1 Banat’s University of Agricultural Sciences and Veterinary Medicine “King Michael I of Romania” from Timisoara, 300645 Calea 119, Timis, Romania
2 Faculty of Health Science, Universidad Arturo Prat, 1110939 Iquique, Chile
3 Departament of Basic Sciences, Faculty of Sciences, Universidad Santo Tomas, 8370003 Santiago, Chile
4 Center of Molecular Biology and Pharmacogenetics, Scientific and Technological Bioresource Nucleus, Universidad de La Frontera, 4811230 Temuco, Chile
5 Department of Analytical Chemistry, Faculty of Sciences, University of Granada, 18071 Granada, Spain
6 Research and Development Functional Food Centre (CIDAF), Bioregión Building, Health Science Technological Park, 18071 Granada, Spain
7 Department of Plant and Soil Sciences, University of Pretoria, 0002 Gauteng, South Africa
8 Applied Microbiology, Biotechnology and Nanotechnology Laboratory, Department of Microbiology, Edo State University Uzairue, Iyamho, 312101 Edo State Nigeria, Nigeria
9 Department of Anatomy, Faculty of Basic Medical Sciences, Edo State University Uzairue, Iyamho, 312101 Edo State Nigeria, Nigeria
10 Institute of Fermentation Technology and Microbiology, Faculty of Biotechnology and Food Sciences, Lodz University of Technology, 90 – 924 Lodz, Poland
11 Phytochemistry Research Center, Shahid Beheshti University of Medical Sciences, 1991953381 Tehran, Iran
12 Molecular and Applied Mycology and Plant Pathology Laboratory, Department of Botany, University of Calcutta, 700019 Kolkata, India
13 Department of Botany, Fakir Chand College, Diamond Harbour, 743331 West Bengal, India
14 Health Science Division, Abu Dhabi Women’s College, Higher Colleges of Technology, 41012 Abu Dhabi, UAE
15 Perdana University-Royal College of Surgeons in Ireland, 43400 Serdang, Serdang, Malaysia
16 Department of Biology, Faculty of Science, Sivas Cumhuriyet University, 58140 Sivas, Turkey
17 Beekeeping Development Application and Research Center, Sivas Cumhuriyet University, 58140 Sivas, Turkey
18 Department of Food Science, Faculty of Food Science and Technology, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia
19 Natural Medicines and Products Research Laboratory, Institute of Bioscience, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia
20 Department of Biochemistry, Bayero University Kano, PMB 3011 Kano, Nigeria
21 Department of Pharmacology, Federal University Dutse, PMB 7156 Dutse, Jigawa State, Nigeria
22 Department of Chemistry, University of Alabama in Huntsville, Huntsville, AL 35899, USA
23 Aromatic Plant Research Center, Lehi, UT 84043, USA
24 Facultad de Medicina, Universidad del Azuay, 14-008 Cuenca, Ecuador
Academic Editor: Marcello Iriti
Abstract
Tradescantia is a genus of herbaceous and perennial plants belonging to the Commelinaceae family and organized into three infrageneric classifications and 12 sections. More than 80 species within the genus have been used for centuries for medicinal purposes. Phytochemical compounds (from various species of the genus) such as coumarins, alkaloids, saponins, flavonoids, phenolics, tannins, steroids and terpenoids have recently been characterized and described with antioxidant, cytotoxic, anti-inflammatory, anticancer or antimicrobial properties. The objective of this review is to describe the different aspects of the genus Tradescantia, including its botanical characteristics, traditional uses, phytochemical composition, biological activities, and safety aspects.
Keywords
- Tradescantia
- phytochemicals
- bioactive properties
- health effects
- safety
The genus Tradescantia, commonly known as ‘Spiderwort’, is the second largest genus within the Commelinaceae family [1] including approximately 80 herbaceous species classified into 12 sections [2, 3, 4, 5, 6, 7, 8, 9, 10]. These species are indigenous to the Neotropics, and the centre of diversity is located in the southern United States and Mexico [3, 5, 8, 9, 11] (Table 1, Ref. [2, 3, 4, 9, 10, 11, 12, 13, 14, 15]). These species are adapted to full sun, deep shade, high or low temperatures, and xeric habitats [16].
