Academic Editors: Shikun He and Graham Pawelec
Background: To observe the ultrastructural outcomes of autologous transplantation of retinal pigment epithelium-partial-thickness choroidal (RPE-PTC) sheets in rabbits after 6 months. Methods: Eighteen pigmented rabbits were used in this study. Among them, nine rabbits were used for autologous transplantation of RPE-PTC sheets. Tissue sections were observed under a transmission electron microscope for one, three, and six months after transplantation, respectively. Results: One, three, and six months after the autologous transplantation of RPE-PTC sheets, the inner and outer segments of photoreceptor cells were arranged regularly, and the connection between the inner and outer segments was normal. The inner structure of the RPE cells and tight junctions among them remained normal. Phagocytosis of outer segment of photoreceptor cells could also be observed in RPE cells. The structure of the Bruch’s membrane appeared loose, rather than being dense as normal, and it was undulated after one and three months, while it became dense after six months. The graft and the bed were healed well, the boundary was unclear, and the graft was vascularized after one, three, and six months, respectively. Conclusions: Our findings revealed that the RPE-PTC sheets could quickly rebuild blood vessels, thereby maintaining the normal physiological functions of RPE cells, as well as the survival and functional status of photoreceptor cells for a long-time.
Age-related macular degeneration (AMD) is the leading cause of blindness among elderly people, particularly in the Western countries [1, 2, 3]. There are two types of AMD, including dry and wet. In recent years, there have been various treatments for wet AMD, including thermal laser, photodynamic therapy, and intravitreal injection of anti-vascular endothelial growth factor (VEGF) [4, 5, 6, 7]. However, these treatment methods only slow down the course of the disease, and they are not effective in patients with retinal pigment epithelium (RPE) tears and subretinal hemorrhage [8, 9]. Thus, surgical restoration of the retinal anatomy is still a therapeutic option for severe wet AMD patients [10, 11, 12, 13]. However, surgical removal of choroidal neovascularization (CNV) did not merely improve or preserve visual acuity (VA) [13, 14]. Surgical excision of CNV combined with autologous RPE transplantation can increase the vision and stabilize it in the long-term [11, 15]. At present, there is no treatment for advanced dry AMD or for cases of severe wet AMD associated with submacular RPE atrophy. RPE transplantation can be an effective therapy for these patients [16].
There are two potential roles for transplantation of autologous RPE cells in AMD patients, including replacement of RPE function and trophic support to dying cells [17, 18, 19]. For autologous RPE transplantation, the grafts consist of RPE, Bruch’s membrane, choriocapillaris, and choroid. A number of scholars have demonstrated the clinical feasibility of the above-mentioned technique [20, 21, 22, 23, 24]. Some clinical cases have shown that RPE-choroidal autologous graft translocation is associated with a high risk of potential complications, while clinical benefits have also been reported in some patients [20, 23, 25, 26]. A 4-year study showed that an autologous-free RPE choroidal graft was stable and improved vision [15].
In a previous study, we attempted to develop a technique by the removal of partial choroidal tissues to decrease the thickness of a RPE choroidal complex, in which we termed it as RPE-partial-thickness choroidal (RPE-PTC) graft. It is suggested that the transplantation of a partial-thickness graft provides better results than a full-thickness one. Under light microscopy, it was revealed that the RPE-PTC graft transplanted to subretinal space could support the neural retina [27, 28]. In the present study, we aimed to report the long-term ultrastructural outcomes of autologous transplantation of RPE-PTC sheets in rabbits. The number of rabbits in each group was different from previous studies, and with the proficiency of the experimental technology, the success rate was also significantly improved compared with before. Therefore, our results are meaningful and can reveal the ultrastructure of RPE-PTC sheets in rabbits that underwent autologous transplantation.
Eighteen pigmented rabbits weighting 2–2.5 kg were used in the present study. Nine rabbits were used for autologous transplantation of RPE-PTC sheets (experimental group), of which three rabbits were examined at three different time points, respectively. In addition, three rabbits were utilized in the control group, which were transplanted with RPE-full-thickness choroidal (RPE-FTC) sheets. In the blank group, RPE cells were gently brushed off by a macular silica gel brush [27], and three rabbits were used in this group. Three rabbits served as the normal control. All surgical procedures were performed on one eye of each animal.
Rabbits were anesthetized by an intramuscular injection of a mixture of ketamine hydrochloride (15 mg/kg) and xylazine hydrochloride (15 mg/kg). Local anesthesia was applied during subconjunctival injection of 2% lidocaine hydrochloride (0.5 mL). Pupils were dilated with topical 1% tropicamide and 2.5% phenylephrine hydrochloride. All procedures performed in the present study were in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research.
