Academic Editors: Neven Zarkovic and Guoyao Wu
Background: In the wild various organisms contribute to daphnids diet. This study, intendeds to evaluate the potential of the concentration of Rhodopirellula rubra as a single or supplementary food source for Daphnia magna. Methods: Feeding assays were performed according to standard guidelines for chronic assays (21 days), and life-history parameters and several biomarkers (protein content, oxidative stress, energetic reserves and pigments) were measured. Five food regimens were conducted with 20 individual replicates (A - R. subcapitata; 0.2 - suspension of R. rubra at 0.2 arbitrary units (AU); 0.4 - suspension of R. rubra at 0.4 AU; 0.2+A - suspension of R. rubra at 0.2+alga; 0.2+A-suspension of R. rubra at 0.4 AU + alga). Additionally, the effects of three diets (A, 0.2, and 0.2+A) on the longevity of D. magna were assessed. Results: The five diets showed a different C, N, and carotenoids composition, with an increase in the mixed diets. The results confirmed that the mixed diets improved D. magna life-history parameters. A decrease in glycogen, and the increase of haemoglobin, protein, and gluthione-S-transferase (GST) were observed. Furthermore, D. magna fed with bacterial single diets, presented worsen life history parameters and a decrease in the protein content. An induction of oxidative stress response (increased catalase and GST), and a significant decrease in lipid peroxidation and an accumulation of glycogen and carotenoids were observed. Overall, an increase in the amount of R. rubra provided to D. magna, from 0.2 AU to 0.4 AU, negatively impacted daphnid performance. No significant effects on Daphnia longevity (a 110-day assay) were observed among the three diets tested. However, a significant survival percentage and fertility (cumulative offspring is more than twice) was observed when D. magna was fed with the mixed diet. Conclusions: Results demonstrated that different diets provided a nutritional diversified food to the daphnids that induced differences in D. magna performance. The mixed diets proved to be beneficial (with increase in offspring) on D. magna performance, independently of the bacterial concentration tested. When in single diet, bacterial concentration is not nutritionally sufficient to raise D. magna even when in increased concentration.
The freshwater crustacean species of the genus Daphnia is a versatile model organism that has long been used in several areas of research [1, 2, 3, 4, 5, 6, 7]. In addition, among the live zooplankton, Daphnia spp. are a valuable food for small freshwater fishes and are also used as an ingredient in the formulation of commercial foods [8, 9, 10]. Furthermore, Daphnia is recognized as a sentinel species of freshwater ecosystems because its decline is an indicator of environmental impacts [3]. This characteristic associated with parthenogenic reproduction, short life cycle, high fecundity, and easy laboratory maintenance, makes them commonly used tool in toxicity assessments [11, 12, 13, 14, 15, 16, 17]. Normally, these organisms are maintained in laboratory conditions during several generations for use in biological research. For the laboratory maintenance, one must consider several factors that influence the performance and the health of these organisms and the responses to experimental conditions tested. Diet, in terms of quality and quantity of food, is a predominant factor that impacts Daphniaperformance due to its effects on life-history parameters such as growth, reproduction, and survival [18, 19], and response in the presence of toxics [20, 21, 22]. The algae-based diet is standardized under laboratory conditions because of its sufficiency, reliability, and simplicity. However, reliance on a single carbon source can lead to fluctuations in Daphnia performance [23, 24]. Indeed, previous studies have shown that Cladocera’s exhibits different responses when subjected to different algae as food source [25, 26]. Jonczyk et al. [27] reviewed notions about culture and maintenance in the laboratory and suggested that survival and reproduction in Daphnia were improved on mixed diets comparatively to a single algal diet. In fact, several studies addressed the effects of mixed diets on Cladocera’s fitness [28, 29, 30], while others evaluate alternative food sources that complement and diversify their diet [31, 32].
Aquatic ecosystems have a considerable amount of bacteria which contribute to the diet of Daphnia spp. since they are non-selective filters. Previous studies conducted by Antunes et al. [33] and Marinho et al. [34, 35] already showed the potential of Planctomycetes, namely Rhodopirellula rubra, to be a good supplementary food source when used in association with the standard food source (the microalgae Raphidocelis subcapitata), and its capacity to improve Daphnia magna life history parameters and in providing pink coloration. The authors also observed that these effects of R. rubra were more relevant at the exponential growth phase than the stationary growth phase. Furthermore, D. magna performance with mixed diets with two different planctomycetes, R. rubra and Gemmata obscuriglobus, was also assessed. Even though G. obscuriglobus produces sterols, molecules fundamental in nutritional terms for D. magna, R. rubra induced a better D. magna performance [34]. Also, an increase of MUFAs content in D. magna fatty acid’ profile associated with the diet rich in R. rubra was found and the pink color displayed by bacteria was retained by mothers and observed in the offspring [35]. However, R. rubra showed not to be sufficient when used as a single food. In face of these results, one may question if the amount of bacterium provided as food has been sufficient for the welfare of D. magna as well as its ability to grow, reproduce and survive? Several studies reported that the amount of food affects growth, longevity, and reproduction in Daphnia spp. [18, 19, 36]. So, the question about bacterial concentration used in our previous studies for D. magna was raised. It is still unclear if the previous concentration of bacterium provided was sufficient regarding the nutritional value, and which are the physiological consequences of the increase of bacterial concentration on D. magna. In natural habitats, organisms are subject to wide seasonal variations in food concentration [37] in contrast to a constant food supply under laboratorial conditions. The responses in life history parameters of aquatic organisms may be related to variation in food availability being indicative of the status of the ecosystem.