| Section | Species | Distribution | |||
| Nº | Name | ||||
| Cymbispatha | 9 | T. comme linoides Schult. & Schult.f. | T. tonalamonticola Matuda | T. gracillima Standl. | From Mexico to Brazil |
| T. cymbispatha C.B.Clarke | T. tonalamonticola Matuda | Bolivia | |||
| T. deficiens Brandegee | T. venezuelensis Steyerm. | ||||
| T. plusiantha Standl. | |||||
| T. standleyi Steyerm. | |||||
| Coholomia | 1 | T. guatemalensis C.B.Clarke ex J.D.Sm. | Guatemala | ||
| Tradescantia. | 29 | T. bracteata Small ex Britton | T. longipes E.S.Anderson & Woodson | T. rozynskii Matuda | USA, Texas |
| T. canaliculata Raf. | T. occidentalis (Britton) Smyth | T. sillamontana Matuda | Mexico (From Chihuahua to Puebla) | ||
| T. edwardsiana Tharp | T. ohiensis Raf. | T. cirrifera Mart. | |||
| T. ernestiana E.S.Anderson & Woodson | T. ozarkana E.S.Anderson & Woodson | T. maysillesii Matuda | |||
| T. gigantea Rose | T. paludosa E.S.Anderson & Woodson | T. monosperma Brandegee | |||
| T. hirsuticaulis Small | T. reverchonii Bush | T. nuevoleonensis Matuda | |||
| T. hirsutiflora Bush | T. roseolens Small | T. pinetorum Greene | |||
| T. humilis Rose | T. subacaulis Bush | T. wrightii Rose & Bush | |||
| T. virginiana L. | T. subaspera Ker Gawl. | T. mirandae Matuda | |||
| T. orchidophylla Rose & Hemsl. | T. tharpii E.S.Anderson & Woodson | ||||
| Setcreasea | 5 | T. brevifolia (Torr.) Rose | T. hirta D.R.Hunt | T. pallida (Rose) D.R.Hunt | Texas |
| T. buckleyi (I.M.Johnst.) D.R.Hunt | T. leiandra Torr. | Mexico (From Chihuahua to Veracruz) | |||
| Separotheca | 1 | T. pygmaea D.R.Hunt | Mexico (Durango) | ||
| Mandonia | 7 | T. ambigua Mart. ex Schult. & Schult.f. | T. llamasii Matuda | T. tepoxtlana Matuda | Mexico (Durango) |
| T. burchii D.R.Hunt | T. peninsularis Brandegee | T. velutina Kunth & C.D.Bouché | |||
| T. crassifolia Cav. | |||||
| Parasetcreasea | 1 | T. andrieuxii C.B.Clarke | Mexico (From Chihuahua to Oaxaca) | ||
| AustroT. | 6 | T. anagallidea Seub. | T. cerinthoides Kunth | T. fluminensis Vell. | Argentina, southern and southeastern Brazil, Bolivia, Paraguay, Uruguay |
| T. umbraculifera Hand.-Mazz. | T. crassula Link & Otto | T. ambigua Mart. ex Schult. & Schult.f. | |||
| Rhoeo | 1 | T. spathacea Sw. | Mexico, Belize | ||
| Campelia | 1 | T. zanonia (L.) Sw. | Tropical America | ||
| Zebrina | 1 | T. zebrina Bosse | From Mexico to Venezuela | ||
| Corinna | 1 | T. soconuscana Matuda | Mexico, Guatemala, Nicaragua, Costa Rica | ||
Tradescantia species are evergreen, perennial or annual, rupicolous or epiphytic, and can reach up to 30–60 cm in height. Roots are thin or thick, and fibrous or tuberous. Stems are aerial, rarely subterranean, prostrate with an ascending or erect apex, forming intertwined dense mats. Leaves are simple, 3–45 cm long, sessile to sub-sessile, distichously or spirally alternate, glabrous, lanceolate to linear-lanceolate to ovate, evenly distributed along the stem or crowded at the apex. The inflorescence is a terminal crescent, pedunculated, sub-umbelled, with cincinni bracts as leaves or reduced (bracteous), spataceous. Flowers are bisexual, actinomorphic or slightly zygomorphic, pedicellate, pedicel glabrous and green. Sepals are green, equal or subequal, polysepalous or connate below and persistent. Petals can be white, pink, or purple, but mostly bright blue, equal or subequal, and free or connate below the floral tube. Stamens are equal or subequal, epipetalous; filaments free, usually bearded at the base, glabrous or with moniliform hyaline trichomes at the base, anthers oblong or reniform, basifixed or dorsifixed. Carpels are pubescent, ovary 3-locular, style straight, cylindrical to sub-cylindrical, glabrous. The fruit is a 3-cell loculicidal capsule. Seeds 1–2 per locule, reniform to ellipsoid usually rugose [2, 12, 13].
T. zebrina Bosse, T. fluminensis Vell., T. spathacea Swartz. and T. albiflora Kunth. are the most widely studied species within the Tradescantia genus. Ethnopharmacological uses of Tradescantia include anti-inflammatory, antioxidant, antibacterial and antiarrhythmic applications. All parts of the plant are potentially useful and, although the phytochemical characterization has been described for some species, most Tradescantia species remain unreported. No species have been tested for drug development aside from toxicity studies. Thus, the objective of this review is to describe all known aspects of the genus Tradescantia including its botanical characteristics, traditional uses, phytochemical composition, biological activities, and safety aspects.
A literature search was performed in PubMed/MEDLINE using the terms ‘Tradescantia’ and ‘global distribution’, ‘traditional uses’, ‘phytochemical’, ‘antioxidant’, ‘inflammatory’, ‘biological properties’ or ‘human health’, individually or in combination. Scientific articles from the last five years including the phytochemical characterization and evaluation of biological properties of Tradescantia species through in vitro and in vivo models, were prioritized for inclusion in this review.
Herbs have traditionally beed used for their fragrance, taste, and therapeutic properties. Fresh or dried herbs have been applied in tablet or capsule form as contemporary methods (powders, teas and extracts) [17]. Some species of the genus Tradescantia have been traditionally used for their presumed anti-inflammatory, antioxidant, antibacterial and antiarrhythmic properties. For example, roots have been used as a drink to heal kidney diseases and digestive system ailments. Leaves have been applied to relieve stings and insect bites. A summary of worldwide traditional uses of the genus Tradescantia is shown in Table 2 (Ref. [18, 19, 20, 21, 22, 23, 24, 25, 26, 27]). Traditional remedies must be interpreted and considered with great caution as possible potentialities of this plant source. Therefore, scientific evidence is needed to demonstrate the cause-and-effect relationship of these properties.