The surgical procedures were carried out, as described in our previous research [27]. Briefly, after anesthesia and pupil dilation, phacoemulsification was performed to extract the lens. Then, the standard three-port vitrectomy was undertaken to remove the vitreous. A retinal detachment was created by injection of balanced saline solution into the superior region. Retinotomy was made in the detachment retina. Then, RPE cells were removed by a hollow silicone tip in the size of approximately two optic discs (ODs).
The grafts were taken from at least 1.5 ODs away inferior to the OD, then, the retina was coagulated by diathermy with the size of about 2 ODs, and the retina was removed. After that, the RPE-choroidal tissue was carefully cut off, and the choroid was separated with a spatula until a translucent RPE-PTC graft was obtained.
Afterwards, the graft was translocated to the bed of Bruch’s membrane. The retina was repositioned by an air-fluid exchange, and silicon oil was tamponade. After surgery, topical antibiotic and steroid were applied for 3 days.
Rabbits in the RPE-FTC sheet group were treated with the above-mentioned method, those rabbits in the blank group did not undergo RPE transplantation, and other procedures were performed the same as above.
After one, three, and six months, three animals were respectively sedated and sacrificed using an intravascular overdose of 2% lidocaine. Each eye was enucleated rapidly postmortem, punctured at the limbus, immersed and fixed in 1:1 mixture of 2.5% glutaraldehyde and 4% paraformaldehyde in 0.1 M phosphate buffer at room temperature for 2 h, and washed with 0.1 M phosphate-buffered solution (PBS). The anterior segment and intraocular silicone oil were removed. Subsequently, the posterior cup was fixed with the same solution for 48 h.
The tissues obtained from the central region of the transplanted area were cut into sections and those sections were fixed with 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) at 4 ℃ for 24 h; after fixing with 1% osmium tetroxide at room temperature for 2 h and twice washing with 0.1 M PBS, the sections were stained, dehydrated in a graded series of acetones, and embedded into Spurr’s resin. Semi-thin sections were stained with toluidine blue and visualized under a light microscope. Ultrathin sections were stained with 2% uranyl acetate and 2% lead citrate, and observed under a transmission electron microscope (JEM1230; JEOL Inc., Tokyo, Japan).
The intraocular pressure of animals did not significantly fluctuate before and after the surgery, and it was in the normal range. Proliferative vitreoretinopathy, retinal detachment or hemorrhage were not observed in all cases. Other surgery-related complications were similar to our previous research [27], and there was no serve complication that influenced the results.
TEM was employed to observe ultrastructure of retina and choroidal blood vessels of rabbits (Fig. 1). The outer segment of photoreceptor was inlaid and combined with RPE cells. The microvilli of RPE cells faced the photoreceptor cells, and the cell polarity was arranged (Fig. 1A,B). The tight junctions among the cells were intact, the void structures in the RPE layer were phagosomes, and the photoreceptor cells that were swallowed could be observed in the RPE cells (Fig. 1C,D). Fig. 1E shows the swallowing process of substances that were discharged by RPE cells and entered the capillaries through a Bruch’s membrane. Under the TEM, five-layer structure of a Bruch’s membrane could be clearly observed. In the outermost layer, the capillary basement membrane and the window-like structure of capillary endothelial cells were clearly visible. However, in the outer layer, the boundary between the medium- and large-sized blood vessels of the choroid was not observable, indicating that the vessels were staggered (Fig. 1E–H).
