1 Laboratory of Feed Biotechnology of Ministry of Agriculture and Rural Affairs, Institute of Feed Research, Chinese Academy of Agricultural Sciences, 100081 Beijing, China
†These authors contributed equally.
Academic Editor: Guoyao Wu
Abstract
Background: Polyamines have been demonstrated to be beneficial to
porcine intestinal development. Our previous study showed that putrescine
mitigates intestinal atrophy in weanling piglets and suppresses inflammatory
response in porcine intestinal epithelial cells, it is still unknown the role of
spermidine in mediating putrescine function. Objective: The current
study aimed to investigate the effect of spermidine on the proliferation,
migration, and inflammatory response in porcine intestinal epithelial cells
(IPEC-J2 cell line). Methods: The effects of spermidine on proliferation
and migration of IPEC-J2 cells were measured. Difluoromethyl ornithine (DFMO) and
diethylglyoxal bis (guanylhydrazone) (DEGBG) were used to block the production of
putrescine and spermidine, respectively. A cell inflammation model was
established with lipopolysaccharides (LPS) stimulation. Gene expression and
protein abundance were determined by real-time quantitative PCR and western
blotting, respectively. Result: Spermidine significantly enhanced cell
proliferation in DFMO (or/and) DEGBG treated
IPEC-J2 cells (p
Keywords
- spermidine
- putrescine
- cell proliferation
- cell migration
- inflammatory response
The intestine plays a critical role in the digestion and absorption of nutrients and host defense. Intestinal epithelial cells form a monolayer physical barrier to prevent the invasion of harmful substances from the intestinal lumen [1, 2, 3]. External stressors such as pathogens, toxins, and weaning affect gut health, which causes damage to the structure and function of the small intestine, resulting in intestinal diseases such as diarrhea and chronic inflammatory disorders [4, 5, 6]. The studies have demonstrated that proliferation and migration of intestinal epithelial cells were directly involved in repairing mucosal damage [7]. Therefore, it is necessary to improve and maintain intestinal health by promoting the growth and migration of enterocytes.
Biogenic amines, including monoamines, diamines, and polyamines, are important nitrogenous organic compounds that have the physiological function of signal transduction in living cells [8, 9]. Polyamines are mainly bound to polyanionic molecules in cells to perform multiple beneficial functions that include anti-inflammation, antioxidation, anti-aging, and enhancing mitochondrial metabolism [10, 11]. Specifically, they have an essential physiological role in cell growth, proliferation, maturation, and regeneration [12]. Spermidine, as a downstream family member of putrescine metabolites, is a biologically active polyamine whose intracellular concentration is strictly regulated by controlling the levels of the two rate-limiting enzymes in polyamine metabolism, ornithine decarboxylase (ODC) and S-adenosylmethionine decarboxylase (SAMDC) [13]. There is an inextricable relationship between spermidine and cell proliferation, migration, and inflammatory response of mammalian cells [13, 14, 15]. Our previous study found that putrescine increased the proliferation and migration of the porcine intestinal epithelial cells (IPEC-J2) and mitigated mucosal atrophy of the small intestine by suppressing inflammatory responses in weanling piglets [16]. Still, it is unknown whether putrescine must be metabolized to spermidine to exert its effects.
Therefore, the primary purpose of this study is to investigate the effect of spermidine on the proliferation, migration, and inflammatory response of porcine intestinal epithelial cells and to detect the role of spermidine in mediating the physiological functions of putrescine. We hypothesized that the conversion of putrescine into spermidine is essential for putrescine to perform its functions in cell proliferation, migration, and anti-inflammation. Difluoromethyl ornithine (DFMO), an irreversible inhibitor of ODC, can effectively block the synthesis of putrescine from ornithine, and diethylglyoxal bis (guanylhydrazone) (DEGBG), a specific inhibitor of SAMDC, can effectively block the synthesis of spermidine from putrescine and spermine from spermidine. They were used to block the production of putrescine and spermidine in the cells, respectively. Results from this study provided new insights into the mechanism of polyamine function in regulating the inflammatory response of porcine enterocytes.
