†These authors contributed equally.
§GAME-HGUGM: Grupo de Apoyo al Manejo de la Endocarditis Infecciosa of Hospital General Universitario Gregorio Marañón. GAME-HGUGM group members are listed in the acknowledgments.
Academic Editor: Suresh G. Joshi
Background: Abiotrophia spp. and Granulicatella spp. are Gram-positive cocci, formerly known as nutritionally variant or deficient Streptococcus. Their role as causative agents of infective endocarditis (IE) is numerically uncertain, as well as diagnostic and clinical management of this infection. The aim of our study is to describe the clinical, microbiological, therapeutic, and prognosis of patients with IE caused by these microorganisms in a large microbiology department. Methods: Retrospective analysis of all the patients with Abiotrophia spp. and Granulicatella spp. IE registered in our centre in the period 2004–2021. Results: Of the 822 IE in the study period, 10 (1.2%) were caused by Abiotrophia spp. (7) or Granulicatella spp. (3). The species involved were A.defectiva (7), G.adiacens (2) and G.elegans (1). Eight patients were male, their mean age was 46 years and four were younger than 21 years. The most frequent comorbidities were congenital heart disease (4; 40%) and the presence of intracardiac prosthetic material (5; 50%). IE occurred on 5 native valves and 5 prosthetic valve or material. Blood cultures were positive in 8/10 patients, within a mean incubation period of 18.07 hours. In the other two patients, a positive 16SPCR from valve or prosthetic material provided the diagnosis. Surgery for IE was performed in seven patients (70%) and in all cases positive 16S rRNA PCR and sequencing from valve or prosthetic material was demonstrated. Valves and/or prosthetic removed material cultures were positive in four patients. Nine patients received ceftriaxone (4 in monotherapy and 5 in combination with other antibiotics). The mean length of treatment was 6 weeks and IE-associated mortality was 20% at one year follow-up. Conclusions: Abiotrophia spp. or Granulicatella spp. IE were infrequent but not exceptional in our environment and particularly affected patients with congenital heart disease or prosthetic material. Blood cultures and molecular methods allowed the diagnosis. Most of them required surgery and the associated mortality, in spite of a mean age of 46 years, was high.
Abiotrophia spp. and Granulicatella spp. are Gram-positive cocci that were first described in 1961 as nutritionally variant or deficient Streptococcus (NVS) [1], due to their need for enriched culture requirements or their characteristic growth as satellite colonies around other microorganisms. They were later reclassified into different species (Streptococcus defectivus, Streptococcus adjacens, Abiotrophia elegans or Abiotrophia balaenopterae) [2, 3, 4, 5]. Finally, and thanks to the use of 16S ribosomal RNA gene (16SPCR) and sequencing, Collins et al. [6] classified these microorganisms into two distinct genera; Abiotrophia (with the only species A.defectiva) and Granulicatella (composed of the three species G.adiacens, G.elegans and G.balaenopterae).
Although their role as causative agents of IE is known [7, 8, 9, 10, 11], their incidence, diagnostic and clinical management is not completely defined. Most data published in the literature report single clinical cases, with heterogeneous characteristics [12, 13, 14, 15], and data on the real impact of this entity are very scarce. Case series described focus on clinical aspects, providing scarce information on the role of microbiological diagnosis [16, 17]. The aim of our study is to describe the prevalence, clinical and microbiological characteristics, treatment and outcome of patients with IE caused by these microorganisms in a series of cases from our institution over the past 18 years, with a special focus on microbiological diagnosis, analysing in detail the diagnostic method used in each of the episodes, taking into account both traditional culture and molecular methods.