As a follow up of our previous studies, we aimed to evaluate the adequacy of a single R. rubra diet and a mixed diet (R. rubra plus R. subcapitata) in two different bacterial concentrations in the D. magnaperformance along a chronic exposure (21 days). This assessment was based on the analysis of life-history parameters, and several physiological parameters: energetic reserves-protein and glycogen content; pigments-carotenoids and haemoglobin content; oxidative stress-antioxidant catalase (CAT) activity, detoxification glutathione S-transferase (GST) activity and lipid peroxidation (thiobarbituric acid assay-TBARS levels), and also on carbon, nitrogen, and carotenoids contents of the diets Furthermore, the effect of diets with the low amount of bacterium in D. magna longevity was also evaluated, regarding the life-history parameters.
The planctomycete R. rubra LF2 was isolated from the biofilm community
of the marine macroalga Laminaria sp. from the north coast of Portugal
[38]. R. rubra strain LF2 was first grown on solid modified M13 medium
[38] at 26 °C and then transferred into liquid modified M13 medium with
continuous stirring at 200 rpm. The culture was upscaled each three days, in
exponential growth phase, starting with a culture volume of 50 mL, passed to 250
mL and finally to 1.5 L using always a 1:10 volume of inoculum. Cells in
exponential growth phase (3 days of growth) were collected by centrifugation at
4000 rpm for 10 min. The cell pellets were resuspended in distilled water and the
optical density adjusted at
D. magna monoclonal cultures were maintained over several generations
of pure parthenogenetic cultures under controlled conditions of temperature (20
Carbon, nitrogen, and carotenoids content were quantified in the five food
regimens tested (Table 1). To quantify carbon in R. rubra, 2.5 mL of the
sample at 0.2 AU and 0.4 AU, was centrifuged at 13,000 rpm for 60 seconds. The
supernatant was discarded, and the pellets were lyophilized for subsequent
quantification For R. subcapitata 3
| A | 0.2 | 0.4 | 0.2+A | 0.2+A | |
| R. subcapitata (cells·mL |
3.0 |
3.0 |
3.0 | ||
| R. rubra (AU·day |
2500 |
2500 |
2500 |
2500 | |
| Carbon (mgC·sample diet |
0.198 | 0.165 | 0.344 | 0.329 | 0.536 |
| Nitrogen (mgC·sample diet |
0.0240 | 0.0423 | 0.0922 | 0.0688 | 0.118 |
| Carotenoids (mg·L |
1.20 |
0.038 |
0.063 |
1.68 |
2.15 |
Carotenoids content was quantified by the method described by Zhang and Hu [45], where 10 mL of acetone (99.5%; V1) was added to the cell pellet from culture cell suspension (V2) and sonicated for 3 min in a Misonix Microson Ultrasonic Cell Disruptor XL, at 20 watts. Then, the tubes were kept in a water bath at 20 °C for 10 min. After centrifugation at 3000 rpm for 10 min, the total carotenoids were determined by reading the absorbance at 480 nm (A) and applying the following equation [45]:
To evaluate the potential of R. rubra as a nutritional and supplementary food source for D. magna, feeding assays were performed according to standard protocols for assessing chronic toxicity [46, 47]. The feeding assays had a duration of 21 days and were conducted under the same conditions as described for cultures maintenance. The experimental setup comprised 5 food regimens as describe in Table 1.
For each food regimen, twenty individual replicates were placed in a single
glass vial filled with 50 mL of the synthetic ASTM hard water medium [39]. Daily,
the daphniids were fed with corresponding food regimen (Table 1), and the medium
renewed was conducted every two days. Mortality and the reproductive state were
checked daily, and the neonates born during the assay were counted and discarded.