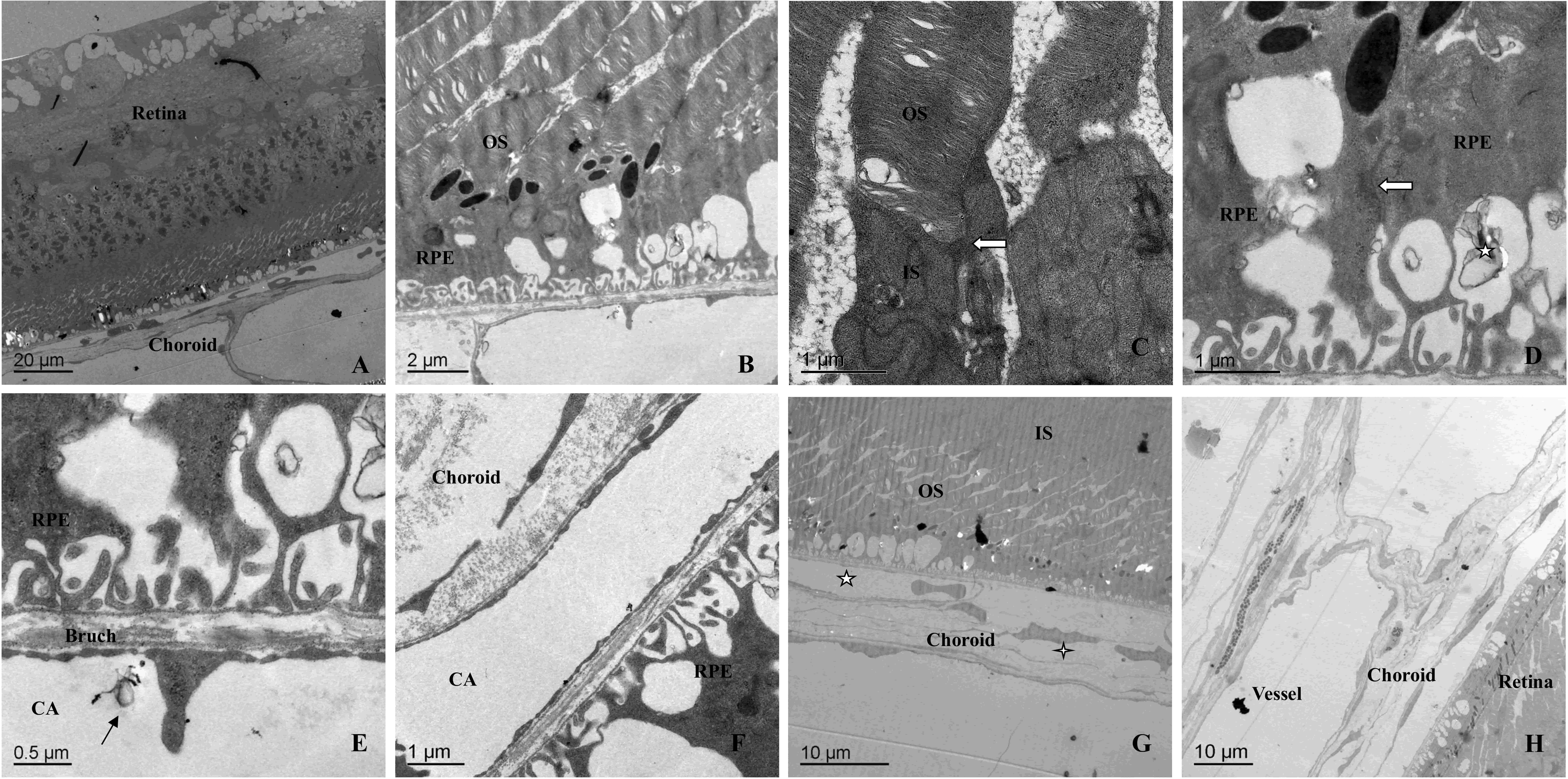 Fig. 1.
Fig. 1.Ultrastructure of normal retina and choroid of a rabbit observed under a transmission electron microscope. (A) Normal retina. (B–F) were obtained from (A). (B) Mosaic combination of normal RPE and outer segment (OS). (C) A normal connection between inner segment (IS) and OS. (D) A tight connection (arrow) and the swallowed photoreceptor cells (five-pointed star) could be observed in normal RPE cells. (E) A normal Bruch’s membrane and phagosome (arrow) that entered capillary (CA) through a Bruch’s membrane. (F) A normal window-like structure of choroidal capillary (CA) endothelial cells. (G) Normal retinal capillaries (five-pointed star) and choroidal stroma (cross star) of a rabbit. (H) Normal choroidal blood vessels of a rabbit.
One month after transplantation of RPE-PTC sheets, the photoreceptor cell nucleus and inner segment could be normally observed under the TEM (Fig. 2A). The membrane of discs in the outer segment was irregularly arranged, and the connection between the inner and outer segments was normal (Fig. 2B). The microscopy of the transplanted site showed the presence of RPE cells with normal shape, polarity, and pigmentation. The chimeric connection between the two segments was initially formed (Fig. 2C). The nucleus, endoplasmic reticulum, Golgi apparatus, mitochondria, and other organelles and intracellular melanin particles of RPE cells were normal (Fig. 2D). Phagocytosis of outer segment of photoreceptor could also be observed in RPE cells (Fig. 2E). The basement membrane of the hypertrophied RPE cells was normal. There were gap junctions, while tight junctions were found (Fig. 2F).