The Hospital General Universitario Gregorio Marañón (HGUGM) is a tertiary care institution, one of the largest hospitals in Spain, serving a population of 700,000 inhabitants, with more than 1200 hospitalization beds. HGUGM belongs to the “Spanish Collaboration on Endocarditis—Grupo de Apoyo al Manejo de la Endocarditis Infecciosa en España (GAMES)”. GAMES study group maintain a nationwide registry of 45 Spanish hospitals that prospectively follow all IE episodes with a common pre-established protocol. Internally, our institution has its own GAME-HGUGM group where all IE cases are discussed. A multidisciplinary group of experts evaluates all patients and review the cases. This is a retrospective descriptive study that analyses the total number of patients with IE by Abiotrophia spp. and Granulicatella spp. diagnosed in our centre in the period 2004–2021 (18 years).
Demographics, underlying conditions, clinical manifestations at IE presentation, affected valves and prosthetic material, microbiological diagnosis, complications, surgical treatment and outcome (until one year of follow up) were analyzed. In order to define IE we used the modified Duke criteria [18]. Place of acquisition of IE was defined following International Collaboration on Endocarditis-Prospective Cohort Study (ICE) and 2015 European Society of Cardiology Guidelines for the management of infective endocarditis recommendations [19, 20].
At our center all the valves and surgical material extracted from patients with
IE are process by conventional cultures and molecular methods. Identification of
the microorganisms was performed by 16SPCR and sequencing from blood culture
isolates and direct valve/prosthetic material at the time of sample processing,
as previously described [21]. Identification was also successfully performed by
matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry
(MALDI-TOF MS, Bruker Daltonics, Bremen, Germany) from blood culture colonies
from 2019. Three sets of aerobic and anaerobic blood cultures, collected from
different sites (peripheral veins +/– catheters) were performed for each patient,
in accordance with our hospital practices. Bottles for blood cultures were
incubated in an automated system (BACTECTM Plus Aerobic/F and BACTECTM
Anaerobic/F, Becton Dickinson). Isolates from valve and blood cultures grew
correctly on Columbia Agar 5% Sheep Blood (aerobic conditions), Brucella Blood
Agar with Hemin and Vitamin K1 (anaerobic conditions) and Chocolate Agar with
Vitox (CO
The resulting different data was incorporated into a Microsoft® Excel 2016 database for Windows. The qualitative variables were described as absolute and relative frequencies and the quantitative variables as median and interquartile range (IQR). All statistical analysis was performed using the IBM SPSS Statistics® version 18.0 software (SPSS Inc., Chicago, IL, USA).
A total of 822 Infective Endocarditis episodes were diagnosed during the 18 years of the study period (From January 2004 to December 2021). Among them, 10 were caused by Abiotrophia spp. (7) or Granulicatella spp. (3), representing 1.22% of all the IE cases. In terms of demographic characteristics, eight patients were males and two females. The mean age was 46 years (18–67 IQR). In four out of ten cases (40%), the age of the patients was under 21 years, with a mean of 13.5 years (8–19 IQR). Regarding previous comorbidities, 4 patients (40%) had congenital heart disease (1 interatrial communication, 1 Tetralogy of Fallot, 1 complex congenital heart disease with major aortopulmonary collateral arteries (MAPCAs), pulmonary branch hypoplasia, pulmonary atresia and ventricular septal defect, and 1 congenital aortic stenosis) and 5 had intracardiac prosthetic material (50%). Other comorbidities included hypertension (4), ischaemic or vascular heart disease (2), dyslipidemia (2) and diabetes mellitus (1).
Overall, 50% of IE occurred on native valves (2 aortic and 3 mitral), and 50% on prosthetic valves or intracardiac prosthetic material (1 biological mitral prosthetic valve, 1 mechanical prosthetic aortic valve, 1 prosthetic conduit and Melody® transcatheter pulmonary valve (TPV), 1 Contegra® conduit (Medtronic Inc, Minneapolis, MN, USA) and 1 polytetrafluoroethylene (PTFE, Goretex®) patch over the atrial septal defect. For patients with a prosthetic valve or prosthetic material, the median time between surgery and the onset of IE was one year. No embolic events or central nervous system involvement were reported in any patient. Regarding cardiac involvement associated with IE, patient #3 presented with ruptured chordae with severe mitral insufficiency, and patient #8 developed a heart failure decompensation in the context of IE. The clinical characteristics, treatment and outcome of the patients are presented in Table 1.
