† These authors contributed equally.
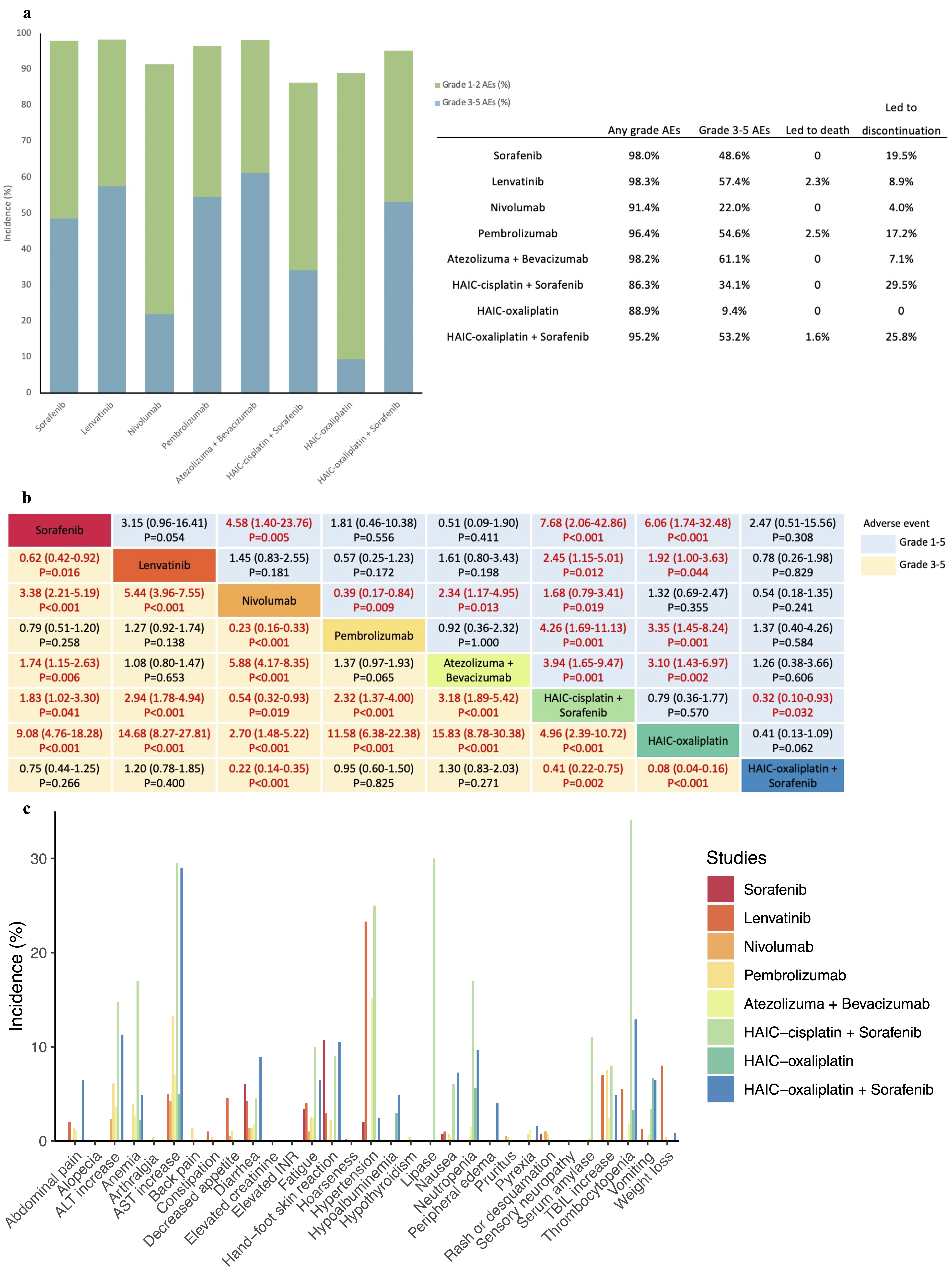
Background: Several recent phase 3 trials have reported manageable safety profiles and promising antitumor activities of molecular-targeted drugs (MTDs; sorafenib, lenvatinib), immune checkpoint inhibitors (ICIs; nivolumab, pembrolizumab, atezolizumab), hepatic arterial infusion chemotherapy (HAIC) and their combinations in advanced hepatocellular carcinoma (AHCC); however, head-to-head comparisons among these regimens are lacking. Methods: We aimed to comprehensively review and compare the efficacy and safety of different MTDs, ICIs, HAIC and their combinations in AHCC. Adverse events (AEs), disease control rates (DCRs), objective response rates (ORRs), overall survival (OS) and progression-free survival (PFS) were assessed. Results: The pooled incidence rates of grade 1–5/3–5 AEs were 98.0%/48.6%, 98.3%/57.4%, 91.4%/22.0%, 96.4%/54.6%, 98.2%/61.1%, 86.3%/34.1%, 88.9%/9.4%, and 95.2%/53.2% for sorafenib, lenvatinib, nivolumab, pembrolizumab, atezolizumab plus bevacizumab, HAIC-cisplatin plus sorafenib, HAIC-oxaliplatin, and HAIC-oxaliplatin plus sorafenib, respectively, which suggested that nivolumab exhibited optimal safety regarding grade 1–5 AEs, whereas HAIC-oxaliplatin monotherapy ranked lowest regarding grade 3–5 AEs. According to RECIST1.1, lenvatinib (72.8%), atezolizumab plus bevacizumab (73.6%), HAIC-oxaliplatin (78.8%) and HAIC-oxaliplatin plus sorafenib (75.2%) showed higher DCRs than sorafenib (57.3%), nivolumab (33.9%), and pembrolizumab (62.3%), whereas only HAIC-oxaliplatin-based treatments demonstrated a higher ORR than the others. Pooled OS and PFS analysis favored the combination regimens other than sorafenib along. Conclusions: Here, we present preliminary evidence for the comparative safety and efficacy of existing MTDs, ICIs, HAIC and their combinations in AHCC, which indicated that HAIC-oxaliplatin monotherapy has acceptable toxicity and efficacy and could be the cornerstone for future combination of systemic treatments in AHCC. Our findings might provide insight into the future design of multidisciplinary treatments in AHCC.