1 Department of Anesthesiology and Perioperative Medicine, The First Affiliated Hospital of Nanjing Medical University, 210029 Nanjing, Jiangsu, China
2 Department of Anesthesiology, Affiliated Stomatological Hospital, Nanjing Medical University, 210029 Nanjing, Jiangsu, China
3 Department of Cardiology, The Third Affiliated Hospital of Soochow University, 212003 Changzhou, Jiangsu, China
† These authors contributed equally.
Abstract
Sarcopenia is the age-related loss of skeletal muscle mass, accompanied by reduced muscle strength or physical function. As the global population continues to age, the prevalence of sarcopenia is gradually increasing. It is conceivable that an increasing number of patients with sarcopenia will be scheduled for surgery and anesthesia in the near future. The complex pathogenesis and clinical features of sarcopenia have brought huge challenges to perioperative management, especially in clinical anesthesia. However, there are currently neither guidelines nor expert consensus on the perioperative management of patients with sarcopenia. In this review, we summarize and elaborate on the pathogenesis, diagnosis, and perioperative precautions of sarcopenia, thereby providing information on the perioperative and anesthestic management of patients with sarcopenia.
Keywords
- Sarcopenia
- Aging
- Anesthesia
- Anesthetic management
- Review
In the 1980s, Rosenberg was the first to describe sarcopenia, which was initially defined as the age-related loss of lean body mass [1]. Subsequently, the concept of sarcopenia continued to develop, and it is currently believed that the decline of muscle function has more clinical significance than the loss of muscle mass. In 2018, the revised European consensus on sarcopenia added low muscle strength to the definition of sarcopenia [2]. The Asian Working Group for Sarcopenia (AWGS) also updated their consensus in 2019, defining sarcopenia as an age-related loss of muscle mass accompanied by either or both low muscle strength and low physical performance, and classifying sarcopenia as the changes in muscle strength and physical function [3]. The prevalence of sarcopenia varies significantly due to differences in the use of diagnostic criteria and tools and in study populations. In Asia, the sarcopenic elderly account for approximately 4.1%–11.5% of the population, while European countries have significantly higher prevalence, ranging from 6.8% to 21.8% [4, 5, 6, 7, 8, 9]. With the global population aging, the prevalence of sarcopenia will gradually increase. It is estimated that by 2050, more than 200 million patients will have sarcopenia, and this estimate has attracted the attention of clinicians [10]. In 2016, sarcopenia was recognized as an independent disease with its own International Classification of Diseases, Tenth Revision, Clinical Modification code (M62.84) [11].
The development of sarcopenia is not limited to elderly lean patients, patients with obesity may also have sarcopenia [12]. It is often believed that the presence of both sarcopenia and obesity can be diagnosed as sarcopenic obesity, although there is currently no uniform definition of sarcopenic obesity. A study applied dual-energy X-ray absorptiometry (DXA) to analyze the body compositions of 4984 patients showed that the prevalence of sarcopenic obesity in men and women were 12.6% and 33.5%, respectively [13]. Interestingly, the prevalence gradually increases with age.
As the average life expectancy increases, the prevalence of sarcopenia was gradually increased. Studies have shown that the pathophysiological changes caused by sarcopenia and aging can significantly increase the risk of perioperative adverse events, including adverse cardiovascular events, adverse drug reactions, severe postoperative complications, prolongation of hospital stay, surgical failure, and even high mortality [14, 15, 16]. However, unlike aging, sarcopenia can be modified before surgery, thereby reducing the incidence of perioperative adverse events and significantly improving the clinical outcomes of such elderly patients. Therefore, the diagnosis, treatment, and perioperative management of elderly sarcopenic patients will be the focus of clinicians in the near future. Regrettably, there are currently no guidelines or clinical recommendations for the perioperative management of patients with sarcopenia. This review will elaborate on the diagnosis, pathogenesis, treatment and perioperative management of sarcopenia, so as to provide reasonable suggestions for clinicians to develop individualized anesthetic management plans.