| Patient | Age | Gender | Underlying conditions | Valve prosthesis or prosthetic material, and year of surgery | Year of IE diagnosis | Affected valve or material | Surgery | Species | Antibiotic treatment | Treatment duration (weeks) | IE recurrence | Exitus after 12 months |
| # 1 | 67 | M | HBP, DM, dyslipidemia, aortic stenosis | No | 2004 | Native aortic valve | Yes | Abiotrophia defectiva | Ceftriaxone and Gentamicin | 6 | No | No |
| # 2 | 55 | M | HBP, dyslipidemia | No | 2009 | Native mitral valve | Yes | Abiotrophia defectiva | Ceftriaxone | 6 | No | No |
| # 3 | 54 | M | HCM, MM | No | 2011 | Native mitral valve | No | Abiotrophia defectiva | Vancomycin, Gentamicin and Rifampicin | * | No | Yes |
| # 4 | 81 | M | Ischaemic and valvular heart disease, AF, dyslipidemia, HBP | Yes (biological mitral valve) | 2012 | Biological mitral prosthetic valve | Yes | Abiotrophia defectiva | Ceftriaxone | 8 | No | No |
| 2010 | ||||||||||||
| # 5 | 1 | M | IAC, Transverse arch hypoplasia with associated aortic coarctation | Yes (Goretex® patch) | 2017 | Pericardial fluid, surgical wound and Goretex® patch | Yes | Abiotrophia defectiva | Ceftriaxone | 5 | No | No |
| 2016 | ||||||||||||
| # 6 | 15 | F | Tetralogy of Fallot, DiGeorge syndrome | Yes (Melody® TPV) | 2019 | Prosthetic duct (RV-PA) and Melody® pulmonary valve | Yes | Abiotrophia defectiva | Ceftriaxone and Gentamicin | Ceftriaxone (6), and Gentamicin (4) | No | No |
| 2018 | ||||||||||||
| # 7 | 20 | M | Congenital heart disease | Yes (Contegra® pulmonary prosthetic lung conduit) | 2021 | Contegra® pulmonary prosthetic lung conduit | No | Abiotrophia defectiva | Ceftriaxone, Gentamicin and Levofloxacin | Ceftriaxone (6), Gentamicin (1), and Levofloxacin from 10/1/22 | No | No** |
| 2001 | ||||||||||||
| # 8 | 86 | M | Aortic stenosis, COPD, HBP, CKD | Yes (mechanical prosthetic aortic valve) | 2011 | Mechanical prosthetic aortic valve | No | Granulicatella adiacens | Daptomycin, Ceftriaxone and Levofloxacin | Daptomycin (3), Ceftriaxone (6) and Levofloxacin (12) | No | Yes |
| 2010 | ||||||||||||
| # 9 | 63 | F | Dyslipidemia, gastritis, hysterectomy, polymyalgia rheumatica | No | 2019 | Native mitral valve | Yes | Granulicatella adiacens | Ceftriaxone | 5 | No | No |
| # 10 | 18 | M | Congenital aortic stenosis | No | 2016 | Native aortic valve | Yes | Granulicatella elegans | Ceftriaxone, Gentamicin, Piperacillin/tazobactam and Vancomycin | 3 | No | Yes |
| HBP, high blood pressure; DM, diabetes mellitus; AF, atrial fibrillation; IAC, interatrial communication; RV-PA, right ventricle-pulmonary artery; HCM, hypertrophic cardiomyopathy; MM, multiple myeloma; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease, *patient only received antibiotic therapy for 1 day due to acute exitus; ** that patient has been followed for 7 months at the time of publication of this paper. | ||||||||||||
In the seven cases where surgery was performed, the valve and/or prosthetic material was analyzed by 16SPCR and sequencing, being positive in all cases. Conventional culture of the valves and/or prosthetic material after surgery was positive in only four cases. Blood cultures were positive in eight patients, in the other two cases (#5 and #10), they were sterile, but they had received empirical broad-spectrum antibiotic therapy prior to blood culture extraction. These patients had positive 16SPCR from valve or prosthetic material.