IPEC-J2
cells were from Dr. Guoyao Wu from Texas A&M University. The cells were cultured
as described previously [17]. Briefly, the cells were cultured
in Dulbecco’s modified Eagle’s medium/F12 (DMEM/F12, Thermo
Fisher Scientific, Waltham, MA, USA) supplemented with 5% fetal bovine serum
(FBS) (Thermo Fisher Scientific, Waltham, MA, USA), 1% penicillin-streptomycin
(Thermo Fisher Scientific, Waltham, MA, USA), 0.01% epidermal growth factor (5
IPEC-J2 cells were seeded at 0.5
IPEC-J2 cells were seeded at 0.5
The cell inflammation model was established in vitro by adding
lipopolysaccharides (LPS, Beyotime technology, Shanghai, China).
IPEC-J2 cells were seeded at 0.5
IPEC-J2 cells were seeded at 0.5
mRNA abundance in the cells was determined by qPCR according to the procedure described by Li et al. [20]. In brief, total RNA was isolated from IPEC-J2 cells by using the Trizol reagent (Thermo Fisher Scientific, Waltham, MA, USA), followed by the determination of the content and quality of total RNA using an Epoch Microplate Spectrophotometer (BioTek Instruments, Inc., Montpellier, VT, USA), and the first-strand cDNA synthesis by TransScript First-Strand cDNA Synthesis Kit (TransGen Biotech, Beijing, China) according to the manufacturer’s protocol. qPCR analysis was performed using the cDNA templates, primer pairs for specific genes of the target, and SYBR Green reagent (Thermo Fisher Scientific, MA, USA) on an ABI 6 flex real-time PCR instrument (Thermo Fisher Scientific, Waltham, MA, USA). The primers used for qPCR are shown in Supplementary Table 1, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a housekeeping gene to normalize target genes. The calculation of fold changes of the target genes relative to those of GAPDH was acquired by the comparative Ct value method.
Western blot analysis was performed using a routine procedure described by Liu
et al. [16] with slight modification. The membranes were incubated with
a primary antibody overnight at 4 °C, followed by washing three times
(10 min per time) with Tris-buffered saline and Tween 20 (TBST) buffer, incubated
with a secondary antibody for 3 h at room temperature. The membranes were
rewashed with TBST buffer before adding the reagents from Western Bright ECL Kit
(Bio-Rad Laboratories Inc., Berkeley, CA, USA). Digital images
were detected by the ChemiDoc MP Imaging System (Bio-Rad
Laboratories, Inc., Berkeley, CA, USA). The antibodies of extracellular
signal-regulated kinase1/2 (ERK1/2) (1:1000), adhesion kinase (FAK) (1:1000),
NF-
Data were expressed as mean
The effect of treatment with different concentrations of spermidine on the
proliferation of IPEC-J2 cells is illustrated in Fig. 1. Compared with the
control treatment, the addition of 2, 4, 8, 16
 Fig. 1.