There are strong correlations between sarcopenia and falls, disability, adverse outcomes, and mortality in the elderly, so the early diagnosis of sarcopenia is particularly important [17]. In clinical practice, we comprehensively evaluate muscle strength, physical function, and muscle mass in order to make a correct diagnosis for elderly patients according to an algorithm (Fig. 1). Because measuring handgrip strength is simple and easy, it is a recommended method with which to measure muscle strength. Gait speed is the clinical test most commonly used to assess physical performance. At present, the main methods used to estimate muscle mass include computed tomography (CT) scan, magnetic resonance imaging (MRI), bioelectrical impedance analysis (BIA), and other imaging techniques. Although CT and MRI are the gold standards for assessing muscle mass, they are relatively expensive and lack precise diagnostic cut-offs; therefore, they are not commonly used in clinical practice [18]. DXA and BIA are inexpensive, safe, and convenient, so the European Working Group on Sarcopenia in Older People (EWGSOP) and AWGS recommend the use of DXA or BIA for muscle mass determination and provide diagnostic cut-offs [2, 3]. However, the precarious conditions of critically ill patients and the relatively limited time before surgery make these examinations impractical for intensive care unit (ICU) physicians and anesthesiologists. Therefore, Mueller et al. [19] proposed using ultrasounds to assess and diagnose sarcopenia in critically ill patients. Although the effectiveness of ultrasounds in measuring muscle mass has been proven, the EWGSOP does not recommend using an ultrasound as a diagnostic tool due to the lack of standardized measurement methods and prediction equations [2, 20]. In addition, for the diagnosis of sarcopenia, the exploration of biomarkers is very meaningful, allowing an anesthesiologist to find patients with sarcopenia conveniently and quickly through laboratory records during preoperative visits. Several biomolecules are confirmed to be related to the onset of sarcopenia, including myostatin, irisin, sarcopenia index (serum creatinine/serum cystatin C), and so forth [21]. However, due to the lack of diagnostic criteria, these are still not widely evaluated in clinical practice.
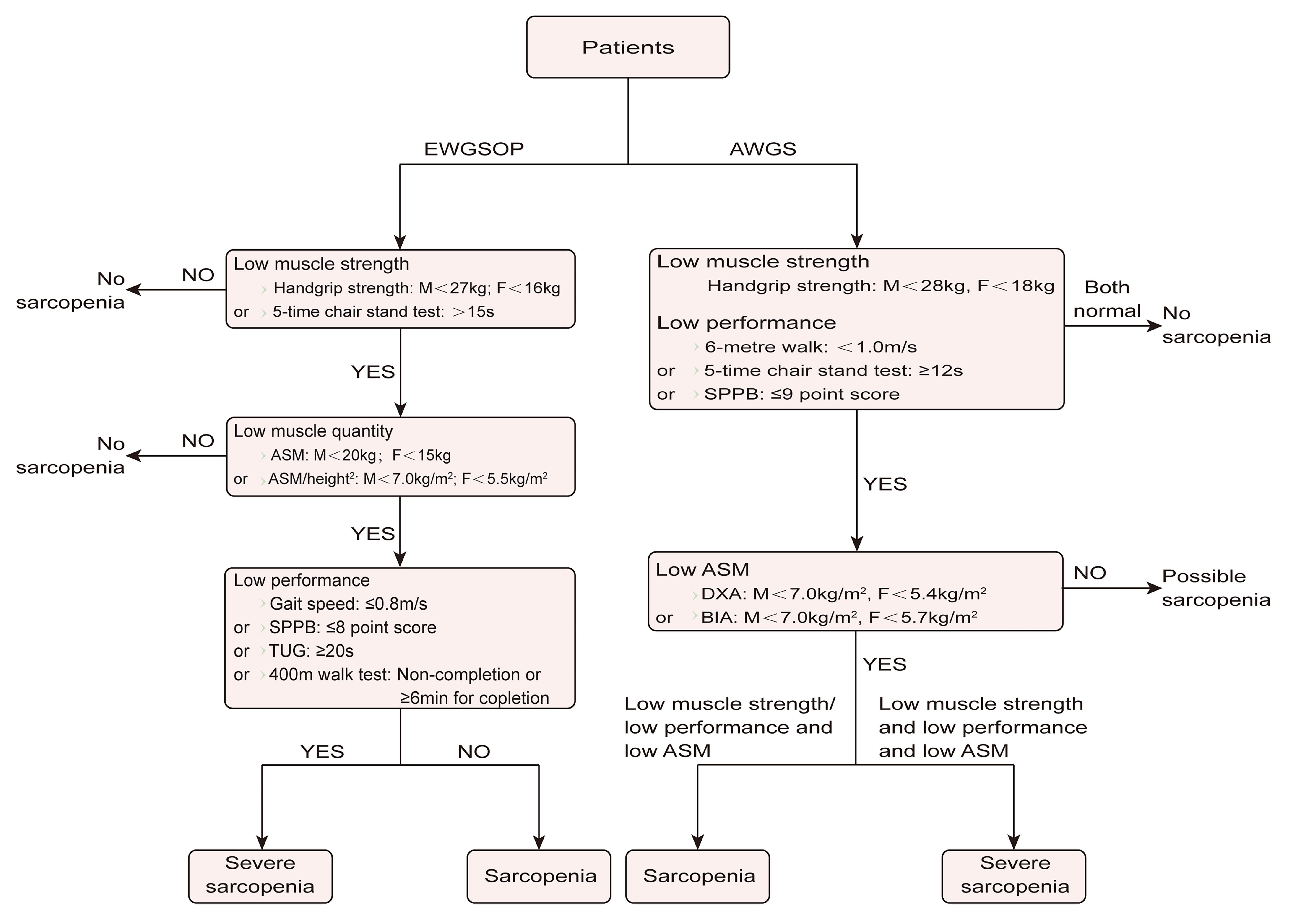 Fig. 1.