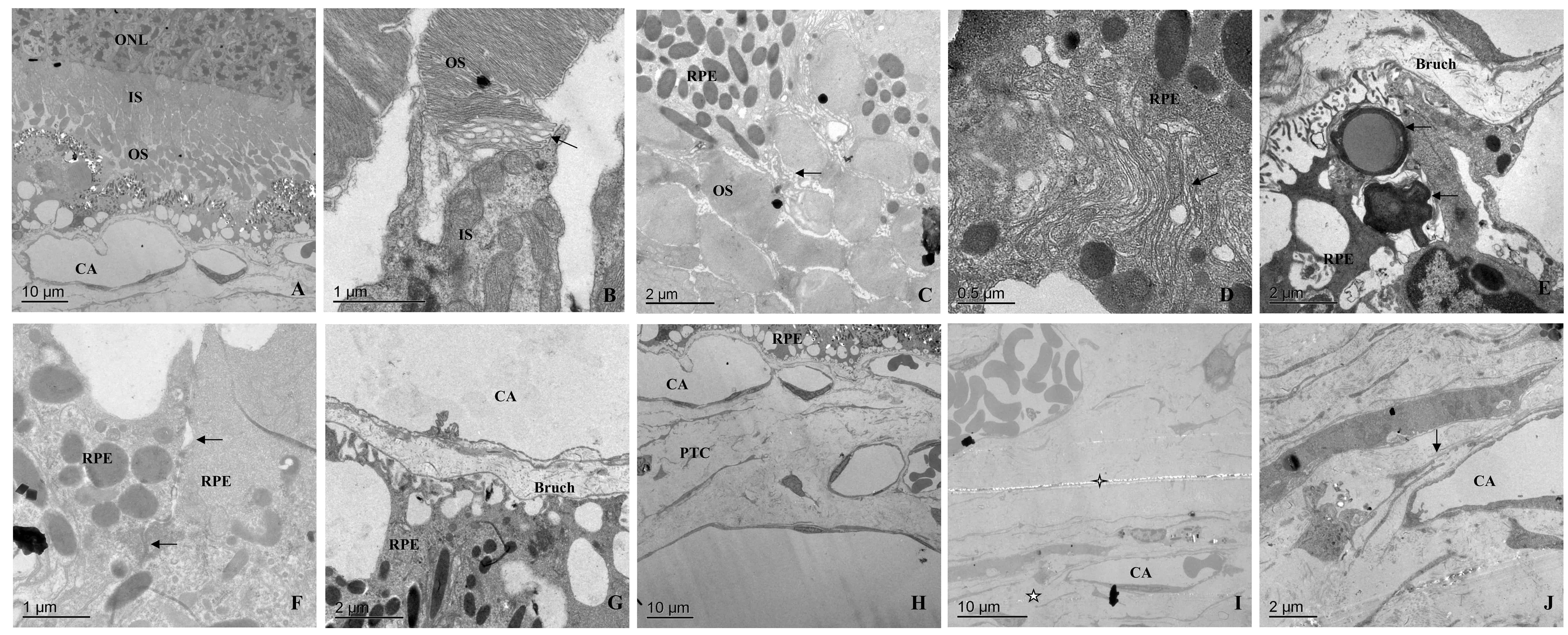 Fig. 2.
Fig. 2.TEM observations of transplanted RPE-partial-thickness choroidal sheets after one month. (A) Transplanted RPE-partial-thickness choroidal RPE-PTC sheets after one month, in which the outer nuclear layer (ONL) was normal, and the outer segment (OS) was slightly disordered. (B–G) were obtained from (A). (B) A connection between inner segment (IS) and OS of photoreceptor cells (arrow). (C) The apical villi of RPE cells were inlaid and combined with the OS (arrow). (D) Ultrastructural imaging of the internal structure of RPE cells. Smooth endoplasmic reticulum (arrow) in RPE cells RPE could be observed. (E) OS and other metabolites (arrow) were phagocytosed in RPE cells RPE, and the above image is the adjacent Bruch’s membrane. (F) There were gaps in the connections between RPE cells and tight junctions (arrows). (G) The Bruch’s membrane was loosened and undulated, and the window-like structure of choroidal capillary (CA) endothelial cells was visible. (H) Revascularization in the transplanted RPE-PTC sheets. (I) It was difficult to distinguish the connection between the implanted (cross star) and the choroidal implanted bed (pentagram), and the original choroidal capillaries (CA) could be observed. (J) The original Bruch’s membrane was loosened (arrow).
On the basal side of the RPE cells, the structure of Bruch’s membrane changed. The structure of Bruch’s membrane was loose, rather than being dense as normal (Fig. 2G). The capillary lumen was normal. The wall of the blood vessel was not collapsed, and the window-like structure of capillary endothelial cells was clear. The difference from the normal situation was that there were some collagen tissues outside the capillaries, and there were medium-sized blood vessels (Fig. 2H). It was difficult to distinguish the boundary between the graft and the implanted bed. The original Bruch’s membrane was begun to loosen. The residual RPE cells and the original capillaries could be observed (Fig. 2I,J).