Fig. 1.Effects of different spermidine concentrations on the growth of
IPEC-J2 cells. IPEC-J2 cells were seeded into 96-well plates at a number
of 0.5
As shown in Fig. 2A, adding 5 mmol/L DFMO significantly inhibited cell
proliferation at 72 h, and adding simultaneously exogenous putrescine at a
concentration of 200 or 400
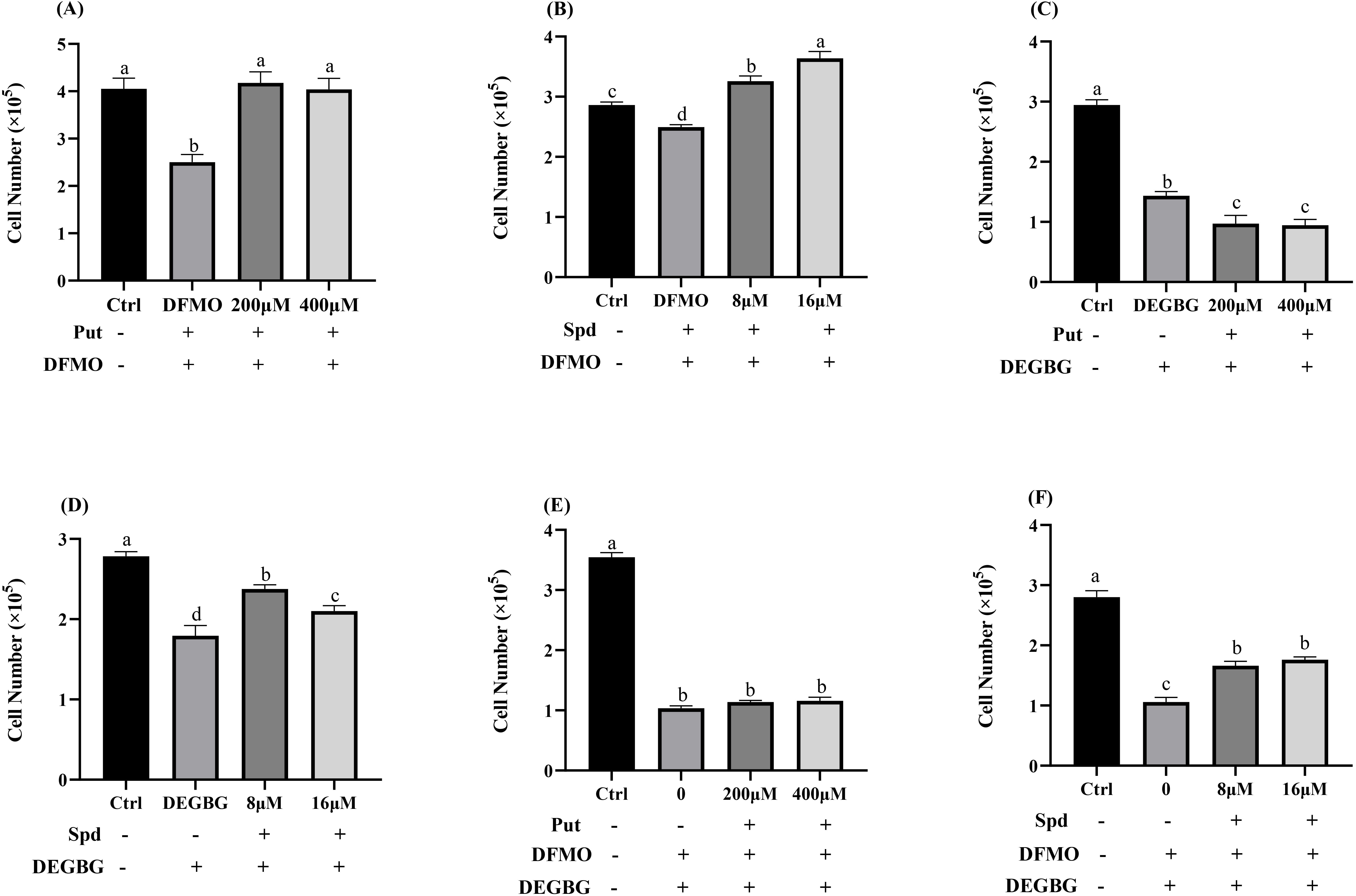 Fig. 2.