The mean minimum time to positivity for blood cultures was 18.07 hours, being 18.07 hours in bottles under aerobic conditions, and 27.77 hours in bottles under anaerobic conditions. Colony identification was successfully performed in all cases by 16SPCR and sequencing. In those cases where preliminary MALDI-TOF identification was performed (cases 6, 7 and 9), it was correct. Both Abiotrophia spp. and Granulicatella spp. were isolated from positive blood cultures on Columbia Blood Agar (low growth and after re-incubation for several days), on Chocolate Agar (optimal growth), and on Brucella Agar (optimal growth), therefore, additional nutritional enrichment of the media was not necessary for the growth of the isolates. The mean time to negative blood cultures after initiation of antibiotic therapy was 10.5 days, but we must consider that in some cases control blood cultures were performed more than 7–10 days after the first positive blood cultures. The microbiological characteristics of Abiotrophia spp. and Granulicatella spp. IE are listed in Table 2.
| Patient | Species | Penicillin (MICs/S, I or R) | Cefotaxime (MICs/S, I or R) | Erythromycin (MICs/S, I or R) | Clindamycin (MICs/S, I or R) | Vancomycin (MICs/S, I or R) | Rifampicin (MICs/S, I or R) | Levofloxacin (MICs/S, I or R) | Microbiological diagnosis* | Time to blood culture positivity (hours) | Total number of positive blood culture bottles over the total |
| # 1 | Abiotrophia defectiva | 0.25 (S) | NA | NA | NA | Blood cultures and 16SPCR from valve | NA | 6/6 | |||
| # 2 | Abiotrophia defectiva | 0.12 (S) | 0.25 (S) | 1 (S) | NA | NA | Blood cultures, 16SPCR and valve culture | 9.72 | 6/6 | ||
| # 3 | Abiotrophia defectiva | 0.25 (S) | 0.5 (S) | 0.5 (S) | NA | Blood cultures | 15.58 | 3/6 | |||
| # 4 | Abiotrophia defectiva | 0.12 (S) | 0.5 (S) | 1 (S) | Blood cultures and 16SPCR from valve | 14.15 | 3/6 | ||||
| # 5 | Abiotrophia defectiva | 1 (I) | 1 (S) | 0.5 (S) | NA | Culture and 16SPCR from pericardial fluid and goretex patch | NA | NA | |||
| # 6 | Abiotrophia defectiva | 0.12 (S) | Blood cultures, 16SPCR and culture from prosthetic conduit and pulmonary Melody valve | 15.98 | 4/6 | ||||||
| # 7 | Abiotrophia defectiva | 1 (I) | Blood cultures | 48.16 | 3/6 | ||||||
| # 8 | Granulicatella adiacens | 0.25 (I) | 2 (I) | 0.5 (S) | NA | Blood cultures | 10.95 | 6/6 | |||
| # 9 | Granulicatella adiacens | 0.25 (I) | 0.25 (S) | 0.5 (S) | Blood cultures, 16SPCR and culture from valve | 11.97 | 6/6 | ||||
| # 10 | Granulicatella elegans | NA | NA | NA | NA | NA | NA | NA | 16SPCR from valve | NA | NA |
| NA, not available; I, susceptible to high doses and/or extended perfusion; MIC, minimum inhibitory concentration, * only those microbiological tests with positive results are mentioned in the table. | |||||||||||
A total of 20 bacteraemias due to Abiotrophia spp. or Granulicatella spp. were detected in our microbiology department during the study period. Eight of these bacteraemias corresponded to patients with IE. Distribution of the number of bacteraemias by microorganism in relation to whether or not they correspond to IE is shown in Fig. 1. A detailed description of the patients with bacteraemia without IE is available in the Supplementary Material section.