Fig. 1.Algorithms for the diagnosis of sarcopenia. ASM, appendicular skeletal muscle mass; AWGS, Asian Working Group for Sarcopenia; BIA, bioelectrical impedance analysis; DXA, dual-energy X-ray absorptiometry; EWGSOP, European Working Group on Sarcopenia in Older People; F, female; M, male; SPPB, Short Physical Performance Battery; TUG, timed-up-and-go.
Neuromuscular degeneration plays an important role in the occurrence and
development of sarcopenia, and is mainly characterized by motor unit remodeling
and neuromuscular junction degeneration [22, 23]. The motor unit is composed of
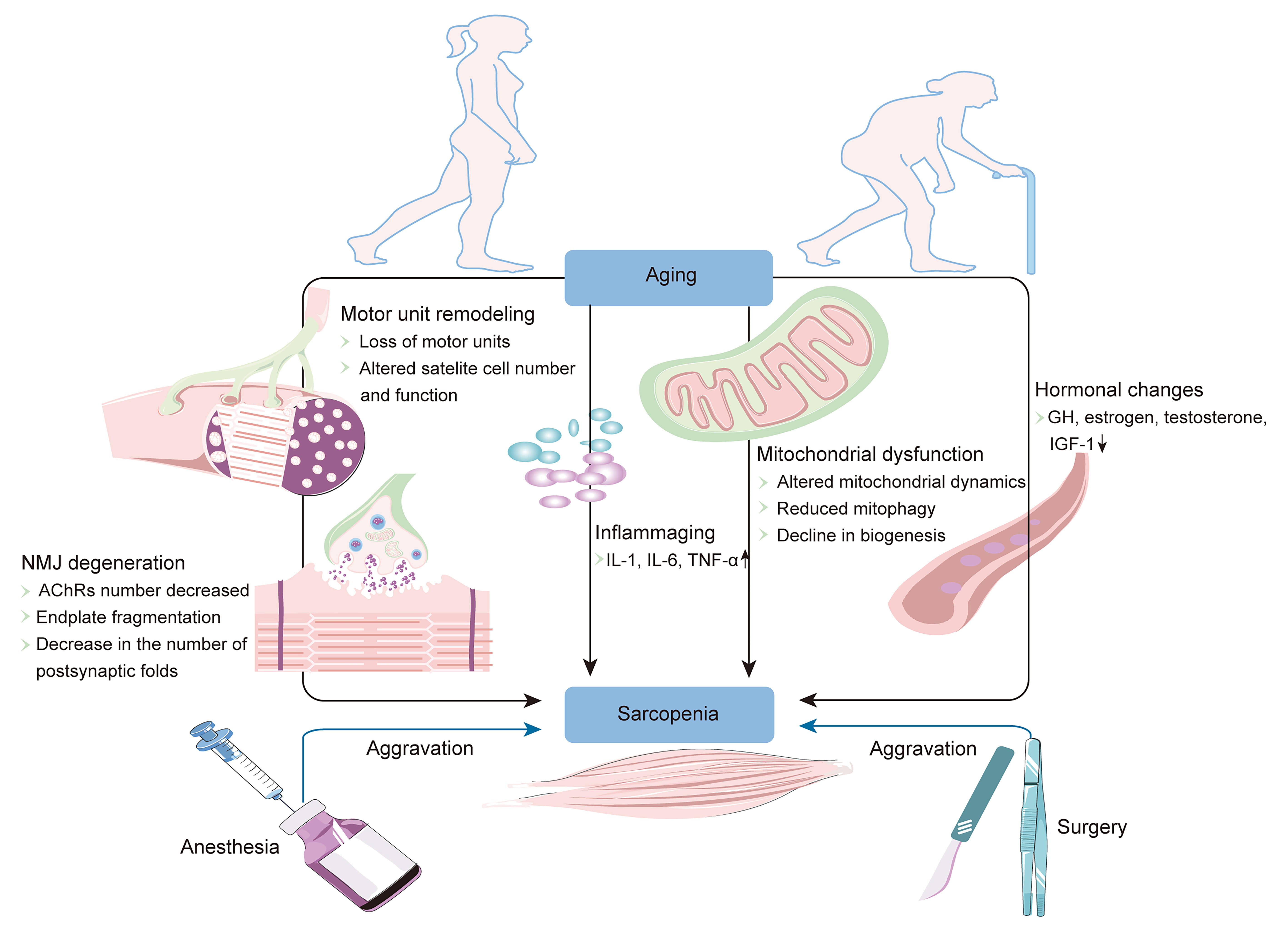 Fig. 2.