Fig. 2.Effect of polyamines (putrescine and spermidine) on
IPEC-J2 cells proliferation in the presence of DFMO (or/and) DEGBG. IPEC-J2
cells were cultured with putrescine (200, 400
Adding 1 mmol/L DEGBG to the culture medium significantly reduced the
intracellular spermidine content and increased the intracellular putrescine
concentrations (p
As shown in Fig. 3, compared with the control treatment, the addition of 8
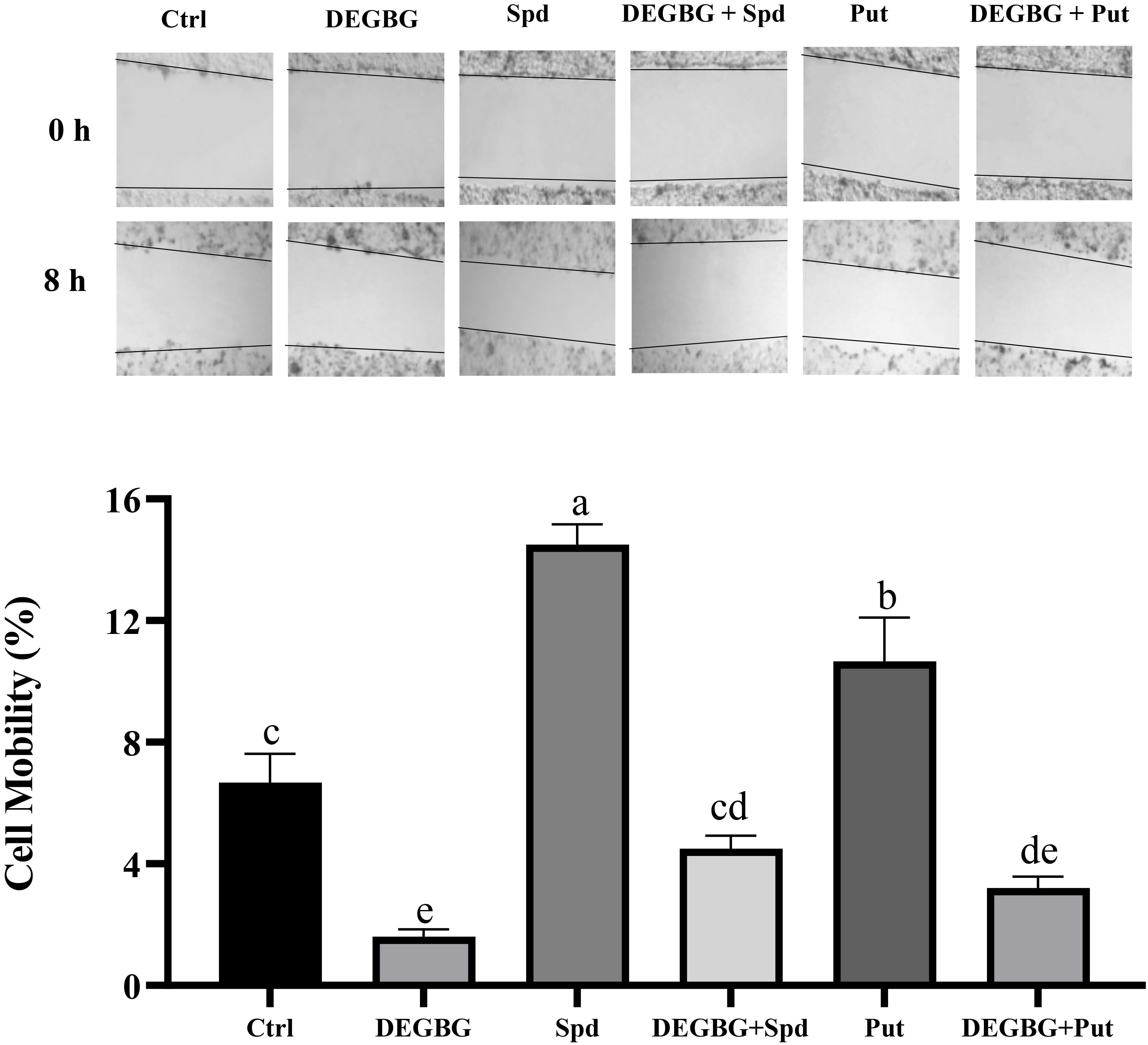 Fig. 3.