Three months after the surgery, the connections between the inner and outer segments of the photoreceptor cells were normal (Fig. 3A,B). The outer segment formed a chimeric structure with the apical villi of RPE cells (Fig. 3C). The inner structure of the RPE cells and tight junctions among them remained normal. Phagosomes were visible in the cells, indicating the phagocytosis of RPE cells (Fig. 3D). The Bruch’s membrane was slightly loose and thickened, the capillaries were not collapsed, the vascular basement membrane was separated from Bruch’s membrane at the fold of Bruch’s membrane, and the window-like structure of capillary endothelial cells was clear (Fig. 3E,F). The graft was revascularized and the vascular structure was rich. It was not easy to distinguish the boundary between the graft and the implanted bed. The structure of original Bruch’s membrane on the implanted bed was not clear. The original choroidal capillaries and the medium- and large-sized blood vessels of the recipient bed could be observed (Fig. 3G,H).
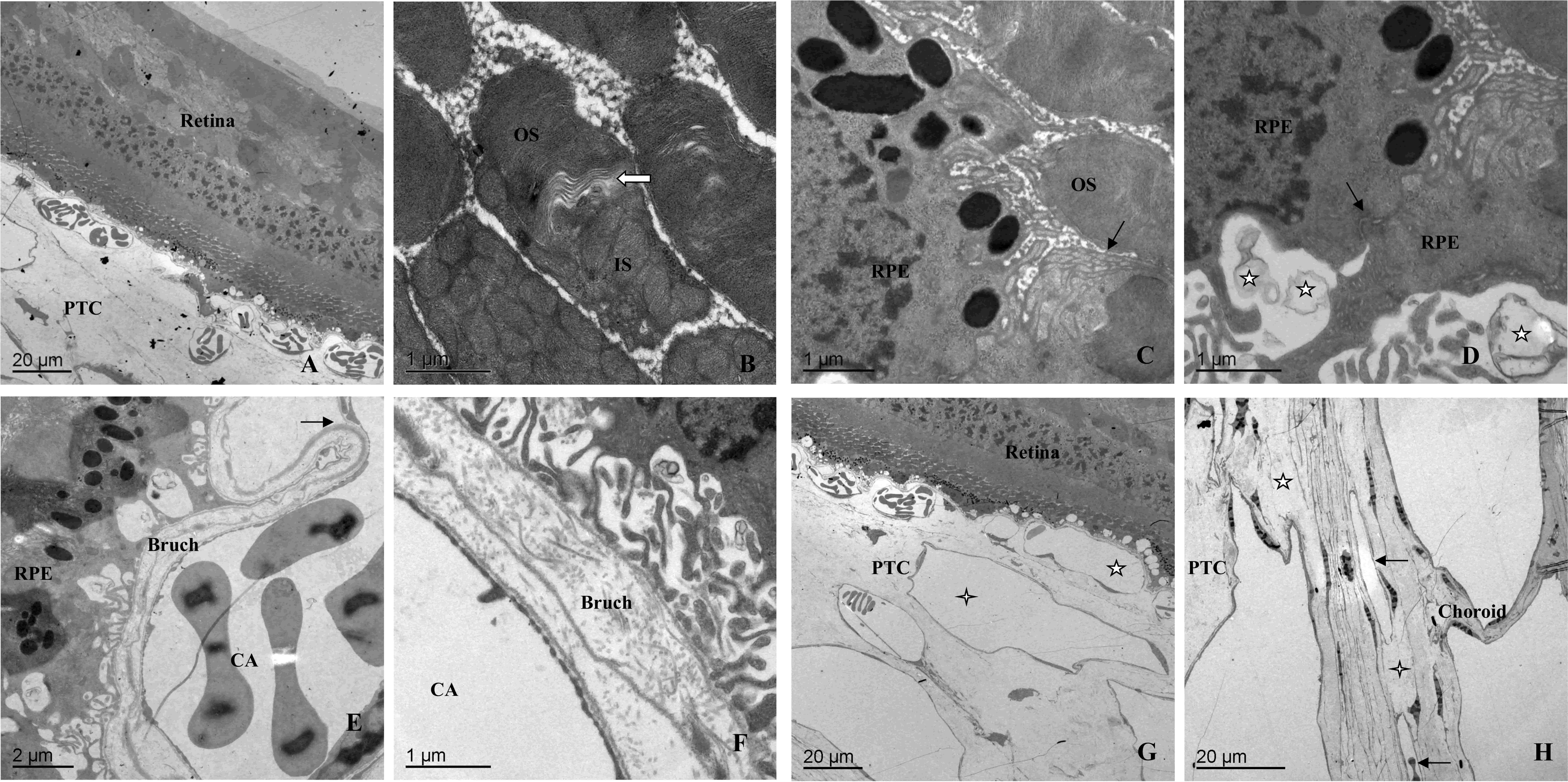 Fig. 3.