Fig. 2.Schematic representation of the main factors involved in the
progression of sarcopenia. AChRs, acetylcholine receptors; GH, growth hormone;
IGF-1, insulin-like growth factor-1; IL-1, interleukin 1; IL-6, interleukin 6;
NMJ, neuromuscular junction; TNF-
Although the exact pathogenesis of sarcopenia is still unclear, mitochondrial
dysfunction, especially impairments in the mitochondrial quality control (MQC)
pathway, plays a major role in the course of sarcopenia [32, 33]. MQC is defined
by mitophagy and mitochondrial biogenesis, a mechanism that maintains
mitochondrial morphology and function through mitochondrial fusion/fission [34, 35]. Mitochondrial fusion can redistribute protein and mitochondrial DNA,
exchanging material and energy to meet the body’s needs [36]. Alternatively,
fusion can reduce the concentration of damaged substances to maintain the normal
functions of the mitochondria [37]. Fission can isolate severely damaged
mitochondria and clear them through autophagy to avoid excessive accumulation of
dysfunctional mitochondria and reduce the generation of reactive oxygen species
(ROS) [38]. Mitochondria form a complex mitochondrial network through continuous
fusion and fission (i.e., mitochondrial dynamics), which plays an important role
in maintaining muscle morphology and contraction function [39]. With the
appearance of aging, the MQC pathway appears abnormal, displaying disturbed
mitochondrial dynamics, decreased mitophagy, and reduced mitochondrial biogenesis
[40, 41, 42, 43]. Unbalanced mitochondrial dynamics and reduced mitophagy cause
mitochondrial dysfunction, damage the mitochondrial network, and ultimately lead
to skeletal muscle loss and muscle function decline [39]. At
the same time, dysfunctional mitochondria continue to accumulate, leading to
reduced adenosine triphosphate production and excessive ROS production. In turn,
the increased production of ROS causes electron transport chain damage and
mitochondrial DNA mutations, and then further aggravates mitochondrial
dysfunction, forming a vicious circle, which finally results in the deterioration
of skeletal muscle quality and function [33, 44]. On the other hand, excessive
ROS reduces the sensitivity of the anabolic signaling pathway mediated by
insulin-like growth factor-1 (IGF-1) and activates skeletal muscle proteolytic
signaling pathways, which cause decreased muscle protein synthesis and increased
protein breakdown. These changes ultimately promote the occurrence and
development of sarcopenia [45, 46, 47]. In addition, mitochondrial dysfunction can
cause neuromuscular junction remodeling and the loss of motor units [48].
Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha
(PGC-1
In the process of aging, patients often have low-grade,
systemic chronic inflammation without corresponding clinical
symptoms: that is, “inflammaging” [53]. These inflammatory factors include but
are not limited to tumor necrosis factor-alpha (TNF-
Currently, sarcopenic obesity is defined as the coexistence of obesity and
sarcopenia [62]. However, the pathogenesis of sarcopenic obesity is not a simple
superposition of the mechanisms of these comorbidities. Obesity and sarcopenia
may have some common pathogenesis and may aggravate each other [63]. In the aging
process, due to the changes in hormone levels (including decreased levels of
estrogen, testosterone, growth hormone, and IGF-1) and a decrease in physical
activity, the resting metabolic rate is reduced, which leads to a decrease in
protein synthesis and a progressive increase in fat mass [12]. The existence of
sarcopenia further reduces the activity levels of elderly patients and aggravates
fat accumulation [64]. Fat accumulates abnormally in adipose tissue, after which
immune cells infiltrate the tissue and release a large number of
pro-inflammatory cytokines, including TNF-
Sarcopenia is associated with falls, disability, longer
hospital stays, and mortality in the elderly, so treatment strategies should be
initiated immediately when sarcopenia is diagnosed [17]. Currently, the treatment
of sarcopenia mainly includes physical exercises, nutritional supplementation,
and pharmacological interventions. Physical exercises are
currently considered to be the most effective of these treatment options [71].
