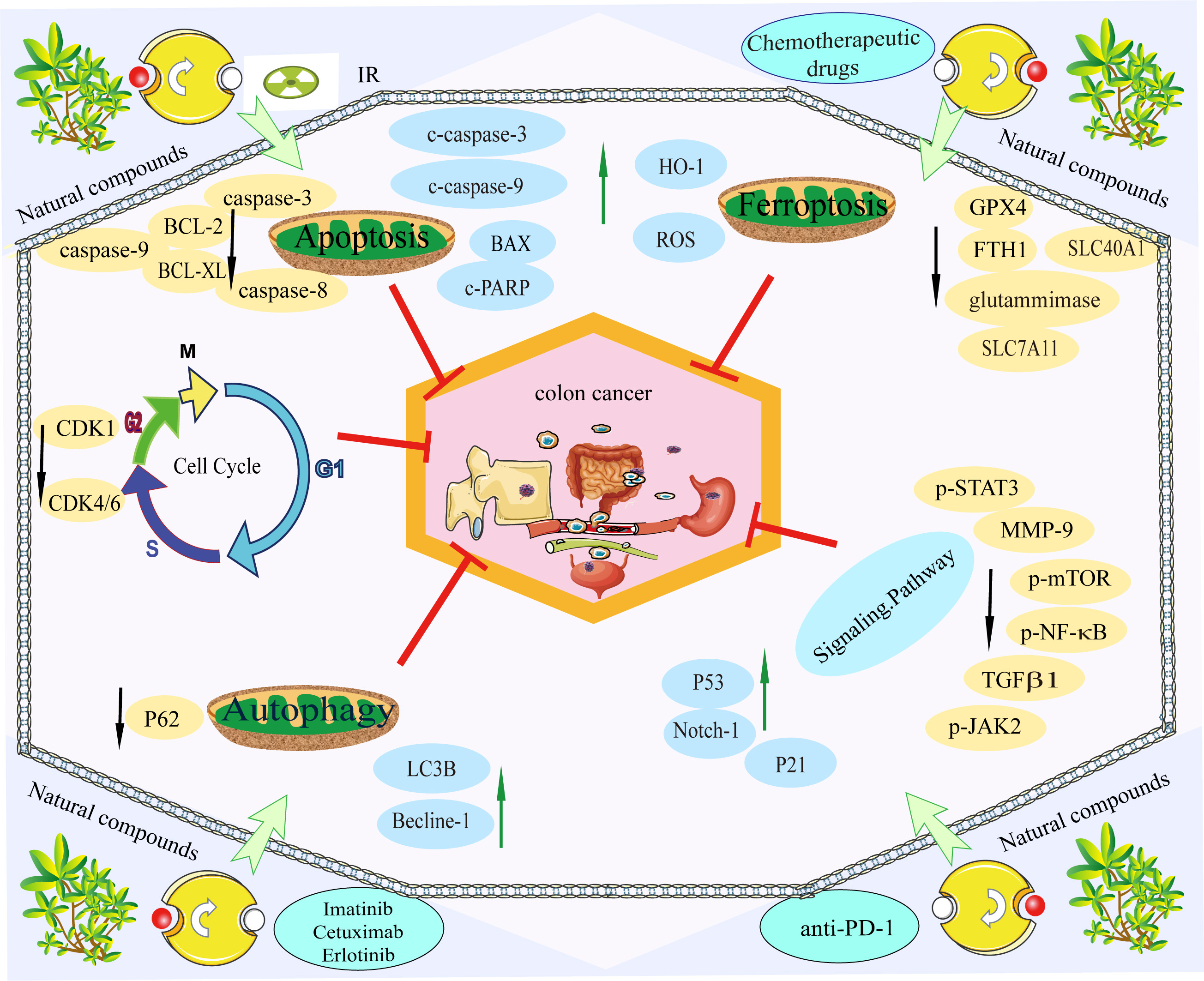 Fig. 4.
Fig. 4.Natural compounds are used in combination with conventional therapies. Natural compounds can improve the efficacy of conventional therapies and reduce side effects. The green arrow indicates promoting protein expression, while the black arrow indicates inhibiting protein expression. The red T bar indicates inhibiting tumour progression and metastasis.
Active natural compounds have anticancer properties in vitro and in vivo through different mechanisms and pathways. Natural compounds combined with conventional therapies can improve the sensitivity of conventional therapies, decrease the dosage of drugs, reduce treatment resistance, and reduce the adverse effects of conventional therapies. It has the potential to become an essential therapeutic tool for treating colorectal cancer progression and metastases.
1C, chalcone derivative; 5-FU, 5-fluorouracil; AdoMet, S-adenosylmethionine; APP,
QW, HC, ZL, and XS designed the research study. ZL, HX, WS, LS, LZ, XH, QZ, and XZ performed the research. ZQ and KL provided advice on data collection. HX analyzed the data. ZL, HX, XH, and QZ retrieved and collected the data. ZL and HX wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Not applicable.
We acknowledge the support of the Science and Technology Development Fund, Macau SAR, and the Science and Technology Planning Project of Guangdong Province.
This study was funded by the Science and Technology Development Fund, Macau SAR (file No.: 0098/2021/A2, 130/2017/A3, and 0099/2018/A3), and the Science and Technology Planning Project of Guangdong Province (2020B1212030008).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.