Fig. 3.TEM observations of transplanted RPE-partial-thickness choroidal sheets after three months. (A) Ultrastructural observations of retina and choroid after transplantation of RPE-partial-thickness choroidal sheets for three months. (B–F) were obtained from (A). (B) A connection between inner segment (IS) and outer segment (OS) of photoreceptor cells (arrow). (C) The apical villi of RPE cells were inlaid and combined with the photoreceptor cell OS (arrow). (D) Tight junctions between RPE cells (arrows) and intracellular phagosomes (pentagrams). (E) The fold of Bruch’s membrane could be observed, where the capillary (CA) basement membrane was separated from the Bruch’s membrane (arrow). (F) The Bruch’s membrane and the window-like structure of choroidal capillary (CA) endothelial cells. (G) Revascularized grafts (PTC), capillaries (five-pointed star), and medium-sized blood vessels (cross star). (H) The graft (five-pointed star) and the choroidal implanted bed (cross star) were healed closely, and the original capillaries (arrows) were still visible.
Six months after transplantation of RPE-PTC sheets, the inner and outer segments of photoreceptors were arranged neatly (Fig. 4A), and the connection between the inner and outer segments was normal (Fig. 4B). RPE and the outer segment followed a mosaic-like pattern (Fig. 4C,D). The structure of RPE cells was normal (Fig. 4E), and the cells were tightly connected and seamless. The structure of Bruch’s membrane was dense and undulated (Fig. 4F). The graft and the bed were healed well, the boundary was unclear, and the graft was vascularized (Fig. 4G,H).
 Fig. 4.
Fig. 4.TEM observations of transplanted RPE-partial-thickness choroidal sheets after six months. (A) The inner and outer segments of photoreceptors were arranged regularly. (B–F) were obtained from (A). (B) The connection between the inner and outer segments (arrow). (C,D) RPE and the outer segment (OS) followed a mosaic-like pattern. (E) Tight junctions among RPE cells (arrow). (F) The structure of Bruch’s membrane was dense and undulated. (G) The graft and the bed were healed well, and the boundary was unclear. CA, choroidal capillary. (H) The graft was vascularized (CA).
One month after transplantation of RPE-FTC sheets, TEM showed that the photoreceptor cells in the outer nuclear layer (ONL) shrank (Fig. 5A). The outer segment of the photoreceptor cells disappeared. The inner segment was directly connected to the RPE cells. The inner and outer segments of some parts disappeared (Fig. 5B,C). The organelles in the RPE cells disappeared. Besides, the majority of microvilli also disappeared, there was no polarity, and the melanin particles were messy.
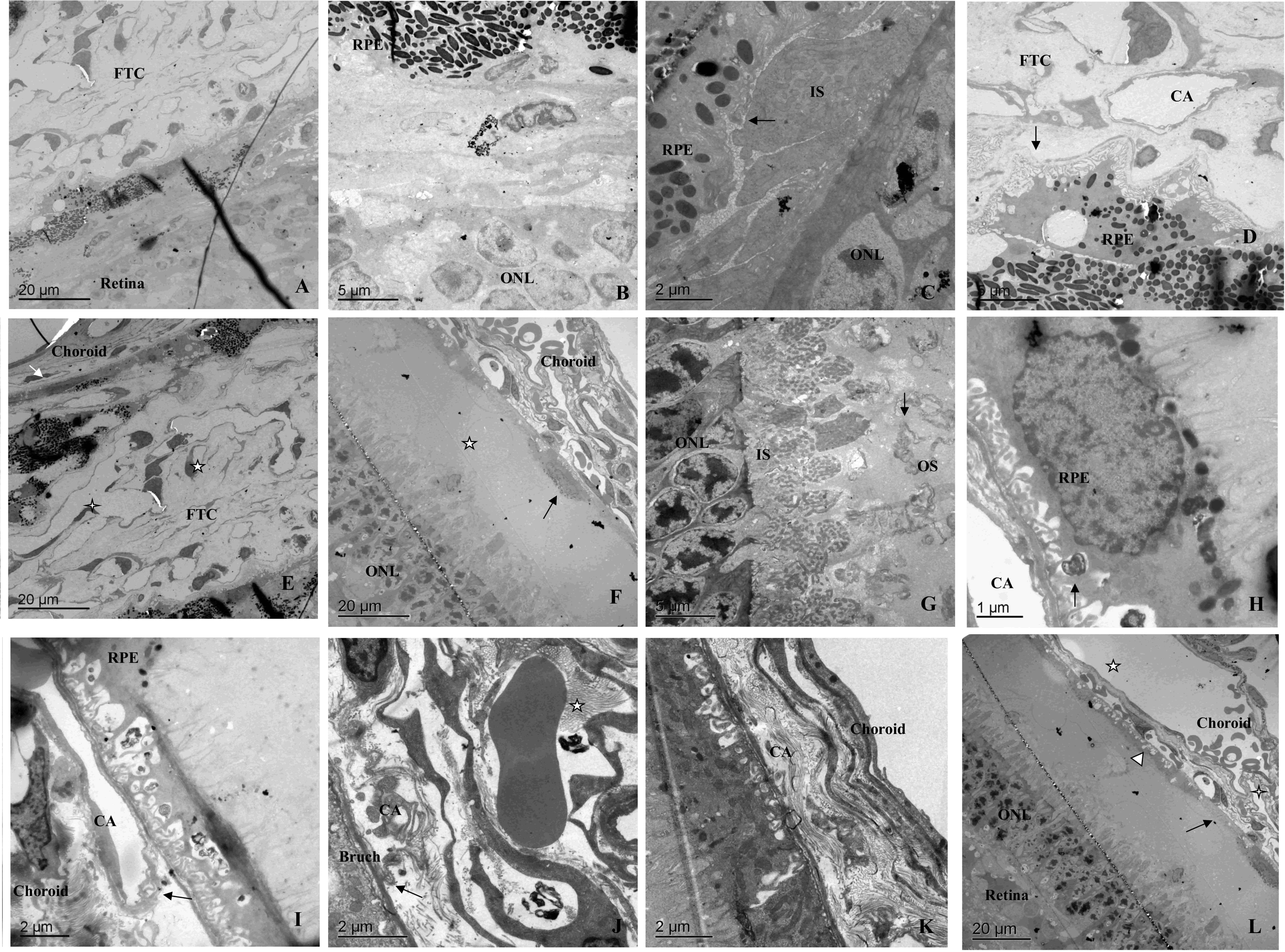 Fig. 5.