At the end of the assay, the following endpoints were quantified: age at first
reproduction (days), reproductive output (mean of offspring produced by all the
mothers), fecundity (mean of offspring produced by the survivor mother at the end
of the assay), number of broods, fecundity of the first brood, somatic growth
rate (day
Somatic growth rate was determined regarding the difference between the initial and final body size of the organisms, measured from the top of the head to the base of the caudal spine, in a binocular stereoscope. At the beginning of the assay, average of initial body length was calculated in a sub-sample of 20 neonates from the same brood of the organisms used in the assay. At the end of the feeding assay all the survivor organisms were measured. The somatic growth rate was calculated, according to the following expression:
where L
Survival and fecundity related data were used for the estimation of the per capita intrinsic rate of population increase (r), which was iterated from the Euler–Lotka equation:
where r is the intrinsic rate of increase (day
At the end of the feeding assay, after body length and weight measurements, 3 daphnids were collected for carotenoids quantification. Firstly, the organisms were placed in a vessel containing ASTM medium for 2 hours to promote gut cleaning, and afterward were storage in Eppendorf microtubes for carotenoids quantification. For each food regimen, the remaining organisms were storage in 5 individual groups at –20 °C for the next day quantifications (protein, glycogen, haemoglobin, carotenoid contents and the activity of catalase (CAT) and of glutathione S-transferase (GST), and levels of lipid peroxidation).
Regarding the results obtained in the feeding assay and to assess the potential
effects of different food regimens on the longevity of D. magna, a new
assay was performed with 20 individualized neonates exposed to the feeding
regimens: A (standard food), 0.2 and 0.2+A (Table 1). The food regimens selected
to assess the D. magna longevity were chosen according to the results
obtained in the life-history parameters of the previous feeding assays. The assay
was performed in vessels with 30 mL of ASTM medium and the organisms were kept
under the same conditions as described for the culture’s maintenance and the
feeding assay. Culture medium were completely renewed three times a week and
daphniids feed at the same time (fed three days a week, as described in OECD
guidelines [47] — “the food provided to organisms should preferably be done
daily, or at least three times a week when the medium is changed”). The
organisms were checked daily for mortality, and if there were neonates, these
were counted and discarded. The endpoints measured at the end of the assay were:
survival, age at first reproduction (days), reproductive output, fecundity of the
first brood, and rate of population increase (r, day
At the end of the feeding assay a set of biomarkers were measured in the organisms exposed to the different food regimens: energetic reserves-protein and glycogen content; pigments-carotenoid and haemoglobin content; oxidative stress-antioxidant catalase (CAT) activity, detoxification glutathione S-transferase (GST) activity and lipid peroxidation (thiobarbituric acid assay-TBARS levels). Organisms’ homogenization was performed in 1.2 mL of cold phosphate buffer (50 mM, pH = 7.0 with 0.1% Triton X-100), and the homogenates were centrifuged at 13,400 rpm for 6 min at 4 °C. The supernatants were recovered for the biochemical analysis. The supernatant was divided into aliquots for subsequent determination of biochemical biomarkers, and all endpoints were determined in triplicate. The absorbances (except for haemoglobin determination) were performed in a microplate reader Thermo Scientific Multiskan GO spectrophotometer, version 1.00.40, with SkanIt Software 3.2.
Haemoglobin was determined by reading the absorbance of 1 mL of supernatant from each homogenized sample in a UV-1600 PC Spectrophotometer from 350 to 500 nm at 1 nm interval [49]. The specific absorbance of haemoglobin at 414 nm was normalized according to the technique described by Williams et al. [49]. The slope of the absorption spectrum within the wavelength intervals of 370–470 nm was determined by linear regression, from which the peak of haemoglobin specific absorbance (394–434 nm) was omitted. As for the 370–395 nm and 430–470 nm intervals, the corresponding values belong to non-haemoglobin materials that are present in Daphnia tissues. The observed absorbance value at the haemoglobin peak (414 nm) was subtracted by the expected absorbance value (calculated by linear regression calculated in the omitted interval of absorbance).
The glycogen quantification was conducted according to Lo et al. [50],
where 100
where Y is the absorbance values in the sample and x is the glycogen content
(
Catalase activity was determined following the procedure by Aebi [51], where the
degradation of H
Glutathione S-transferase activity was measured according to the method by Habig et al. [52]. GST catalysis the conjugation of glutathione with the substrate 1-chloro-2,4-dinitrobenzene (CDNB), forming a thioether that increases absorbance at 340 nm. The results were expressed considering the equivalent one unit of GST activity to the number of moles of thioether produced per minute per milligram of protein.
Lipid peroxidation was measured by the quantification of the concentration of thiobarbituric acid reactive substances (TBARS), according to Buege and Aust et al. [53]. The main by-products of oxidative damage to lipids membranes caused by reactive oxygen species (ROS) are malondialdehyde (MDA) and MDA-like compounds. This methodology is based on the reaction of compounds, such as MDA, formed by degradation of initial products from lipid membranes by free radical attack, with 2-thiobarbituric acid (TBA). Absorbance readings of each sample were measured at a wavelength of 535 nm and expressed as MDA equivalents per milligram of protein.