Fig. 3.Effect of putrescine and spermidine on IPEC-J2 cells
migration in the presence of DEGBG. IPEC-J2 cells were seeded into 6-well plates
at a concentration of 0.5
Compared with the control treatment, DEGBG supplementation decreased the
phosphorylation of ERK1/2, but this passive effect was reversed by the
simultaneous addition of spermidine (p
 Fig. 4.
Fig. 4.Effect of putrescine and spermidine on activation of
the ERK1/2 protein in IPEC-J2 cells. IPEC-J2 cells were incubated with
putrescine or spermidine for 24 h in the presence or absence of DEGBG. Then, the
protein abundance of ERK1/2 and P-ERK1/2 proteins were detected by western
blotting. (A) ERK1/2 protein abundance and (B) the phosphorylated ERK1/2 protein
abundance at 24 h after being pretreated with 200
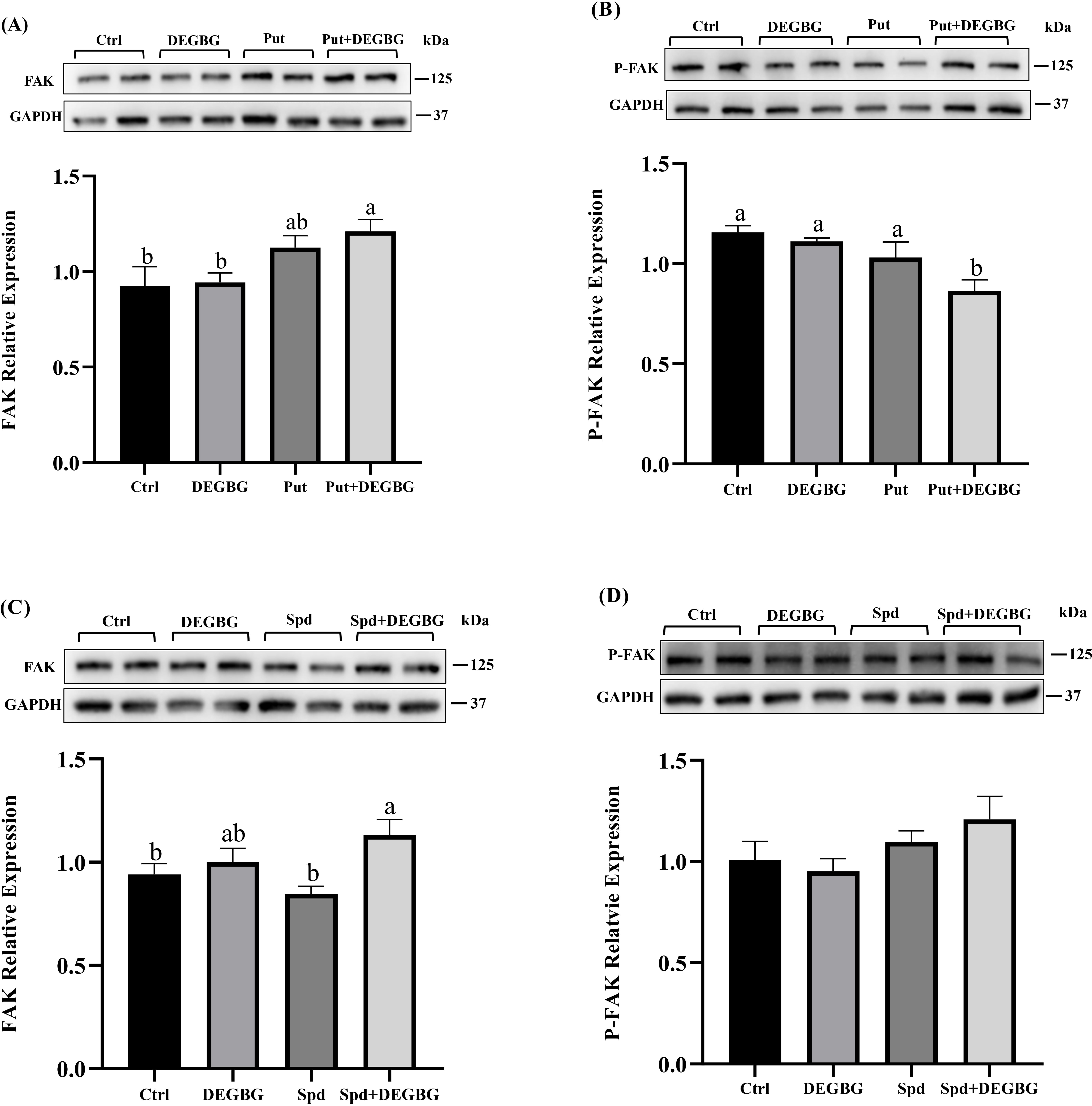 Fig. 5.