| Plant species | Country/Region | Plant part(s) | Traditional usage for treatment of | Instruction | Ref. |
| T. spathacea | Southern Mexico | Whole plant | Coughs and loosen mucus | 1 teaspoon of plant extract obtained from grinding mixed with a little sugar (3 times/day) | [18] |
| Southern Mexico | Stem and Leaves | Vomiting of blood | 1 tablespoon of decoction (3 times/day) | [18, 19] | |
| Southern Mexico | Leaves | Burns, scalds and dysentery | poultice | [18] | |
| China | Whole plant | Wounds | poultice | [18] | |
| China | Flower | Dysentery | - | [18] | |
| China | leaves and flowers | Dysentery, entrorrhagia and hemoptysis | Decoction | [20] | |
| Singapore | Leaves | fever, cough and bronchitis, decorative | Decoction | [21] | |
| Puerto Rico | Leaves | Psoriasis | Decoction | [21] | |
| Cuba | Leaves | Grazes | poultice | [21] | |
| T. zebrina | China | Leaves mixed with other types of herbs | Blood tonic and to treat amenorrhea | - | [22] |
| Caribbean | Leaves | To flush gravel out of the kidneys and bladder, break the crisis of colitis | Decoction | [23] | |
| Philippines | Leaves | Cleansing blood and treating influenza, | Tea | [24] | |
| Mexico | Leaves | - | Cold iced tea drink | [25] | |
| Caribbean | Leaves | To flush gravel out of the kidneys and urinary system, improves bowel inflammation | Decoction | [23] | |
| T. pallida | Philippines | Leaves | Sore eyes | - | [26] |
| T. virginiana L. | America | Roots | Insect stings to reduce pain and itching | Ointment | [27] |
T. zebrina (Fig. 1) has widely been used as traditional Chinese medicine for renal system diseases. In Jamaica, high blood pressure, cough and tuberculosis have been treated with this plant. Specifically, the leaves are considered to have beneficial properties to reduce inflammation and treat haemorrhoids and kidney infections. In Mexico, a decoction of the leaves are traditionally consumed as a cold drink called ‘Matali’ [25]. In the Caribbean, leaf extracts are consumed to relieve kidney and urinary problems, and reduce intestinal inflammation [23]. In the past, Filipinos used the leaves as tea to cleanse the blood and relieve influenza symptoms [24]. Malaysians recommended a decoction of the plant to improve kidney function and was believed to have advantages for treating venomous snake bite, leucorrhoea, urinary tract infection, nephritis and bowel inflammation. T. pallida D.R.Hunt has also been traditionally used for its anti-inflammatory and antitoxic potential, as well as improving blood circulation and prevent sore eyes [26]. T. spathacea, also known as Rhoeo spathacea or Rhoeo disccolor, has been used in China in a decoction of dried or fresh leaves and flowers to treat dysentery and haemoptysis [20]. In Singapore, traditional medicinal uses of this plant focus on treating fever, cough, and bronchitis. In spite of all these properties, its sap is considered to have adverse effects related to itching of the skin and eyes, and abdominal and oral pain when ingested [28].
These traditional uses may suggest the presence of potential bioactive compounds (in their chemical composition) capable of preventing or alleviating certain diseases. The lack of scientific evidence necessary to prove such applications must be carried out through appropriate scientific methods. Recent studies allow these traditional uses to be translated into future applications of modern medicine.
The therapeutic value and pharmacological effects of medicinal plants largely depend on their phytochemical composition. In the case of Tradescantia, the phytochemical composition has only been explored for a few species. Entire characterization studies are based on phytochemicals qualitative analysis or simple screening of compound families. Phytochemicals quantitative studies in Tradescantia species have not yet been carried out.
Alkaloids, flavonoids, phenolics and saponins have been the most identified
phytochemical compounds in Tradescantia species [29, 30, 31, 32]. Apigenin,
luteolin and flavonols, 6-hydroxyluteolin and tricin, have been the main
identified flavonoids (C-glycosides). The geographical origin of
Tradescantia species has been described as a factor that affects the
content of these compounds. Lee Tan et al. [33] determined the total
phenolic content (TPC), total tannin content (TTC) and total flavonoid content
(TFC) of T. spathacea, T. pallida and T. zebrina
methanolic extracts (Table 3, Ref. [33]). Cheah. et al. [34]
also determined the TPC (33.5
| TPC (mg GAE/100 g sample) | TTC (mg TAE/100 g sample) | TFC (mg RE/100 g sample) | |
| T. spathacea | 203.9 |
20.6 |
10.8 |
| T. pallida | 153.1 |
13.6 |
10.6 |
| T. zebrina | 620.9 |
57.6 |
17.1 |
| GAE, Gallic acid equivalent; RE, Rutin equivalent; TAE, Tannic acid equivalent. | |||
Del Pero Martínez et al. [35] were the first to qualitatively analyse the flavonoid composition in 42 Tradescantia species. Luteolin, apigenin, 6-hydroxyluteolin and tricin glycosides were the most commonly characterized flavonoids.
Phytochemical screening analyses of T. spathacea leaf extracts showed the presence of coumarins, alkaloids, saponins, flavonoids and terpenoids [31]. In another screening study, alkaloids, steroids, flavonoids, saponins, cardiac glycoside, terpenoids, tannins and phenolic compounds were detected in a T. spathacea leaf extract [29]. Flavonoids, alkaloids, tannins, phenols and steroids were characterized from T. zebrina leaf extracts. T. fuminensis leaf extracts analysis revealed the presence of saponins, phenolic compounds and flavonoids [30, 32]. Huq et al. [36] carried out a phytochemical screening of T. pallida extracts, detecting the presence of alkaloids, tannins and carbohydrates, in high, moderate and trace amounts, respectively. However, no specific metabolites were isolated or identified.
Studies on T. zebrina, T. spathacea and T. albiflora have focused on
specifically characterising the phytochemical compounds of the species (Table 4).