Physical exercises can significantly increase muscle mass, improving the muscle
function and physical performance [72, 73]. Exercise interventions mainly include
resistance training and endurance training. The current focus is mainly on
resistance training. Resistance training can inhibit mitochondrial-mediated
apoptosis and regulate the IGF-1/Akt/mammalian target of the rapamycin signaling
pathway, thereby promoting protein synthesis [74]. A meta-analysis of 14 trials
showed that there is high-quality evidence that resistance training can improve
muscle mass, strength, and physical performance [75]. Importantly, high-intensity
training may provide greater benefits than other exercises. An 18-month
randomized controlled study involving 43 participants confirmed that
high-intensity resistance exercise can increase the skeletal muscle mass and grip
strength of patients with sarcopenia and can inhibit the progression of
sarcopenia [76]. Endurance training can also improve mitochondrial function by
increasing PGC-1
Although exercise is an effective measure with which to intervene in sarcopenia, it requires professional guidance and is often difficult for the elderly to maintain. Therefore, more and more researchers are turning their attention to nutritional interventions and pharmacological approaches. Nutritional interventions mainly include supplementation of protein, vitamin D, creatine monohydrate, and so forth. [78]. Protein and vitamin D supplementation can significantly improve muscle strength and function [79, 80]. A multicenter randomized controlled trial involving 1240 elderly people showed that vitamin D and leucine can significantly improve the muscle mass and function of patients with sarcopenia [81]. However, as the optimal dosage ranges of these nutritional supplements have not yet been determined, their clinical use has been limited. Combining exercise and nutritional supplementation is among the most promising strategies [82]. Although some trials showed that the combination is beneficial for sarcopenia, no recommendations can be proposed owing to inconsistent evidence [83]. Expert guidelines believe that the time of nutritional intake before and after exercise is critical to the increase of muscle mass [84]. Studies have found that giving nutrition after exercise can produce a good synergistic effect on muscle protein synthesis [85]. Therefore, supplementation with nutrition after exercise may be more conducive to sarcopenia. Regrettably, there is little study on the effect of nutritional intake time on sarcopenia, and some of these have not shown the benefits of supplementation with nutrition after exercise for sarcopenia. A 26-week three-arm randomized controlled trial showed that compared with whey protein pre-resistance training group, the skeletal muscle mass, functional capacity, and total strength gains of the whey protein post-resistance training group were higher, but there was no significant difference between the two groups [86]. In this regard, there is still a lack of definitive clinical evidence on the effect of nutritional intake time on sarcopenia. Further studies are still needed to provide better clinical evidence.
There are currently no drugs for the treatment of sarcopenia. Potential therapeutic drugs mainly include testosterone, selective androgen receptor modulators, and anti-inflammatory drugs [87]. Although these drugs can partially improve muscle mass and function, most of them have side effects that greatly limit their clinical usage. Studies have shown that testosterone can increase muscle protein synthesis and myogenesis, thereby improving muscle mass and strength [88, 89]. However, long-term use of testosterone may cause various complications, such as cardiovascular and prostate diseases [47, 90]. Although selective androgen receptor agonists have fewer side effects than testosterone, they have not shown significant advantages in improving muscle mass and function [91]. Therefore, testosterone is still the most effective and safest drug for sarcopenia. There is great potential for treating sarcopenia by repurposing drugs, such as angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. A study enrolling 130 participants showed that compared with placebo, perindopril significantly improved the 6-min walking distance and physical functions of the elderly [92]. However, whether it can achieve definite curative effects in the treatment of sarcopenia still needs further verification.