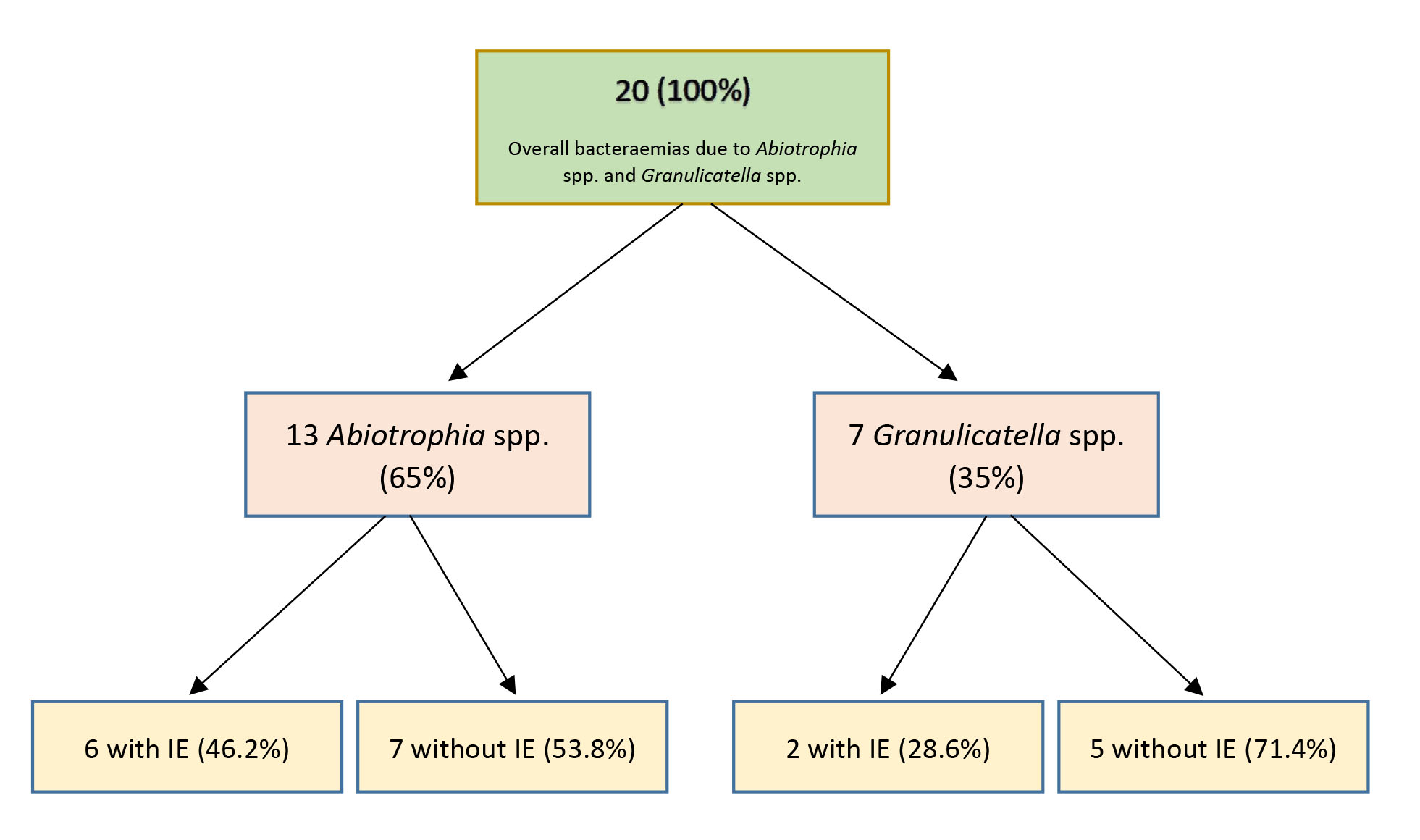 Fig. 1.