Fig. 5.Effect of putrescine and spermidine on activation of
FAK protein in IPEC-J2 cells. IPEC-J2 cells were incubated with putrescine (200
Detection of the mRNA abundance of pro-inflammatory cytokines in IPEC-J2 cells
was conducted by q-PCR, and the results were shown in (Fig. 6).
Compared with the control treatment, a
significant augment in the abundance of IL-8, IL-6,
TNF-
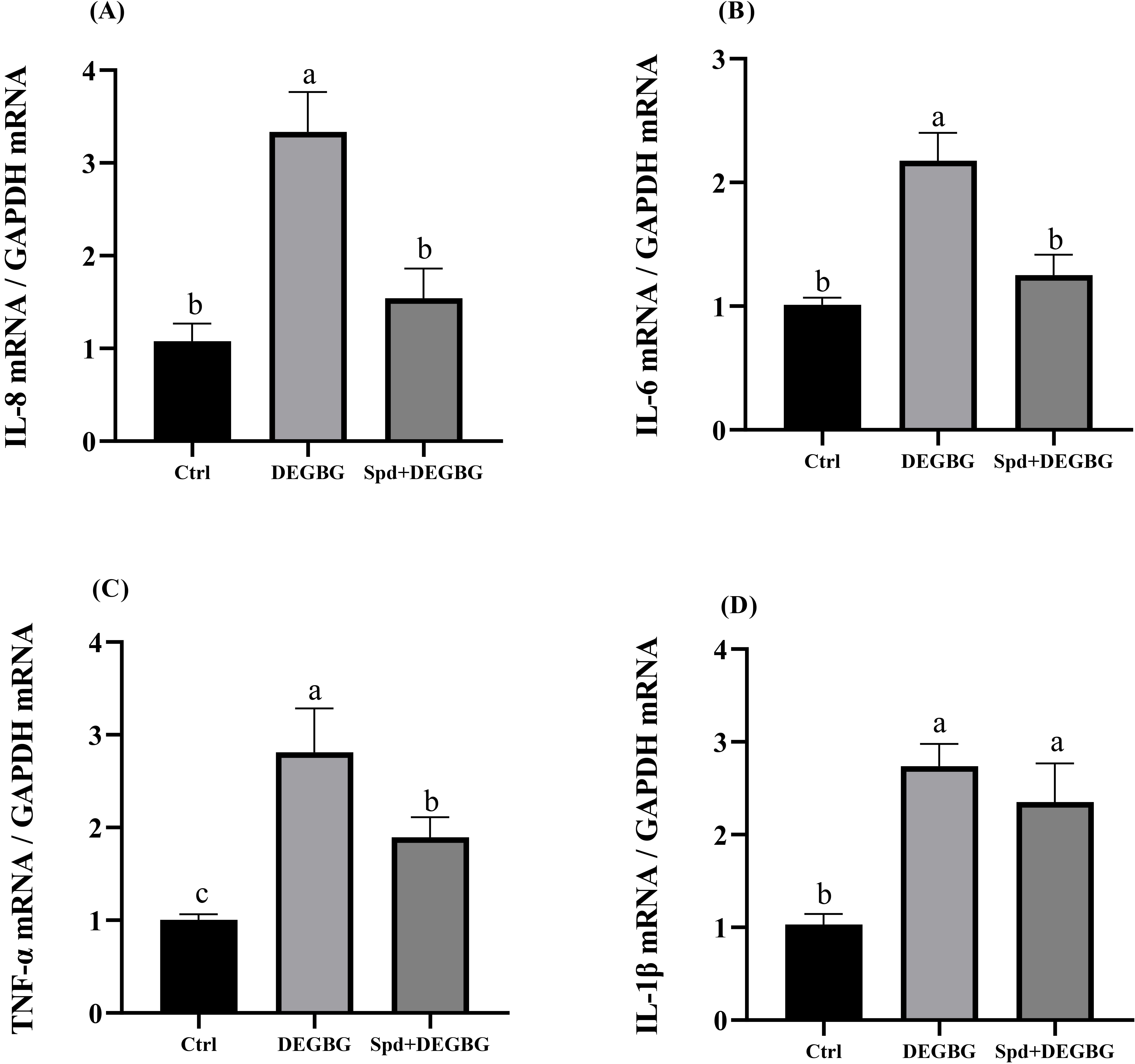 Fig. 6.
Fig. 6.Effect of spermidine on the expression of inflammatory
genes in IPEC-J2 cells induced by DEGBG. IPEC-J2 cells were incubated with
spermidine (8
 Fig. 7.
Fig. 7.Effect of spermidine on the expression of inflammatory
genes in IPEC-J2 cells induced by DEGBG. IPEC-J2 cells were incubated with
putrescine or spermidine for 24 h in the presence or absence of DEGBG. Then, the
abundance of NF-
Weaning stress is one of the important factors which cause small intestinal
mucosal damage in piglets. The repair of intestinal mucosal injury is mainly
divided into two independent processes. Firstly, mucosal epithelial cells make up
the damaged site through migration, while the cells do not proliferate in this
process. Then, the cells supplement the lost cells through proliferation and
differentiation [21]. Our
previous study found that dietary supplementation with 0.2% putrescine promoted
the repair of intestinal mucosal injury and reduced the diarrhea rate of piglets
[16]. Moreover, adding 200
In the present study, we found that spermidine supplementation stimulated cell proliferation in a dose-dependent manner. This result is consistent with the previous report in which murine erythroleukemia cells proliferated faster and had higher spermidine levels than normal cells [14]. Considering the mutual conversion of polyamines during synthesis and catabolism, DFMO and DEGBG were used here to inhibit the activities