With the population aging, there is an increasing demand for surgical procedures
among the elderly. However, due to sarcopenia’s comorbidities, the benefits may
not outweigh the complications of surgery and anesthesia for such patients, and
surgery may even cause a serious decline in the quality of life. Increasing
evidence indicates that sarcopenia is closely related to postoperative
complications, mortality, and lengths of hospital stays [93, 94]. Fukuda
et al. [95] found in a cohort study that although there was no
significant difference between sarcopenia and non-sarcopenia groups in the
overall incidence of complications after gastric cancer surgery, the incidence of
serious complications was significantly higher in the sarcopenia group. A
multivariate analysis suggests that sarcopenia is a risk factor for severe
postoperative complications. In 2018, a meta-analysis of 29 studies involving
7176 patients also showed that sarcopenia is an independent predictor of an
increased risk of complications after gastrointestinal tumor resection [96].
Therefore, elderly patients with sarcopenia who need surgery should attract the
special attention of anesthesiologists. At present, an American Society of
Anesthesiologists (ASA) classification is often used in clinical practice to
assess the risk of anesthesia in perioperative patients. However, the ASA class
is mainly based on the subjective judgment of the anesthesiologist, lacks
relatively objective indicators, and may not be used to predict the risks of
postoperative complications, mortality, and other outcomes. [97]. The combination
of the ASA classification and other objective indicators can effectively assess
the patient’s baseline functional status and predict
perioperative risks [98]. In addition, because of the obesity characteristics of
patients with sarcopenic obesity, anesthesiologists may ignore the coexisting
sarcopenia and follow the guidelines for the anesthetic
management of ordinary obese patients, which may lead to serious consequences.
Therefore, it is particularly important to identify and diagnose sarcopenia
before surgery. The 2019 expert guidelines on liver transplantation also
recommend that a sarcopenia assessment should be included in the preoperative
liver transplantation evaluation [99]. On one hand, preoperative sarcopenia
screening can combine an ASA classification with objective indicators of
sarcopenia to effectively stratify patients before surgery, guiding clinicians in
decision-making and in formulating the best anesthesia plan. On the other hand,
appropriate interventions (including nutrition and exercise, among others) should
be carried out during the perioperative period to increase the functional reserve
of a patient with sarcopenia, thereby improving the postoperative clinical
course. A study showed that after a preoperative exercise and nutritional support
program (i.e., prehabilitation), 4 patients in an experimental group became
non-sarcopenic, and even no patients in the sarcopenia group had a higher grade
of Clavien–Dindo classification or a higher incidence of postoperative
complications [100]. Therefore, it is necessary to routinely perform sarcopenia
screens for patients’
Perioperative anesthesia management for elderly patients with sarcopenia is a huge challenge owing to their reduced functional reserves, comorbidities, cognitive impairments, and advanced age. One of the first issues that must be faced is the choice of an anesthesia method. There is a dispute as to whether regional anesthesia or general anesthesia is better. A study enrolling 96,289 patients aged 65 years or older who underwent hip fracture surgery showed that after propensity score matching, the mortality rate, delirium rate, and incidence of ICU admission in the regional anesthesia group were significantly lower than those in the general anesthesia group [101]. Therefore, the authors believe that regional anesthesia is more suitable for elderly patients. Subsequent studies also confirmed that regional anesthesia is associated with a reduced risk of multiple complications compared with general anesthesia when performing total hip arthroplasty surgery in frail elderly patients [102]. The International Consensus on Anaesthesia-Related Outcomes after Surgery group also recommends the use of neuraxial anesthesia during hip arthroplasty [103]. Therefore, under appropriate circumstances, regional anesthesia may be a better choice for the elderly. But this does not mean that regional anesthesia is always better than or can replace general anesthesia. There are still many studies showing that compared with general anesthesia, regional anesthesia has no significant difference in postoperative morbidity, readmission, and in-patient mortality rates [104, 105]. There is still no consensus on which anesthesia mode is more beneficial to the elderly. Some guidelines suggest that regional anesthesia can be used as a supplement to general anesthesia, thereby reducing the use of intraoperative anesthetics and opioids and improving clinical outcomes [106]. In any case, the anesthesiologist should develop an individualized anesthesia plan based on the preoperative evaluation of a patient with sarcopenia and the advantages and disadvantages of different anesthesia methods.