For example, Dash et al. [22], identified the compounds ecdysone (1),
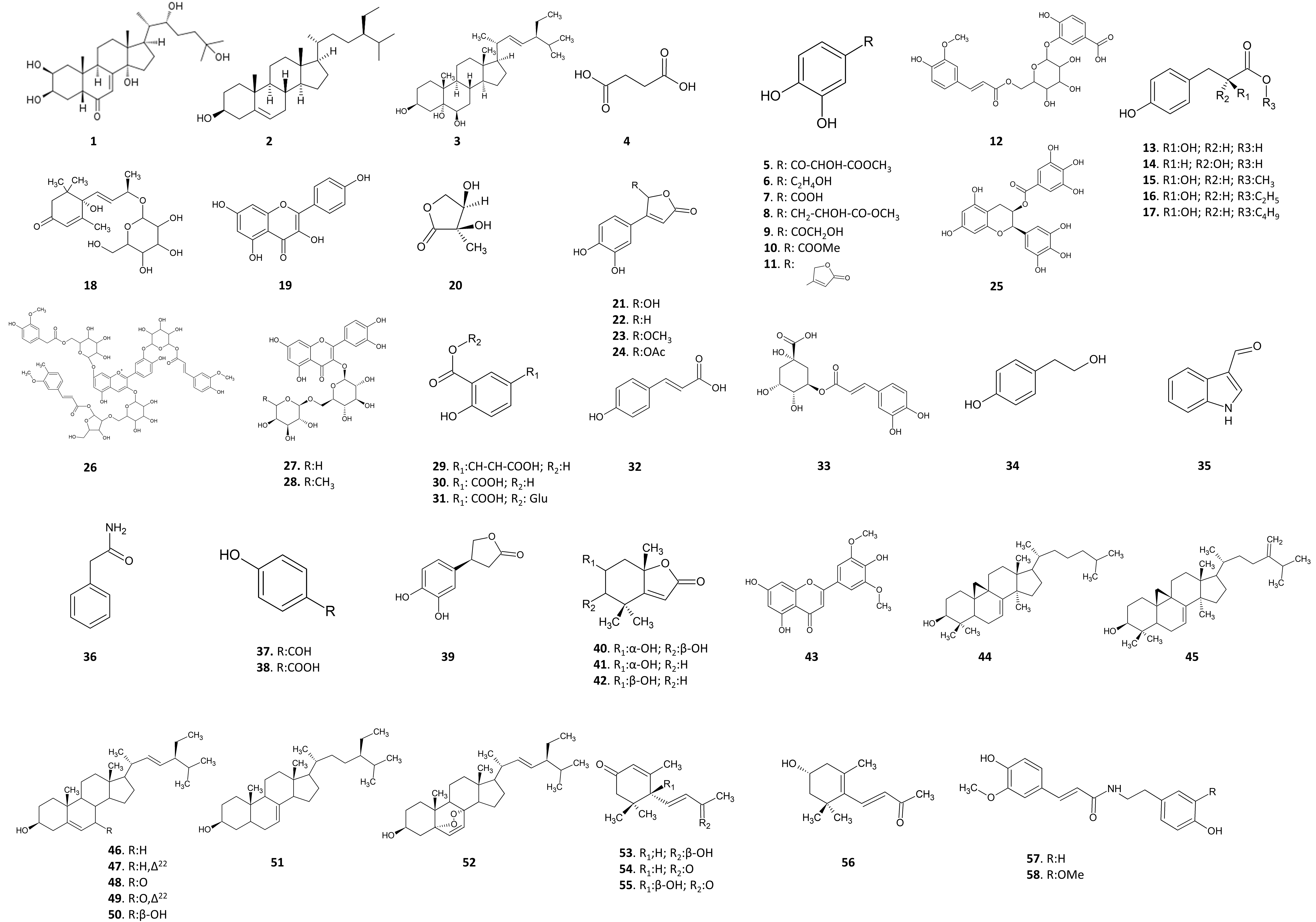 Fig. 2.
Fig. 2.Phytochemical compounds characterized in different T.
species (T. zebrina, T. albiflora, T. pallida): 1.
ecdysone; 2.
| Nº | Name | T. Zebrina | T. spathacea | T. albiflora | Nº | Name | T. spathacea | T. albiflora |
| 1 | Ecdysone | X | 30 | vanillic acid | X | |||
| 2 | X | 31 | glycosylated vanillic acid | X | ||||
| 3 | 3 |
X | 32 | p-coumaric acid | X | |||
| 4 | Succinic acid | X | 33 | chlorogenic acid | X | |||
| 5 | ( |
X | X | 34 | tyrosol | X | ||
| 6 | hydroxytyrosol | X | X | 35 | indole-3-aldehyde | X | ||
| 7 | protocatechuic acid | X | X | 36 | 2-phenylacetamide | X | ||
| 8 | oresbiusin A | X | 37 | p-hydroxybenzaldehyde | X | |||
| 9 | 2-hydroxy-3′,4′-dihydroxyacetophenone | X | X | 38 | p-hydroxybenzoic acid | X | ||
| 10 | 3,4-dihydroxymethylbenzoate | X | 39 | rosmarinosin B | X | |||
| 11 | 3-(3′,4′-dihydroxyphenyl)-butenolide | X | 40 | 2 |
X | |||
| 12 | tradecantoside | X | 41 | isololiolide | X | |||
| 13 | (S)-2-hydroxy-3-(4′-hydroxyphenyl) propanoic acid | X | 42 | loliolide | X | |||
| 14 | (R)-2-hydroxy-3-(4′-hydroxyphenyl) propanoic acid | X | 43 | tricin | X | |||
| 15 | latifolicinin C | X | 44 | 3-epicyclomusalenol | X | |||
| 16 | latifolicinin B | X | 45 | 24,25-dihydrocimicifugenol | X | |||
| 17 | latifolicinin A | X | 46 | sitosterol | X | |||
| 18 | (6S,9R)-roseoside | X | 47 | stigmasterol | X | |||
| 19 | kaempferol | X | 48 | 7-ketositosterol | X | |||
| 20 | (2R,3R)-2,3-dihydroxy-2-methylbutyrolactone | X | 49 | 7-ketostigmasterol | X | |||
| 21 | bracteanolide A | X | X | 50 | 7 |
X | ||
| 22 | 4-(3′,4′-dihydroxyphenyl)furan-2(5H)-one | X | X | 51 | schottenol | X | ||
| 23 | bracteanolide B | X | 52 | ergosterol peroxide | X | |||
| 24 | 5-O-acetyl bracteanolide A | X | 53 | (6R,7E,9R)-9-hydroxy-4,7-megastigmadien-3-one | X | |||
| 25 | epigallocatechin | X | 54 | (E)-3,5,5-trimethyl-4-(3-oxobut-1-en-1-yl)cyclohex-2-enone | X | |||
| 26 | rhoeonin | X | X | 55 | (S)-dehydrovomifoliol | X | ||
| 27 | peltatoside | X | 56 | (3R)-3-hydroxy- |
X | |||
| 28 | rutin | X | 57 | N-trans-feruloyltyramine | X | |||
| 29 | ferulic acid | X | 58 | N-trans-feruloyl-3-methoxytyramine | X |
Shi et al. [41] found that T. pallida contained two main
anthocyanins with natural food colorant potential. One was cyanidin
3,7,3
Wang et al. [42] discovered that particular compounds isolated from
T. albiflora extracts were able to reduce serum uric acid levels in
oxonate-induced rats. The methanol extract of T. albiflora was
fractionated successively using different polarities solvents such as hexane,
ethyl acetate and n-butanol. Repeated column chromatography of the ethyl
acetate extract resulted in the purification of the following twelve aromatic
compounds: hydroxytyrosol (6), protocatechuic acid (7),
2-hydroxy-3
Although crucial, these findings are not sufficient for a general understanding of the phytochemical compounds found in most Tradescantia species. These studies are based on qualitative characterizations, and therefore the concentration levels of these phytochemical compounds in Tradescantia species are currently unknown.
In spite of the large number of existing Tradescantia species, there is still a lack of knowledge regarding their qualitative and quantitative phytochemical composition. Considering the advantages of high-resolution analytical techniques, quantitative and qualitative phytochemical studies are necessary to clarify the cause-effect relationship of the beneficial properties.
The different bioactive properties described for Tradescantia species might be related to their phytochemical composition. For instance, several studies have reported the bioactive properties of epigallocatechin, such as antioxidant, antidiabetic, anti-inflammatory or antitumor activities [44]. Hydroxytyrosol is also a phytochemical compound to which antioxidant, anti-inflammatory, anticancer, antidiabetic or cardioprotective properties, have been described [38]. Much of the bioactive properties of specific compounds present in Tradescantia plants have recently been reviewed by Tan et al. [45], highlighting the following compounds: rutin, epigallocatechin, (6S,9R)-roseoside, kaempferol, oresbiusin A, hydroxytyrosol, protocatechuic acid, latifolicin (A, B and C) and ferulic, vanillic, chlorogenic, and p-coumaric acids. Kaempferol, protocatechuic acid, rutin, epigallocatechin, have been described in other plant sources as antioxidant and anticancer compounds [46]. Although individual compounds may have proven individual bioactive properties, it is also important to consider the bioactive potential of Tradescantia extracts in case of possible synergistic effects between compounds. However, only few studies have been conducted to evaluate the bioactivity of Tradescantia extracts (Table 5, Ref. [30, 32, 33, 34, 37, 39, 43, 47, 48, 49, 50, 51, 52, 53, 54]). The following subsections describe the main studies carried out on the evaluation of the bioactive properties of Tradescantia extracts.