Protein was determined according to the methodology described by Bradford [54],
adapted to microplates. This involves the binding of a dye (Bradford reagent) to
the total protein, giving rise to a stable and colored complex that can be
quantified at 595 nm. The
For the quantification of the carotenoid content 0.5 mL of 100% ethanol was
added to the samples. The microtubes were wrapped in aluminum foil for protection
from light and placed in the refrigerator overnight at –4 °C, according
to Moeller et al. [55]. The day after, the total carotenoid absorption
of the supernatant was measured in the spectrophotometer at
where V is the extract volume (mL), W is total dry weight (mg) of organisms and
OD = optical density (
One-way Analysis of Variance (ANOVA) was applied at all the endpoints measured
in the feeding and longevity assays: age at first reproduction, fecundity,
reproductive output, number of broods, number of N1 offspring, somatic growth
rate and rate of population increase (these endpoints were log (x + 1)
transformed prior to the ANOVA, to comply with ANOVA requirements), glycogen,
protein, TBARS, GST and CAT. When ANOVA results showed significant differences a
post-hoc Tukey test was conducted to assess statistical differences between the
different food regimens. For all analyses, the level of significance (
One of the main questions addressed in this work was if an increase in the amount of R. rubra provided to D. magna as food would enhance its growth. As previously observed by Marinho et al. [34, 35], R. rubra at a concentration of 0.2 AU proved not to be sufficient to provide the nutritional requirements needed by D. magna. In fact, a significant delay of the age at first reproduction, a decrease of broods, and an increase of somatic growth rate, fecundity, reproductive output, and the rate of population increase were observed (Fig. 1). The obtained results suggest that 0.2 R. rubra concentration, when provided as exclusively food source, is below the “incipient limiting level” (ILL—the external level above which there is no limiting effect of food supply, terminology described by Fry et al. [56], when the daphnids filter at a maximum rate [1, 57]. Regarding the double R. rubra concentration (0.4), D. magna performance was not improved, and the parameters of the life history even aggravated (Fig. 1). The cell aggregate formation typical of R. rubra may justify this result, as the feeding structures of D. magna may be inadequate to efficiently filter the bacteria. In fact, and as already demonstrated by Gliwicz et al. [58], filtration rate and particle size affect the amount of food collected and ingested by Daphnia as well as in other organisms (e.g., rotifers [59]). On the other hand, the higher amount of food in diet 0.4 may reduce the ability of Daphnia to ingest the bacteria due to occlusion of the filter apparatus, fact already observed by Martínez-Jerónimo et al. [60]. Both food regimens (0.2 and 0.4) presented to be poor diets compared to the mixed diets (0.2+A and 0.2+A) and the diet with only alga (A).
 Fig. 1.
Fig. 1.Life-history parameters; (A) age at first reproduction; (B) offspring of
first brood; (C) number of broods; (D) somatic growth rate; (E) fecundity and
reproductive output; and (F) rate of population increase, results of D.
magna after exposure for 21 days to several food regimens (see Table 1). Error
bars represent standard error (n = 20) and different letters (a,b,c,d) represent
significant differences between food regimens (Tukey test, p
On the other hand, when D. magna was fed with the mixed diet 0.2+A, a
significant reduction of the age at first reproduction, and a significant
increase of somatic growth rate, fecundity, reproductive output and rate of
population increase were recorded (Fig. 1), corroborating the data from Marinho
et al. [34, 35]. Moreover, when comparing the two mixed diets (0.2+A and
0.2+A), no differences were observed (a similar D. magna behavior was
obtained; Fig. 1). This implies that, under the same laboratorial conditions,
D. magna did not need an additional quantity of bacteria to achieve the
same levels of reproduction and growth. The elemental analyses of the levels of C
and N in the five diets showed an increase from the single to the mixed diets
(Table 1). This carbon increase in the diets, from A to 0.4 or from 0.2+A to
0.2+A, showed to be insufficient to improve D. magna life-history
parameters. On the other hand, the quality of the food provided by the alga
(although comparatively lower) is essential to D. magna rearing.
Curiously, the levels of N present in the bacterium are higher in all diets than
in the algal diet. The level of this element may explain the important role of
this bacterium in the N availability to D. magna in the mixed diets.