of ODC and SAMDC, two key enzymes of polyamines metabolism, respectively [13]. Interestingly, supplementation with exogenous putrescine or spermidine alone completely recovered the impaired growth of IPEC-J2 cells induced by DFMO, which indicated that both putrescine and spermidine played a critical role in regulating cell proliferation. However, putrescine did not alleviate the growth inhibition induced by DEGBG, but spermidine effectively mitigated this inhibition, which meant that putrescine could not rescue cell proliferation in the case of blocking the synthesis of spermidine from putrescine. Similarly, the combined addition of DFMO and DEGBG also remarkably inhibited cell growth; spermidine partly recovered the growth of porcine enterocytes, whereas exogenous putrescine did not. These results demonstrated that putrescine exerted its function through converting to spermidine; similar results have been found in IEC-6 cells [22]. Available evidence suggests that the extracellular signal-regulated kinase1/2 (ERK1/2) signaling pathway is tightly involved in regulating cell proliferation, maturation, and differentiation [23, 24]. We therefore investigated the activation of the ERK1/2 protein and found that DEGBG treatment significantly decreased ERK1/2 phosphorylation in the IPEC-J2 cells. However, the addition of spermidine restored the phosphorylation of ERK1/2. On the contrary, putrescine failed to recover the phosphorylation of ERK1/2 caused by DEGBG. Taken together, these results indicated that spermidine played a vital role in regulating the proliferation of porcine intestinal epithelial cells, the metabolism of putrescine to spermidine was essential for putrescine to perform its functions, and this beneficial effect of spermidine and putrescine was at least partly mediated by the activation of the ERK1/2 pathway.