| Activity | Specie | Extract (Dose) | Model | Results | REF |
| Antioxidant activity | T. zebrina, T. pallida | Methanolic leaf extract | FRS, FRP, FIC | T. zebrina showed the highest antioxidant activity | [33] |
| T. spathacea | Aqueous and methanol leaf extracts | FRS, FRP, FIC | Decoction and infusion extracts showed an antioxidant activity similar to herbal teas | [39] | |
| T. zebrina | Methanolic leaf extracts (0.1 mg/mL) | DPPH | A radical scavenging activity of 18.1 |
[34] | |
| Anti-inflammatory activity | T. fluminensis | Ethanolic leaf extract (Dose: 150, 300 mg/kg) | Egg albumin induced paw edema model. Adult Wistar albino rats | A significant dose-dependent inhibition of inflammation | [32] |
| T. albiflora | Isolated compounds from T. albiflora | LPS-stimulated NO production in RAW 264.7 cells | Bracteanolide A, methyl 3,4-dihydroxybenzoate and hydroxytyrosol show inhibitory potential | [43] | |
| T. fluminensis and T. zebrina | Methanol leaf extracts (0.2 µg/mL) | 15-Lipoxygenase inhibitory assay | Higher than 50% of inhibition. | [30] | |
| T. fluminensis showed higher inhibitory potential (87.2%) | |||||
| Anticancer activity | T. fluminensis and T.zebrina | Methanol and aqueous extracts (2.5, 5%) | A549, SCC-13y and HFF-1 cells | Inhibitory effects on cancerous lines (lung adenocarcinoma, squamous cell carcinoma and human foreskin fibroblasts) | [47] |
| T. zebrina | Aqueous and methanol extracts | SCC-13y malignant keratinocyte cells and A-549 lung carcinoma cells | Effects of T. zebrina on cancerous cells | [48] | |
| T. spathacea | Leaf extract | Human breast adenocarcinoma (MCF-7) | IC |
[49] | |
| T.spathacea | Aqueous crude extract (20 mg/kg) | Rat resistant-hepatocyte carcinogenesis model | A reduction of the number and area of preneoplastic lesions. | [37] | |
| T. pallida | ZnO nanoparticles using T. pallida leaf extract | HeLa cervical cancer cell line | Cytotoxicity againt cercival cancer cell line | [50] | |
| (0–1000 µg/mL) | |||||
| Cytotoxic activity | T. zebrina | Extracts obtained with different solvents. (5–640 |
NRU and MTT assays. Monkey kidney epithelial (Vero) cells. | A concentration dependent and extraction solvent dependent cytotoxic effect | [51] |
| Insecticidal activity | T. zebrina | Infusion extract of dry plant (2.5–10% m/v) | Anopheles benarrochi larvae | LC |
[52] |
| Antibacterial/Antimicrobial activities | T. zebrina, T. pallida and T. spathacea | Leaf extracts (0.02–10 mg/mL) | 6 species of Gram-positive (Methicillin-Resistant Staphylococcus aureus, Proteus vulgaris, Bacillus cereus, Aeromonas hydrophila, Bacillus subtilis, Enterococcus faecalis, Micrococcus luteus, Staphylococcus epidermidis) | The three species showed antibacterial activity. T. zebrina exhibited the best antibacterial potential. | [33] |
| T. spathacea | Fresh and dried extracts | Moniliophthora roreri | A prevention of the growth of M. roreri at concentrations of 40–50% | [53] | |
| Acetylcholinesterase inhibitory activity | T. zebrina | methanolic leaf extracts (100.00 and 10.00 µg/mL) | Acetylcholinesterase activity assay using the standard Ellman method with slight modifications | Significant decreases in acetylcholinesterase activity (p |
[34] |
| Modulation of the immune response | T. spathacea | Aqueous leaf extract | Lymphoproliferation assay and NK cell activity assay | Stimulation of the human lymphocyte proliferative response | [54] |
| (1 ng/mL–100 |
The antioxidant capacity of vegetable or food sources has become increasingly
important as a strategy to combat oxidative stress. Oxidative stress is a
biological process produced by an imbalance between the production of reactive
oxygen species (ROS) in tissues and cells, and the ability of a biological system
to repair the resulting damage by antioxidant agents. This imbalance can cause