Taipale et al. [28] and Freese et al. [29], also recorded that
when bacteria [Micrococcus luteus, Methylomonas methanica,
Methylosinus trichosporium (2.5 mg
The effects of the different diets on D. magna physiological parameters
were also evaluated and are presented in Figs. 2,3. The protein levels recorded
in 0.2 and 0.2+A were similar to the A diet, while in 0.4 and 0.2+A a significant
decrease (0.7
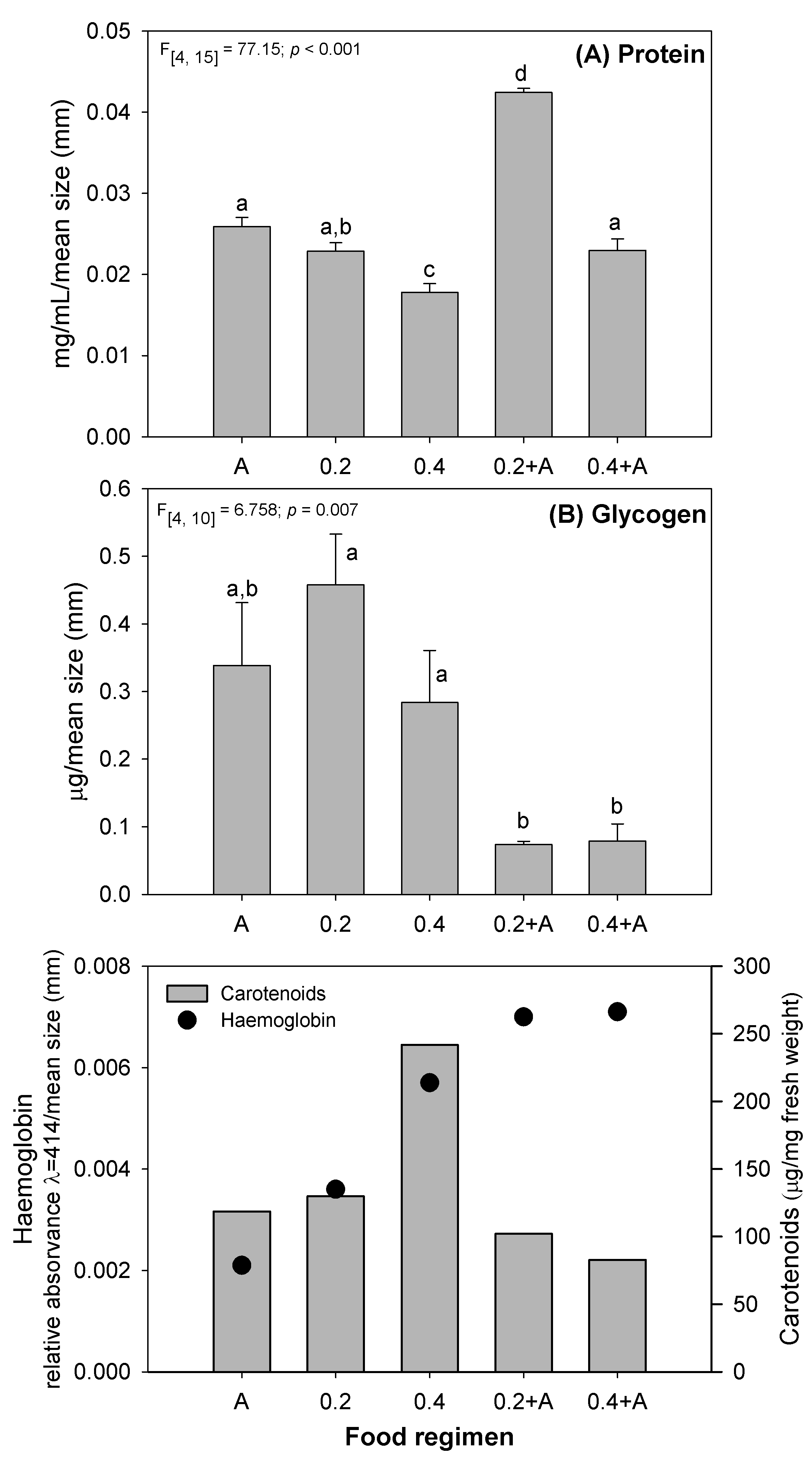 Fig. 2.