The changes in the body composition of patients with sarcopenia, including
decreased skeletal muscle, increased fat, and organ function declines, have
impacts on the pharmacodynamics and pharmacokinetics of intravenous
anesthetics [107]. Propofol is currently the most commonly used
intravenous anesthetic in clinical practice. An observational study involving 40
patients showed that compared with other adult patients, the dose of propofol
required for loss of consciousness was lower and the concentration of propofol in
plasma was higher in those aged
The effectiveness of volatile anesthetics is also different in patients with sarcopenia. With age, the minimal alveolar concentration (MAC) of inhaled anesthetics decreases, and the MAC of 80-year-old patients is about half that of infants. However, this is still based on data obtained in the general population, and there is a lack of relevant research on the use of volatile anesthetics in patients with sarcopenia. However, the decreased functional reserves in these patients may further increase their sensitivity to volatile anesthetics. In any case, in clinical practice, the anesthesiologist should pay attention to this change and adjust the dose of inhaled anesthetics according to end-tidal concentration monitoring [110].
Studies have shown that elderly patients are significantly more sensitive to adverse reactions to opioids. A retrospective analysis of 8855 patients showed that the risk of respiratory depression with opioids gradually increases with age [115]. Compared with healthy elderly patients, patients with sarcopenia have a more significant decline in the tolerance of opioids [116]. Therefore, perioperative analgesia in elderly patients with sarcopenia is mainly based on non-opioid analgesia [117]. Evidence supports the use of regional anesthesia for perioperative analgesia. Regional anesthesia can effectively relieve postoperative pain, reduce postoperative complications, and promote patient recovery [118]. A Cochrane systematic review conducted in 2018 found that there is high-quality evidence supporting the use of a regional blockade to effectively reduce postoperative movement pain, and there is moderate-quality evidence supporting the use of a regional blockade to reduce the risk of pneumonia [119]. In addition, the use of regional anesthesia can also significantly reduce the dosage and side effects of opioids. Leeper et al. [120] found that compared with receiving systemic analgesia, the average use of morphine was reduced by 41% in the fascia iliaca block. The guidelines also recommend the use of peripheral nerve blocks for analgesia [106]. Therefore, anesthesiologists can use regional blockades for perioperative analgesia in patients with sarcopenia, thereby reducing the use of opioids, avoiding unnecessary complications, and improving perioperative safety.
Since the introduction of neuromuscular blocking agents into clinics, postoperative residual neuromuscular blockage remains a major problem that plagues clinicians. An observational study of 415 patients found that 31% had residual paralysis after surgery, and the incidence was higher, about 44%, in elderly patients [121]. In addition, hypoxia and ventilation support were frequent in the elderly group. Incomplete recovery of neuromuscular function can cause dysphagia, aspiration, and upper airway obstruction and can increase the risk of pulmonary complications. Sarcopenia not only affects the quality and function of the skeletal muscles of the limbs, but also causes atrophy of respiratory and swallowing muscles, leading to respiratory insufficiency and dysphagia [122, 123]. This may further aggravate the above-mentioned residual neuromuscular blockade-related complications and increase the risks associated with anesthesia. Therefore, neuromuscular blocking agents should be used cautiously in patients with sarcopenia. At present, the commonly used neuromuscular blocking agents mainly include rocuronium and cisatracurium. Rocuronium is a steroidal muscle relaxant that depends on liver and kidney elimination. Therefore, its pharmacokinetics is easily affected by age and liver and kidney functions. A number of studies have shown that the duration of action and the average recovery index time of rocuronium are prolonged in elderly patients, and the variability in these parameters is greater [124]. Therefore, Pietraszewski et al. [121] believe that a dose of rocuronium that is less than 30% of the recommended dose for young people is safer for the elderly. Unlike rocuronium, cisatracurium is eliminated by Hofmann degradation, which is organ-independent, making it less affected by age and liver and kidney functions [125]. Moreover, in elderly patients, the variability in the duration of action is lower after receiving 2 times the 95% effective dose (ED95) of cisatracurium compared with an equipotent dose of rocuronium or vecuronium [126]. This helps the anesthesiologist predict the recovery of neuromuscular function after the patient uses cisatracurium, which may be more important in the elderly. Studies based on healthy elderly patients indicate that aging does not affect the pharmacodynamics of muscle relaxants [127]. However, research has shown that due to the decrease in the number of nicotinic acetylcholine receptors, myasthenia gravis patients have a significant increase in sensitivity to non-depolarizing muscle relaxants, with a longer effect duration [128]. The efficacy of non-depolarizing muscle relaxants in myasthenia gravis patients is also significantly enhanced, and one-fourth of the standard intubating dose of rocuronium can achieve a complete neuromuscular block [129, 130]. In addition, the use of muscle relaxants can aggravate myasthenia gravis and cause respiratory distress [131]. The pathogenesis of sarcopenia is complicated, especially the presence of motor unit remodeling; neuromuscular junction degeneration, including a reduction in the number of acetylcholine receptors; endplate fragmentation; and reduction in postsynaptic folds, which may significantly change the pharmacological properties of muscle relaxants. In addition, due to the high risk of a residual neuromuscular blockade in patients with sarcopenia, it is particularly important to use antagonists when appropriate to completely reverse the relaxation at the end of anesthesia. The timing of administration mainly depends on the depth of the neuromuscular blockade. Therefore, in the process of anesthesia management for sarcopenic patients, it is strongly recommended that anesthesiologists use neuromuscular function monitoring, carefully titrate the dose of muscle relaxants, avoid the use of excessive muscle relaxants, and timely antagonize muscle relaxants to reduce the incidence of residual paralysis and ensure patient safety.