toxic effects through the production of peroxides and free radicals that damage
all components of the cell, including proteins, lipids, and DNA. The biological
effects of ROS are controlled in aerobic organisms by a range of physiological
antioxidant defense mechanisms, which involve a complex group of processes aimed
at preventing or retarding excess oxidation at the cellular level and in some
cases reversing the oxidative damage of the affected molecules. There are
different molecules involved in the antioxidant system which are mainly
classified into endogenous or exogenous antioxidants. Endogenous antioxidants
include several enzymes (e.g., glutathione peroxidase, catalase, superoxide
dismutase, etc.) and non-enzymatic (e.g., glutathione, bilirubin, uric acid,
etc.) compounds. Exogenous antioxidants are mainly obtained from the diet,
including vitamins (e.g., vitamins A, C, E), carotenoids (e.g., lutein,
zeaxanthin, and lycopene), phenolic compounds (e.g., flavonoids, phenolic acids),
glucosinolates and organosulfur compounds. Due to the different families of
compounds with possible antioxidant properties present in natural sources such as
phenolic compounds or carotenoids, many investigations have focused on studying
their antioxidant, anticancer and anti-inflammatory potential through in
vitro cell lines and animal models [55]. The antioxidant activities of phenolic
compounds are based on their ability to be excellent donors of hydrogen, which
are accepted by reactive radicals to yield much less active radical and
non-radical species [56]. There are different methods for evaluating antioxidant
capacity such as the oxygen radical absorbance capacity assay (ORAC), Trolox
equivalent antioxidant capacity assay (TEAC), ferric reducing antioxidant power
assay (FRAP) and 2,2-diphenyl-1-picrylhydrazyl assay (DPPH) [57]. The DPPH and
ORAC assays involve a hydrogen atom transfer reaction. The ORAC assay measures
the radical chain-breaking ability of antioxidants by monitoring the inhibition
of peroxyl radical-induced oxidation. Peroxyl radicals are the predominant free
radicals found in lipid oxidation in foods and biological systems. DPPH radical
scavenging test is a sensitive antioxidant assay and depends on substrate
polarity. The presence of multiple hydroxyl functions could be considered as an
option for hydrogen donation and/or radical scavenging activity. Ferroptosis is
one of the effects of ROS overproduction, which is a type of iron-dependent
oxidative cell death caused by a variety of factors. Ferroptosis is different
from apoptosis but is also the result of dysfunction of antioxidant defense,
leading to the loss of cellular redox homeostasis. In contrast, TEAC and FRAP
assays are based on single-electron transfer. The FRAP method is based on the
reduction of Fe (III) to Fe (II) [58]. Antioxidants capable of chelating and
reducing iron (III) ions are potential candidates for controlling ferroptosis and
their destructive effects on healthy cells [46]. Tan et al. [33] studied
the antioxidant activity of T. zebrina, T. pallida and
R. spathacea by DPPH free radical scavenging (FRS), ferrous ion
chelating (FIC), and phenolic ferric reducing power (FRP) assays. Their results
revealed that T. zebrina showed the highest antioxidant capacity values
(FRS: 906.5
Inflammation is defined as an immune response of a living organism against numerous infectious agents such as viruses or bacteria. Some examples of common signs of inflammation are redness, pain or fever. The use of synthetic drugs against inflammatory processes has shown efficacy, but side effects are also often produced. Due to the phytochemical composition of many plant sources with anti-inflammatory potential, the use of these plants, including several Tradescantia species, has been studied as possible alternatives to alleviate inflammatory processes. For example, T. fluminensis, commonly known as Wandering Jew, has been identified as one of the species with anti-inflammatory potential. Tu et al. [43] evaluated the anti-inflammatory potential of compounds isolated from T. albiflora, finding that bracteanolide A, methyl 3,4-dihydroxybenzoate and hydroxytyrosol have great inhibitory potential against nitric oxide (NO) production in a RAW 264.7 cell model. NO production occurs during inflammatory processes and is, therefore, a good indicator of the extent of inflammation. Among the three compounds evaluated, 5-O-n-butyl bracteanolide A showed the highest anti-inflammatory potential.