Fig. 2.Variation in protein (A) glycogen, (B) haemoglobin and carotenoids in
D. magna after exposure for 21 days to several feeding regimes (see
Table 1). Error bars represent standard error (n = 20), different letters
(a,b,c,d) represent significant differences between food levels (Tukey test,
p
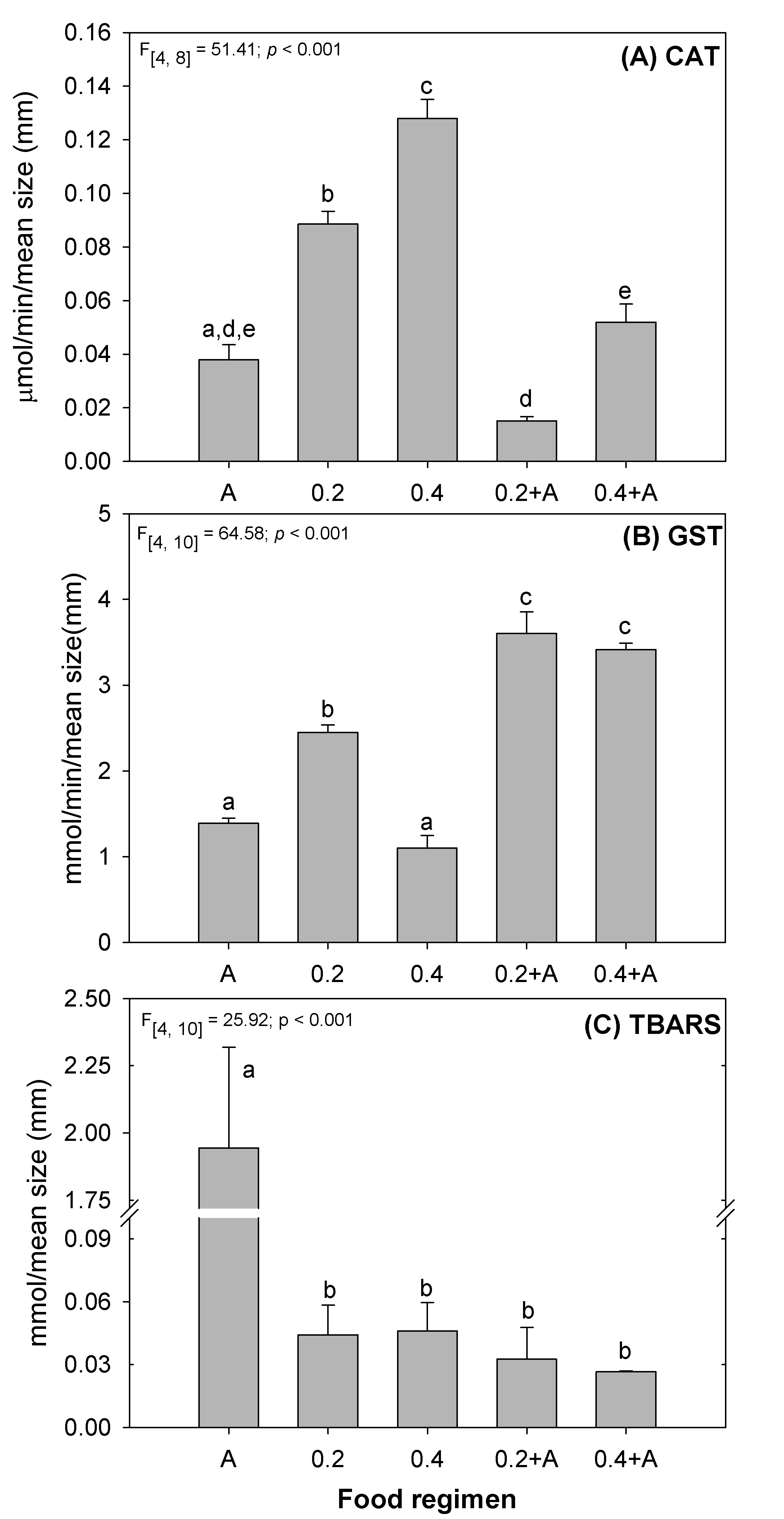 Fig. 3.
Fig. 3.Results of oxidative stress biomarkers (A) CAT; and (B) GST activities; (C)
TBARS concentrations, in D. magna after exposure for 21 days to several
feeding regimens (see Table 1). Error bars represent standard error (n = 20) and
different letters (a,b,c,d,e) represent significant differences between food
levels (Tukey test, p
Glycogen represents the main form of glucose storage in animal organisms, which fuels glycolysis as a first response in the case of a lack of food [69, 70]. In the 0.2 and 0.4 diets, D. magna had no difference in the glycogen levels comparatively to A diet (Fig. 2B). Indeed, a significant lower somatic growth rate and reproduction values (see Fig. 1D–F) were observed in the single bacterial diets, showing that the organisms allocated the energy available for self-maintenance instead of supporting growth and reproduction. Several studies already demonstrated that under conditions of low feeding, daphnids increase the allocation of energy for self-maintenance [71, 72]. Regarding the glycogen contents in the mixed diets, a significant decrease was observed relatively to the bacterial single diets (Fig. 2B). As a significant increase of somatic growth rate and reproductive parameters was observed in the mixed diets (Fig. 1D, E), D. magna was not able to accumulate glycogen due to consumption for reproductive needs. This indicates that D. magna energy allocation strategy was directed for growth and reproduction having not the possibility for reserve storage. When glycogen is used for energy production it is rapidly catabolized, leading to large losses of this energy reserve [68].
Haemoglobin in Daphnia is a respiratory pigment, which is part of the
oxygen transport system [73]. In invertebrates, haemoglobin is involved in
adaptive response to changes in environmental conditions, such as oxygen
availability, pH, salinity, CO
Several different types of carotenoids are present in algae and bacteria.