Fig. 1.Distribution of bacteraemias by Abiotrophia spp. and Granulicatella spp.
Regarding the antibiotic treatment received, ceftriaxone was administered in all patients in a targeted regimen once the antibiogram of the microbiological isolates was known (patient #8 received 3 weeks of daptomycin prior to ceftriaxone as the isolate had a high MIC to ceftriaxone and the clinical situation was severe). In four out of eight patients, ceftriaxone was administered as monotherapy, with a favorable evolution in all of them. In four patients, ceftriaxone was administered in combination with gentamicin. No adverse effects to the antibiotic treatments were reported in any case. The average duration of intravenous antibiotic treatment was 6 weeks. Patient #3 only received antibiotic therapy for 1 day due to acute exitus the day after hospital admission. Patient #7 received 6 weeks of ceftriaxone, the first week in combination with gentamicin. After completing treatment with ceftriaxone, he was started on suppressive long-term treatment with oral levofloxacin, due to the impossibility of performing surgery for IE. Patient #10 temporarily received piperacillin/tazobactam and vancomycin for other infectious complications during hospital admission.
Surgery for IE was indicated in all cases, but could be performed in seven patients (70%). The description of the non-surgical patients is detailed in the Supplementary Material section.
At one-year follow-up, no patient had recurrence of IE due to Abiotrophia spp. or Granulicatella spp. Patient #9, who had presented with an IE due to G.adiacens on native mitral valve in 2019, underwent valve replacement surgery and presented with another IE on prosthetic mitral valve due to methicillin-sensitive Staphylococcus aureus in 2021. IE-associated mortality was 20% (patients #3 and #10). Another patient died due to another infectious complication five months after IE. A detailed description of the patients who died can be found in the Supplementary Material section.
Here we present one of the largest case series of Abiotrophia spp. or Granulicatella spp. IE diagnosed in a single institution (10 cases), over 18 years. Most of the previous reports are based on a review of previously published individual cases with one or a few cases of their own [22, 23, 24]. Although the percentage of IE due to Abiotrophia spp. or Granulicatella spp. is low compared to the total number of IE diagnosed in our hospital in the same period (1.22%), it is not an exceptional entity, especially if we compare it with the percentage of IE caused by microorganisms from the HACEK group (1.58%) or the Streptococcus viridans group (14.84%) in our institution.
Our study shows that isolation of these microorganisms do not require special media supplementation or the prolongation of the incubation time as previously described [1, 25], something that should lead us away from the old idea that these microorganisms are difficult to culture. MALDI-TOF preliminary identification was correct in the three cases in which it was performed, as our group described before [26], and was verified by subsequent 16SPCR and sequencing. We routinely performed 16SPCR and sequencing on all isolates from blood cultures or valve/prosthetic material. In addition, we performed 16SPCR and sequencing directly on all surgical material (valves and/or prosthetic material) in IE, the role of which was previously described by Marin et al. [27]. In cases in which blood cultures were negative, 16SPCR on valve or prosthetic material provided the diagnosis. This work highlights the role of molecular methods in the diagnosis of endocarditis, and reinforces the idea that they should be implemented in routine practice in the diagnosis of this entity.
MICs to penicillin and cefotaxime were generally low, with some resistance to erythromycin and clindamycin. MICs to vancomycin, rifampicin and levofloxacin were low in all isolates tested, with 100% susceptibility. These data are in accordance with the susceptibility profile published in the literature for these microorganisms [28].
Information on the literature available on the role of Granulicatella spp. and Abiotrophia spp. infection outside of IE is very limited [29, 30, 31]. In this work, we reviewed all the bacteraemias due to Abiotrophia spp. or Granulicatella spp. in our institution in the study period (2004–2021). We detected 20 Abiotrophia spp. or Granulicatella spp. bacteraemias during the study period, of which 8 episodes were IE. In our opinion, the presence in blood of these microorganisms requires the exclusion of IE.