Due to the existence of common pathophysiological mechanisms such as chronic inflammation and hormonal pathways, sarcopenia is an important risk factor for cognitive impairment and dementia. A meta-analysis of 22 studies found that sarcopenia is associated with an increased risk of cognitive impairment, and this effect is independent of the study population and the definitions used for sarcopenia and cognitive impairment [132]. An advanced age and preoperative cognitive impairment are both important risk factors for perioperative cognitive dysfunction [133]. Among the cognitive complications related to anesthesia, sarcopenia is closely related to the occurrence of postoperative delirium. Hiraoka et al. [134] found that the decreased muscle function group showed a higher incidence of postoperative delirium after liver cancer surgery. A retrospective cohort study of 251 patients found that compared with patients without a low skeletal muscle mass (LSMM), patients with an LSMM had a higher incidence of postoperative delirium [135]. A multivariate analysis showed that LSMM is associated with postoperative delirium in patients with colon cancer, and this correlation is more significant when patients also have malnutrition or physical dependence [135]. Therefore, patients with sarcopenia should be identified during the perioperative period, we should be vigilant about possible postoperative delirium, and we should take necessary measures to prevent postoperative delirium. Exercise and nutritional interventions are used to improve sarcopenia before surgery and reduce the occurrence of postoperative delirium. Multi-modal analgesia is used during the perioperative period to avoid postoperative delirium caused by insufficient analgesia or an overdose of opioids. If possible, the use of drugs that can cause postoperative delirium should be avoided or reduced during the perioperative period, including benzodiazepines, opioids, ketamine, antihistamines, and atropine [114].
The high prevalence of sarcopenia and the increasing demand for surgery have brought great challenges to clinical anesthesiologists. Due to its complex pathophysiological process, sarcopenia may cause pharmacological changes in anesthetic drugs, such as a decreased effective dose and a relatively prolonged duration of action. Therefore, in the selection of anesthesia methods and medications, it is necessary to comprehensively consider the basic conditions of patients with sarcopenia and the changes in the characteristics of anesthetic drugs and then make an individualized anesthetic management strategy. The current data are mostly based on studies of healthy elderly people, and future research needs to pay more attention to the effects of sarcopenia on the pharmacodynamics and pharmacokinetics of anesthetics, to provide a reasonable basis through which clinicians can achieve precise anesthesia.
CL and CY devised the structure of the review. The manuscript was drafted by YS, and YS and LZ reviewed the manuscript. EY and LY edited the manuscript. All authors have read and agreed to submit the manuscript.
Not applicable.
Not applicable.
This study was funded by the National Natural Science Foundation of China (No. 81703482 and 81974171 to C.Y., 82070405 to L.Y.) and the Science and Technology Support (Social Development) Project of Bureau of Science and Technology of Changzhou (No. CE20195044 to L.Y.).
The authors declare no conflict of interest.
AChRs, acetylcholine receptors; ASA, American Society of
Anesthesiologists; ASM, appendicular skeletal muscle mass; AWGS, Asian Working
Group for Sarcopenia; BIA, bioelectrical impedance analysis; CT, computed
tomography; DXA, dual-energy X-ray absorptiometry; EWGSOP, European Working Group
on Sarcopenia in Older People; F, female; GH, growth hormone; ICU, intensive care
unit; IGF-1, insulin-like growth factor-1; IL-1, interleukin 1; IL-6, interleukin
6; LSMM, low skeletal muscle mass; M, male; MAC, minimal alveolar concentration;
MQC, mitochondrial quality control; MRI, magnetic resonance imaging;
NF-