Fig. 5.TEM observations of transplanted RPE-full-thickness choroidal sheets and the observations in the blank group. Ultrastructural observations of retina and choroid after transplantation of RPE-full-thickness choroidal RPE-FTC sheets for one month: (A) The low-resolution image of the RPE-FTC and retina. The structure of retina (Retina) was disordered. (B), (C), and (D) were obtained from (A). (B) The inner and outer segments of photoreceptor cells, and microvilli of RPE cells disappeared. (C) Outer segment (OS) of the photoreceptor cell disappeared, inner segment (IS) directly contacted with the RPE cells (arrow), and microvilli of RPE cells disappeared. (D) The RPE basement membrane of the Bruch’s membrane was broken (arrow), the organelles disappeared, and the capillary (CA) wall was destroyed. (E) The large-sized blood vessels (cross star) and middle-sized blood vessels (five-pointed star) in the graft were collapsed and shrunk, and the capillaries of the choroidal implanted bed were still visible (arrow). The blank group: (F) Once local RPE was removed after one month, a gap (five-pointed star) appeared between the photoreceptor and the residual RPE (arrow) and Bruch’s membrane. (G-K) were obtained from (F) and (L). (G) Outer nuclear layer (ONL) of photoreceptor cell nucleus was normal, IS was abnormal, and OS was obviously damaged (arrow). (H) Some RPE cells could be observed, the remaining microvilli of RPE cells still existed, and phagosomes were visible in the cells (arrow). (I) The basement membrane was retained, and there were microvilli. The capillary (CA) basement membrane of the Bruch’s membrane was ruptured (arrow). (J) Bruch’s membrane destruction (arrow), capillary destruction (CA), and collagen fiber proliferation in the outer blood vessel (pentagram). (K) Capillary (CA) atresia and fibrosis. (L) Where RPE cells were fallen off (arrow), the choroidal capillaries were atretic, and the medium-sized blood vessels were collapsed (cross star); where the RPE cells remained (triangle), the capillaries were still open, and large- and medium-sized choroidal vessels were still normal (pentagram).
The Bruch’s membrane of the implant was wavy. Basement membrane of local RPE cells was broken (Fig. 5D). The capillary basement membrane on the choroidal side was destroyed. The capillary wall shrank, and the window-like structure of capillary endothelial cells disappeared. The large- and medium-sized blood vessels of the graft were also significantly collapsed (Fig. 5E). There was a large amount of collagen in the implanted tissue, accompanied by pigment cells. The boundary between the graft and the original choroidal tissue could be distinguished by the original capillaries and residual RPE cells. There was no gap between the graft and the choroidal tissue (Fig. 5F).
RPE cells were gently brushed off. After one month, TEM showed that the ONL of the photoreceptor cells remained normal, while the outer segment disappeared and the inner segment was destroyed (Fig. 5G). Although RPE cells were severely damaged, the organelles disappeared, the cell morphology became rounded and blunt, and the damaged RPE cell nucleus was still normal. There were still microvilli standing upright toward the photoreceptor cells, and the basement membrane attached to the Bruch’s membrane remained intact (Fig. 5H).