In another study, the inhibitory activity of lipoxygenase produced by T. fluminensis and T. zebrina leaf extracts was assessed [30]. Lipoxygenase was involved in the production of lipid mediators, which played an important role in inflammatory processes. T. fluminensis showed the highest potential with an inhibition value of 87%.
Chan et al. [51] evaluated the cytotoxicity of six medicinal plants,
including T. zebrina, using neutral red uptake (NRU) and 3-(4,5
dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays. In this
study, six extracts obtained with different solvents (chloroform, ethanol,
methanol, water, ethyl acetate and hexane) were evaluated. The cytotoxicity
activity was evaluated in a monkey kidney epithelial cell (Vero) model using
extract concentrations ranging from 5 to 640
Li et al. [50] synthesized zinc oxide nanoparticles (ZnO NPs) using an
aqueous leaf extract (TPALE) of T. pallida to evaluate their cytotoxic
and fluorescent properties. Specifically, 20 mg of TPALE were mixed with 80 mL of
zinc acetate (1 mM) for nanoparticle formation. Subsequently, the solution was
centrifuged and the pellet heated at 350 ºC for 5 hours. The
synthesized ZnO NPs were characterized using several techniques (e.g., scanning
electron microscopy, transmission electron microscopy, X-ray diffraction). The
resulting rod-shaped particles had a size of 25
Brauner et al. [47] evaluated the anticancer effects of methanolic and aqueous extracts of T. fluminensis and T. zebrina on A549 (lung adenocarcinoma), SCC-13y (squamous cell carcinoma) and HFF-1 (human foreskin fibroblasts) cell models [47]. The results showed a decrease in the cancer cell proliferation rate, especially when T. zebrina was added to the treatment. Therefore, this study confirmed the inhibitory capacity of T. fluminensis and T. zebrina on cancer and non-cancer cells. Moehring et al. [48] evaluated the anticancer properties of methanolic and aqueous extracts of T. zebrina on SCC-13y and A-549 cell models. The number of cells was counted for five days to assess the rate of inhibition by the plant extract. The results showed a reduction in cell growth in both cell lines. In addition, the extracts were evaluated against a non-cancerous cell line (HFF-1) to assess the relative toxicity of the extracts. T. zebrina showed an inhibitory potential against both non-cancer and cancer cells.
Rosales-Reyes et al. [37] evaluated the protective effects of a crude
T. spathacea aqueous extract against liver cancer using the
carcinogenesis model of resistant hepatocytes in rats. The aqueous crude extract,
with dose below 20 mg/kg of body weight, reduced the number and area of
preneoplastic lesions. The administration of the aqueous extract produced no
promoter or initiator effects, nor induce the development of altered hepatocytes
foci. In another study, Prakash et al. [49] evaluated the anticancer
potential of T. spathacea leaf extract against a human breast
adenocarcinoma cell line (MCF-7). The results showed that a 50% inhibition of
MCF-7 was detected at a dose of 299.7
Cocoa has been identified as a large cash crop for the production of chocolates, with social, economic and environmental impacts. Pests and diseases are factors that significantly affect the quality of cocoa beans. Moniliophthora roreri has been identified as one of the main pathogenic fungi that affect the quality of cocoa plants. To prevent this phenomenon, España et al. [53], evaluated the inhibitory effects of T. spathacea, Origanum vulgare and Zingiber officinale extracts against the growth of M. roreri. The results showed that all three plants prevented the growth of M. roreri conidia, using concentrations of 40–50% of both fresh and dry plants.
T. zebrina, T. pallida and T. spathacea leaves methanolic extracts showed antibacterial activity against methicillin-resistant Staphylococcus aureus, Proteus vulgaris, Bacillus cereus, Aeromonas hydrophila, Bacillus subtilis, Enterococcus faecalis, Micrococcus luteus, and Staphylococcus epidermidis [33].
Other biological activities have also been studied for various species of
Tradescantia. For instance, the larvicidal effect against
Anopheles benarrochi has been studied using different concentrations of
a T. zebrina tea extract (2.5–10% m/v). A median lethal concentration
(LC
Chunxin et al. [60] evaluated the antiarrhythmic activity of
Cheah et al. [34], evaluated the in vitro acetylcholinesterase
inhibitory activity of T. zebrina leaves methanolic extract [34]. This
enzyme has been associated with many diseases, especially neurodegenerative
disorders such as Alzheimer’s disease. The results indicated a significant
decrease in acetylcholinesterase activity (p
Sriwanthana et al. [54] evaluated the effect of T. spathacea and seven medicinal plants species, against natural killer (NK) cells, a type of cell of the innate immune system. All cells were found to significantly enhance human lymphocyte proliferative responses when exposed to high concentrations in a dose-dependent manner. The authors found that the studied plant species had stimulating effect on human lymphocytes, playing an important role in modulating the immune response.
Despite the numerous species within this genus Tradescantia, very few have been biologically explored, with T. zebrina, T. spathacea, T. fluminensis, and T. pallida being the most studied. In general, it is necessary to continue studying the biological activities of Tradescantia species, increasing the number of assays in in vivo models to allow a greater advance in knowledge. Further studies should be performed to ensure the results reproducibility detailed in this review. In addition, the bioactivity, bioavailability, and bioaccessibility studies of the phytochemical compounds are also necessary. It is key to understand the compounds capable of reaching the target tissues to exert beneficial properties. There is still a lack of knowledge in relation to the bioactive properties of these extracts or compounds, to be used in modern medicine applications. More studies should therefore be performed on the bioactive properties, bioavailability, metabolism, and long-term effects of Tradescantia extracts to increase scientific evidence and translate the traditional uses of these plants into food or modern medicine applications.