Téllez et al. [16] published the largest case series available to date of IE caused by these microorganisms (12 cases of their own), and review the literature of IE collected until 2015. In their review, focused on clinical aspects, the lower rate of congenital heart disease (10.5%) compared to ours (40%) is remarkable. Our data suggest that IE due to Abiotrophia spp. or Granulicatella spp. should be especially considered in patients with any type of cardiac structural damage, and that it is exceptional in patients without previous cardiac disease. Regarding the species involved, most of their cases were due to the genera Granulicatella (9/12), while in our cases, the genera Abiotrophia was predominant (7/10). No specific predisposing factors for IE due to these particular microorganisms have been described, although some authors suggest that previous oral colonization could be considered [32].
In absence of comparative studies, ceftriaxone seems to be the treatment of choice, with a good safety profile and early negative blood cultures. The association of gentamicin to ceftriaxone therapy did not seem to provide an evident benefit in terms of mortality or cure of IE. The high need for surgery in these patients (70%) in our case series is also very remarkable, which probably is well explained by the frequency of prosthetic material and underlying heart disease.
The main limitations of our study lie in the small number of cases available, probably due to the rare condition of this entity. Another aspect to be taken into account is that this is a descriptive study carried out in a single centre, and our results should be treated with caution when extrapolating them to other cases of IE due to Abiotrophia spp. or Granulicatella spp. in different patient profiles with other characteristics than ours.
It is essential to keep studying the role of Abiotrophia spp. and Granulicatella spp. IE, which, although uncommon, is not exceptional. Further analysis of larger case series, ideally in a multicentre manner, is needed to better understand this infection and to improve the management of these patients in clinical practice.
IE due to Abiotrophia spp. or Granulicatella spp. are infrequent, representing around one-percent of all IE cases in our series. It mainly affects patients with previous heart disease (congenital or acquired) and/or carriers of intracardiac prosthetic material, occurring in younger patients compared with IE due to other aetiologies, mainly due to the role of congenital cardiopathy. Blood cultures and 16SPCR and sequencing allowed the diagnosis. The antimicrobial therapy of choice was ceftriaxone. Most of them required surgery and the IE-associated mortality was considerable.
AE, MV—formal analysis, conceptualization, data curation, writing-original draft and editing. MeM, CS—microbiological methodology, data curation. MaM, LA, BP, AD, VG, AP, MMS, EB, PM—conceptualization, data curation, formal analysis, clinical methodology, writing and editing.
The study and the common case report form were approved by the local and national institutional review boards and ethics committees (Comité ético de Investigación Clínica Regional de la Comunidad de Madrid CEIC-R; EC 18/07; date 11/01/2008). Informed consent was not required as this was a retrospective study with data extracted from the hospital’s official medical records, anonymized at the time of writing this paper.
GAME-HGUGM study group: Iván Adán, David Alonso, Juan Carlos Alonso, Ana Álvarez-Uría, Javier Bermejo, Emilio Bouza, Gregorio Cuerpo Caballero, Antonia Delgado Montero, Agustín Estévez, Ramón Fortuny Ribas, Esther Gargallo, Ana González Mansilla, Mª Eugenia García Leoni, Francisco Javier González Moraga, Víctor González Ramallo, Martha Kestler Hernández, Amaia Mari Hualde, Marina Machado, Mercedes Marín, Manuel Martínez-Sellés, Rosa Melero, Patricia Muñoz, Diego Monzón, María Olmedo, Álvaro Pedraz, Blanca Pinilla, Ángel Pinto, Cristina Rincón, Hugo Rodríguez-Abella, Marta Rodríguez-Créixems, Eduardo Sánchez-Pérez, Antonio Segado, Neera Toledo, Maricela Valerio, Pilar Vázquez, Eduardo Verde Moreno, Sofía de la Villa.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