At the site, where RPE cells were fallen off, the Bruch’s membrane was damaged. The damaged area could be mainly observed on the side of the capillary basement membrane, which was manifested by the exfoliation of the capillary basement of the Bruch’s membrane (Fig. 5I). In addition, the wall of blood vessels was destroyed. Collagen fibers proliferated inside and outside the capillaries, and the vascular cavity was atretic (Fig. 5J). However, as long as there were remnants of RPE cells (even if they were injured), the capillaries were not atresia, and the diameter of the large- and medium-sized blood vessels in the choroidal tissue on the outside remained normal (Fig. 5K,L).
Although anti-VEGF drugs have provided a convenient and effective option for the treatment of exudative AMD in recent years, for the treatment of severe exudative AMD with or without RPE tears, submacular hemorrhage, and advanced dry AMD, there is still no effective strategy [6, 7, 8]. Autologous RPE-choroidal transplantation is an option for advanced wet AMD or atrophic AMD [11, 21].
Due to the difficulty of ultrastructural observation in humans, there are few electron microscopy observations of autologous RPE transplantation. Our previous study confirmed the survival of autologous RPE-PTC grafts, while the time of observation was short [27, 28]. In the present study, we conducted observations for up to 6 months, and it was revealed that autologous RPE-PTC transplantation could maintain the morphology and function of RPE cells, and provide blood supply for the long-term survival, which is consistent with the clinical observations. Lu et al. [11] studied on 80 patients with hemorrhagic AMD, and found that autologous RPE transplantation could improve vision compared with surgical excision of CNV alone. Transplantation of a RPE-Bruch’s membrane complex graft and a RPE monolayer graft both improved visual acuity and stabilized central fixation.
TEM images of RPE-FTC sheets showed that the transplanted RPE cells lost polarity and function. This is also consistent with most clinical and histological observations. In a study of 8 patients with submacular hemorrhage who underwent transplantation of RPE-FTC sheets, although the submacular hemorrhage and neovascular membrane were removed and no re-bleeding occurred over the long-term, the visual acuity was not improved [29]. In the assessment of 30 AMD patients who underwent autologous transplantation of RPE-FTC sheets, there was no significant improvement in visual acuity 1 year after surgery, and 11 patients developed CNV recurrence [30]. Histology indicated that although some areas of the transplanted RPE maintained a monolayered structure, the expressions and distributions of RPE65, CRALBP, and GFAP were diminished and modified during the follow-up [31]. Proliferative vitreoretinopathy was developed in all cases [31]. The autologous transplantation of RPE-PTC sheets was better than that of RPE-FTC sheets, which was confirmed through ultrastructural observations in the present study.
Contrarily, another study did not yield similar results. Parolini et al. [32] investigated 88 eyes of 84 patients who underwent autologous RPE choroidal transplantation for 2–10 years. The results showed that in patients with CNV, the increased best-corrected visual acuity (BCVA) and central fixation could be maintained for a longer time. However, only transient visual benefit was found in patients with atrophic macular degeneration. A longer follow-up may still be necessary.
Compared with the damage caused by implanting RPE-FTC grafts, it was revealed that after mechanical brushing of RPE cells, the secondary damage of photoreceptor cells was remarkably lighter and the development was slower. Using a light microscope, we previously observed that some photoreceptor cells existed at three to six months after surgery [27]. It can be concluded that the death of photoreceptor cells after tissue injury is a relatively slow process, and the influence of very thick implants under the retina on photoreceptor cells was more serious than RPE cells were brushed off alone. Hence, it can be pointed out that when AMD patients experience decreased vision, and if the photoreceptor cells are still alive, after an effective treatment, it is possible to partly restore patients’ vision.
In summary, according to TEM observations, when the RPE-PTC sheets were implanted under the retina, the grafts could quickly rebuild blood vessels, thereby maintaining the normal physiological functions of RPE cells, as well as the survival and functional status of photoreceptor cells. This technique has a potential role to treat AMD.
YH (Yuntao Hu), ZM—Research design; YH (Yuntao Hu), XC, YH (Ying Hong), TZ, YL—Experiments; XD, YH (Yuntao Hu), XC, YH (Ying Hong), TZ, YL—Data analysis; XD—Manuscript writing; YH (Yuntao Hu), ZM—Manuscript review; YH (Yuntao Hu)—Grant acquisition. All authors have read and approved the manuscript.
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
We would like to thank Baihe Hu for his assistance in transmission electron microscopy.
This work was supported by the National Natural Science Foundation of China (grant no. 81641080). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
The authors declare no conflict of interest.