Plants contain many different biologically active substances, some of which have proven healing properties, while others are harmful to human health and cause adverse effects [61]. The latter are usually low molecular weight endogenous toxins or secondary metabolites produced by plants for protection from animals, insects or microorganisms [62]. Plant toxins are generally classified into the following four main groups based on the chemical structure: alkaloids, glycosides, proteins, and saponin glycosides [61, 62]. The presence of phytochemicals in Tradescantia extracts, such as alkaloids, flavonoids, tannins, and phenols, indicate potential toxic properties of plant, although, reports on adverse reactions of compounds from this plant are scarce [22, 32]. This is due to the fact that the harmful effects of herbaceous plants are pourly controlled in comparison to the pharmaceuticals counterpart [63].
According to Filmer et al. [64], Tradescantia spp. belong to the fourth class of toxicity, which means that the juice, sap or thorns of this plant can cause skin rash or irritation. This potential toxicity may be attributed to calcium oxalate crystals found in the parenchymal tissues of the stem, leaves, roots, and flowers [65]. Plants in humid environments can cause the plant cells to secrete these crystals, which can then come into contact with the skin [66]. In December 2019, the FDA’s Poisonous Plant Database listed 5 titles that mention the toxicity of this plant. Only one case of a 32-year-old patient allergic to Tradescantia spp. (T. albifloxia and T. fluminensis) has been confirmed in humans [67]. The patient exhibited an itchy face, throat, and conjunctiva, swollen lips, and dyspnea [68] in addition to previous atopic dermatitis. In a recent study, Wang et al. [69] revealed that a methanolic extract of T. zebrina leaves could induce cytochrome (CYP) 3A4, an enzyme activity that metabolizes drugs in human liver microsomes. Some studies have suggested that T. fluminensis may show adverse effects in dogs, causing allergic reactions (red and itchy skin) [70]. More in vivo studies should be conducted to further confirm the effect of Tradescantia. spp. in the human body system.
Despite these few examples of possible negative effects on human health, these plants have been used in beverage production and in folk medicine. However, the lack of specific information on the negative impact of whole plants or extracts obtained from these plants on human health, it can only be determined that their main bioactive compounds have a negative effect on human health.
The 3-epicyclomusalenol, a phytochemical found in Tradescantia spp.,
has also been identified as a phytochemical compound of banana (Musa
sapientum) [71] and brown algae (Kjellmaniella crassifolia) [72].
According to the Human Metabolome Database (HMDB) (http://www.hmdb.ca/),
no side effects data has been reported for this compound. Ergosterol peroxide,
also obtained from Ananas comosus (pineapple) and fungus
Ganoderma lucidum [73], represents a promising new reagent to overcome
drug resistance of tumour cells, though no information on the biological side
effects and interactions of this compound have been described. Methyl
3,4-dihydroxybenzoate is an antioxidant polyphenol (found in green tea) that
attenuates fluoride toxicity on lung epithelial cells [74]. 4-Hydroxybenzoic acid
is a low toxicity, antioxidant capable of stimulating the oestrogenic response in
human breast cancer cells [75]. Some phytosterols have been reported to have more
or less pronounced cytotoxic effects on normal cells. Despite the fact that
In conclusion, no relevant toxicological data are available on plants belonging to the genus Tradescantia, though long-term history use in humans show no safety concerns for the oral or oromucosal use of the preparations of these plants are apparent. Based on these plant matrices, further toxicological studies are necessary to establish their safety prior to commercialization of products.
T. zebrina, T. fluminensis, T. spathacea, and T. albiflora are the most widely studied species within the genus Tradescantia. Although research has mainly focused on the phytochemical composition and/or biological activities of Tradescantia species, most of the literature is based on traditional applications, descriptive analyzes, and non-medicinal uses. T. zebrina has been the most commonly studied species within the genus concerning bioactivity.
To develop potential therapeutic applications based on Tradescantia compounds, it is necessary to increase the knowledge regarding the biological mechanisms of action of these compounds. More studies should be conducted to better explore the phytochemical composition both quantitatively and qualitatively, as well as the corresponding tests to examine their bioactive activities. The application of modern approaches, such as genomics, proteomics, metabolomics, and transcriptomics, should be considered for future studies of this genus. Given the large number of species in the genus Tradescantia, a more systematic approach is necessary to ensure data are consistently collected in future studies.
AA, Ascorbic acid; DPPH, 2,2-Diphenyl-1-picrylhydrazyl assay; FIC, ferrous ion chelating; FRAP, ferric reducing antioxidant power assay; FRS, Free radical scavenging; FRP, phenolic ferric reducing power; GAE, Gallic acid equivalent; MS, Mass spectrometry; MTT, 3-(4, 5 dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; NMR, Nuclear magnetic resonance; NRU, neutral red uptake; ORAC, oxygen radical absorbance capacity assay, RE, Rutin equivalent; TAE, Tannic acid equivalent; TEAC, Trolox equivalent antioxidant capacity assay; TFC, total flavonoid content; TPC, total phenolic content; TTC, total tannin content.
MB, CQ, JH-B, ÁF-O, SE-Y, COA, AEM, AO, PB, HA, KT, NB, JMB, SS, KA, AS-C, Mde la LC-G, SHEL, MP, IS, SDD, AFAR, US, RMK, WN.S, JS-R equally contributed to this work. ÁF-O, M de la LC-G, SHEL, AFAR and JS-R. critically reviewed the manuscript. All authors contributed to the editorial changes in the manuscript. All authors read and approved the final manuscript.
Not applicale.
We acknowledged Universiti Putra Malaysia (UPM) for article processing fee payment.
This research received no external funding.
The authors declare no conflict of interest.