R. subcapitata belongs to the phylum Chlorophyta which contains, among
others,
It is known that food deprivation or lack of nutritional factors, such as
vitamins, causes changes in the activities of tissue antioxidant enzymes [83].
The effect of diet on free radicals’ formation varies in function of the type of
organism, age, physiological status, and ingested food [84]. For example, a study
of fed and fasted mammalian species (male Wistar rats) showed that malnutrition
accelerated the production of free radicals with the consequent depletion of the
liver antioxidant stores [85]. If vitamins C and E were added to mice
erythrocytes previously incubated with hydrogen peroxide (H
Results from the evaluation of oxidative stress in D. magna under the
different feeding regimens are provided in Fig. 3. The levels of CAT activity in
the mixed diets were similar to A diet, showing a non-oxidative stress response.
CAT activity was significantly increased in D. magna fed with the two
bacterial diets (Fig. 3A). This result may be justified by a potential increase
in hydrogen peroxide levels and consequent oxidative stress due to D.
magna undernutrition status (Fig. 3A —0.2 and 0.4). Im et al. [92]
also demonstrated that under low food concentration and high temperatures
D. magna showed increased activity of antioxidant enzymes (SOD and CAT)
and reduced adult somatic growth rate. Regarding the GST activity, a significant
increase in D. magna fed with the diets 0.2 (1.8
A longevity study (from birth to natural death) was also performed with D. magna fed with 3 feeding regimens (A, 0.2 and 0.2+A; Figs. 4,5). No death occurred in the first 25 days of the assay for any of the feeding regimens (Fig. 4A). After this period, survival felt abruptly from day 30 to day 60 in the A diet (with a slope of –1.039) with 50% death recorded at day 50 (Fig. 4A). In the 0.2 feeding regimen, death occurred from day 40 to day 90 (with a slope of –1.243) with 50% mortality around day 70, with complete organisms’ death recorded at day 107 (Fig. 4A). In the 0.2+A feeding regimen, the decrease survival was from day 30 to day 100 (with a slope of –0.831) with 50% death occurring at day 70. No significant differences were recorded in D. magna survival (days) regarding the three feeding regimens tested (Fig. 4A). The reproductive output along the 110 days of experiment is shown in Fig. 4B. A significant decrease of reproductive output was recorded for the 0.2 diet (with a slope of 1.479), while a significant increase was observed for 0.2+A diet (with a much higher slope of 6.671) and for A diet (a slope of 3.405) (Fig. 4B). Although this assay was performed for 110 days instead of 21 days, comparable results were obtained (for comparison see Fig. 1). A significant delay in the age at first reproduction (Fig. 5A), and a significant decrease of N1 fecundity (Fig. 5B) were observed in 0.2 feeding regimen. The rate of population increase showed similar results obtained in the 21-day assay (Fig. 1F) where a significant decrease was observed for 0.2 while for 0.2+A a significant increase was recorded (Fig. 5C).
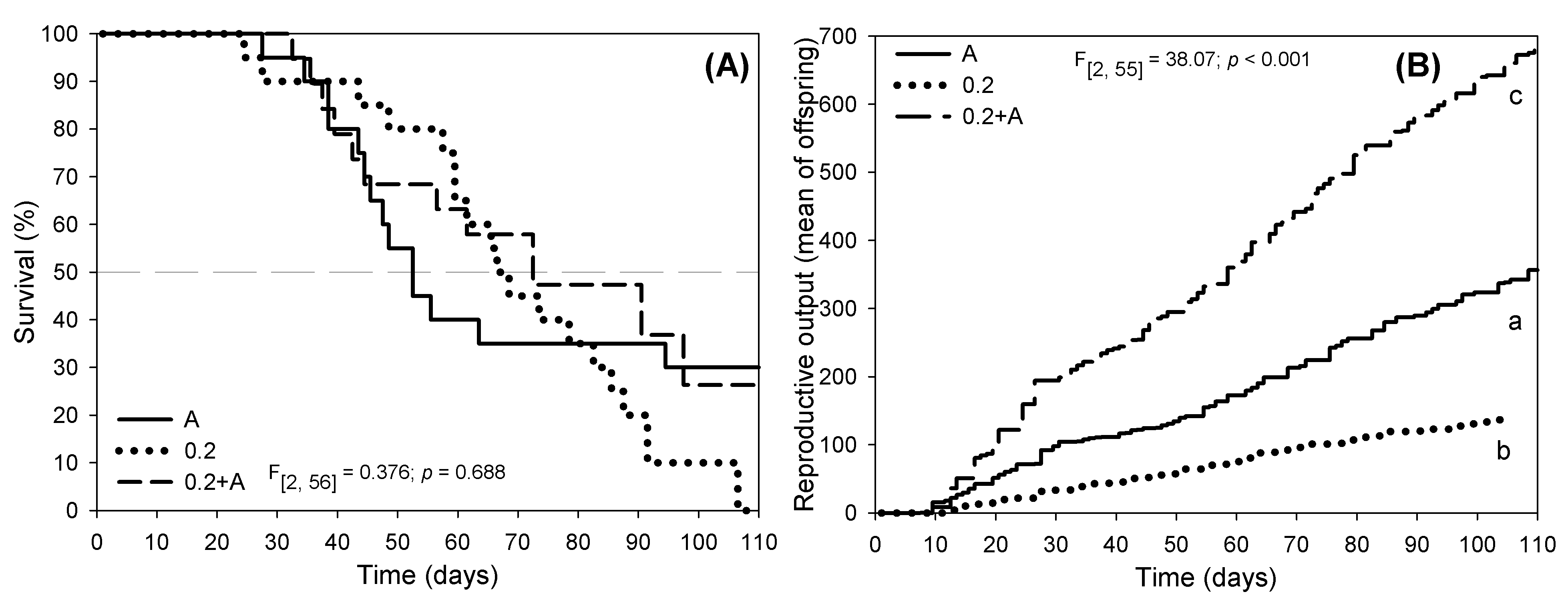 Fig. 4.