Evidence showed that the level of ODC and SAMDC rapidly increased at the wound edge, which indicates that polyamines play a vital role in the acceleration of wound healing [13]. Cell migration is involved in repairing mucosal damage [25]. The current study used a model with scratching porcine intestinal epithelial cells to mimic wound healing after intestinal mucosa damage in vivo. This study showed that treating cells with DEGBG caused depletion of spermidine and accumulation of putrescine in cells, resulting in inhibiting the migration of porcine enterocytes. Exogenous spermidine supplementation partly restored cell migration caused by DEGBG. Consistent with our results, it has been reported that the rate of scratch wound closure of mouse embryo fibroblasts is significantly improved in the presence of spermidine [26]. However, the administration of putrescine failed to restore the migration of IPEC-J2 cells, which suggested that putrescine itself cannot rescue migration in DEGBG-treated cells. These results corroborate the previous study in which putrescine itself is incapable of functioning to restore cell migration in IEC-6 cells [22]. Focal adhesion kinase (FAK) is a critical member of integrin-mediated signal transduction, mainly involved in regulating extracellular matrix signal transduction dependent on protein tyrosine kinase (PTK) activity, thus affecting cell adhesion, movement, and migration [27, 28]. Previous studies have revealed that polyamines enhanced cell migration by upregulating the phosphorylation levels of FAK protein in DFMO-treated cells [16]. The present study showed that adding putrescine downregulated the levels of phosphorylated P-FAK in DEGBG-treated cells, which partially suggested that putrescine cannot activate FAK phosphorylation when the amount of spermidine is deficient, excess putrescine due to the inhibition of its metabolism even reduced FAK phosphorylation. Moreover, DEGBG alone did not downregulate FAK phosphorylation and adding spermidine in DEGBG-treated cells numerically increased FAK phosphorylation but was not significant indicated that it might need to take a longer time to affect FAK phosphorylation by DEGBG and spermidine, the treating time is 24 h for present study while treating with DFMO for 72 h has been found to reduce FAK phosphorylation [16]. Collectively, these findings demonstrated that putrescine itself might not be essential for the migration of IPEC-J2 cells, but its metabolite spermidine enhanced cell migration. The mechanism of regulating cell migration by spermidine may be through activation of the FAK signaling pathway. Future studies are warranted to test this hypothesis.
There was accumulating evidence showing that the increased inflammatory response
is closely related to mucosal damage [29, 30]. Spermidine has been demonstrated
to perform anti-inflammatory effects in vitro and in vivo via
regulating immune responses [31, 32]. The current study found
that spermidine depletion by DEGBG remarkably increased the mRNA expressions of
the inflammatory mediators IL-8, IL-6, and TNF-
In conclusion, the current study demonstrated that putrescine needs to be converted into spermidine to efficiently promote IPEC-J2 cell growth and migration, which is associated, at least in part, with the activation of ERK1/2 and FAK signaling pathway. More importantly, our results highlight new insights into spermidine in immunomodulation. Thus, exogenous spermidine supplementation may constitute an attractive strategy to improve intestinal health and treat inflammation-related diseases.
ZW and XL conceived and designed this study; ZW and LC performed the experiments; XZ and XJ analyzed the data; ZW and LC prepared the figures; ZW, LC, and XL interpreted the results of the experiments; LC and ZW drafted the initial manuscript; XJ and XL guided and revised the manuscript. All authors read and approved the final manuscript.
Not applicable.
We thank Zhaolai Dai for his technical assistance with polyamine determination.
This study was supported by the National Natural Science Foundation of China (31672438), the Elite Youth Program of the Chinese Academy of Agricultural Sciences (to XL).
The authors declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/j.fbl2706194.