Fig. 4.Survival curves (A) and reproductive output (B) for D. magna
fed with the green microalgae R. subcapitata (A), the planctomycete
R. rubra suspension at 0.2 AU (0.2) and a mixed diet of R.
rubra suspension at 0.2 AU plus R. subcapitata (0.2+A). Different
letters (a,b,c) stands for significant differences between the food regimens
(Tukey test, p
 Fig. 5.
Fig. 5.Life history parameters (A) age at first reproduction; (B) offspring of
first brood; and (C) rate of population increase in longevity assay of D.
magna fed on the green microalgae R. subcapitata (A), the planctomycete
R. rubra suspension at 0.2 AU (0.2) and a mixed diet of R.
rubra suspension at 0.2 AU plus R. subcapitata (0.2+A). Error bars
represent standard error (n = 20), and letters (a,b,c) stands significant
differences between the food regimens (Tukey test, p
Vijverberg [98] observed that well fed D. hyalina lived for 43.4 days
while poorly fed organisms lived for 64.4 days. Ingle [99] reported that well fed
D. longispina organisms had an average life duration time of 29.9 days
while malnourished organisms lived longer, for 41.4 days. Indeed, previous
studies with D. magna also reported that survival increased under
limiting food conditions [60]. D. magna fed with 0.05, 0.15, 0.5, 1.5,
and 4.5 mgC
The elemental analyses of the levels of C, N, and carotenoids in the five diets showed overall an increase from single diets to the mixed diets. These results demonstrated that different diets (algae and bacterium) provided a diversified and different nutritional food to the daphnids that represent differences in the performance of D. magna, even in laboratory conditions.
The bacterium (R. rubra) proved to be a good supplement food for growing D. magna since the mixed feeding regimens 0.2+A and 0.2+A (bacteria + algae) significantly improved its performance, reinforcing the previous results obtained by our group. We also showed that a food regimen with higher R. rubra levels (from 0.2 to 0.4) is not enough to fill D. magna nutritional needs. Moreover, these results are supported by the longevity assay. The high protein content observed showed that the mixed diet tested (0.2+A diet) is, in fact, the best diet for D. magna. Low levels of lipid peroxidation were registered in all bacterial diets which may be due to the levels of activity of the antioxidant enzymes (CAT and GST) observed, and the intake of carotenoids (non-enzymatic antioxidant defense) provided by the bacterium. Thus, our results evidenced that biological, biochemical, and physiological processes are affected by the food conditions to which organisms are subjected.
Thus, and reinforcing our previous studies, we can conclude that R. rubra is a good diet supplement for D. magna that improve the growth, fecundity and survival of D. magna under laboratorial conditions enhancing this daphnid’ performance. Although different food sources are already used in the maintenance of Daphnia spp, a diversified diet, that includes the bacterium R. rubra, can be adopted to improve D. magna performance in laboratory maintenance.
MM, GJ, OML and SCA contributed to the study conception and design. MM, GJ, JC, LS and SCA performed all material preparation, data collection and analysis. MM and GJ have written the first draft of the manuscript and all authors commented on the various versions of the manuscript. All authors read and approved the final manuscript.
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Not applicable.
Conceição Marinho received a PhD fellowship (SFRH/BD//146190/2019) from Foundation for Science and Technology (FCT - Government of Portugal). This work was supported by National Funds (through the FCT - Foundation for Science and Technology) and by the European Regional Development Fund (through COMPETE2020 and PT2020) through the strategic program UIDB/04423/2020 and UIDP/04423/2020. Sara Antunes is hired through the Regulamento do Emprego Científico e Tecnológico – RJEC from the Portuguese Foundation for Science and Technology program (CEEC-IND/01756/2017).
The authors declare no conflict of interest.
