1 Department of Structural and Functional Biology, Institute of Biology, University of Campinas (UNICAMP), 13083-970 Campinas, SP, Brazil
2 Department of Medicine, University of Miami Miller School of Medicine, Miami, FL 33136, USA
3 Department of Urology, Albert Einstein College of Medicine and Montefiore Medical Center, Bronx, NY 10461, USA
4 Laboratory of Nerve Regeneration, University of Campinas (UNICAMP), 13083-970 Campinas, SP, Brazil
5 Institute of Biomedical Sciences, Department of Physiology, Federal University of Uberlandia (UFU), 38408-102 Uberlandia, MG, Brazil
§Current address: Department of Anesthesiology, University of California San Diego, La Jolla, CA 92093, USA.
Abstract
Background: Pannexin1 (Panx1) is a membrane channel expressed in
different cells of the nervous system and is involved in several pathological
conditions, including pain and inflammation. At the central nervous system, the
role of Panx1 is already well-established. However, in the periphery, there is a
lack of information regarding the participation of Panx1 in neuronal
sensitization. The dorsal root ganglion (DRG) is a critical structure for pain
processing and modulation. For this reason, understanding the molecular mechanism
in the DRG associated with neuronal hypersensitivity has become highly relevant
to discovering new possibilities for pain treatment. Here, we aimed to
investigate the role of Panx1 in acute nociception and peripheral inflammatory
and neuropathic pain by using two different approaches. Methods: Rats
were treated with a selective Panx1 blocker peptide (
Keywords
- DRG
- Panx1
- inflammatory pain
- neuropathy
- capsaicin
Pannexin 1 (Panx1) is a cell surface single-channel expressed in different types
of cells in the nervous system, including nociceptive neurons, macrophages, and
satellite cells [1, 2, 3]. They can be activated by mechanical stimulation,
intracellular calcium, high extracellular potassium concentration, and
N-methyl-D-asparate receptor (NMDA) activation [2, 4, 5, 6]. Studies have shown an
interactive network between Panx1 channels and ATP signaling in the dorsal root
ganglion (DRG), where these channels trigger inflammatory pain cascades through
caspase-1 maturation, interleukin-1
The DRG is an essential site for the transduction of nociceptive signals. Within the ganglia reside the cell bodies of neurons that innervate a vast portion of the peripheral tissue of our body. Each neuron is enveloped by a layer of glial cells, forming units interspersed by macrophages [13]. Besides sharing space in the DRG, this group of cells also exchanges molecules and neurotransmitters that have been proven critical for developing and maintaining pain. Our research group demonstrated that the communication between neurons and satellite glial cells in the DRG mediated by ATP is essential for inflammatory pain development and acute nociceptive response [14, 15]. These findings indicate that DRG is a promising target for pain modulation. Taking the sustained link between Panx1 and ATP/inflammation signaling at the DRG, we investigated the implications of these channels in modulating inflammatory pain induced by carrageenan, nociception by capsaicin or formalin, and neuropathic pain induced by chemotherapy.
Studies were conducted using male rats and female or male mice. Animals were
housed on a 12 h light/dark cycle with controlled humidity and temperature (22
Male Wistar rats (200–250 grams) were used for behavior assessments and primary DRG neuronal cell culture. Rats were acquired from the Multidisciplinary Center for Biological Research (CEMIB) of University of Campinas, Campinas, Brazil, and maintained under standard housing conditions in the Laboratory of Pain Studies animal facility at the Department of Functional and Structural Biology of State University of Campinas, Campinas, Brazil.
Adult female or male mice (6 months old) with global deletion of
Panx1
Drug inoculation into the DRG of rats was performed using the direct injection
method as previously described [19]. Briefly, animals were anesthetized with
2.5% isoflurane (Cristalia, Itapira, Brazil), and the fur was shaved over the
lower back region at the level of the iliac crest. Animals were placed in a prone
position over a cylinder, causing the lower back to become hyper flexed. A guide
cannula (hypodermic needle, 25
The subcutaneous injection of the inflammatory agent carrageenan (Cg) was
administered into the central plantar region of the right hind paw, which
corresponds to the peripheral field of the L5 DRG [20]. For rats and mice, the
dosage used was as follows:
Panx1-KO and WT mice received a single systemic injection of paclitaxel (i.p., 2 mg/kg, #33069-62-4, Cayman, Ann Arbor, MI, USA) or four injections of the same drug, at the same concentration, on alternate days (1, 3, 5, 7 days) [21]. The control group received systemic administration of vehicle solution: 0.1% Dimethyl sulfoxide (DMSO) (#67-68-5, Sigma Aldrich) in Saline, at the same time points as the treated group.
Electronic von Frey (for rats: Insight, #EFF301, Ribeirão Preto, Brazil;
and for mice: Bioseb, #BIO-EVF-WRS, Pinellas Park, FL, USA)
was used in this study to measure the mechanical withdrawal threshold of the hind
paw, as previously described [22]. This test was applied to the
following groups: paclitaxel and Cg treatments. Rats and mice were kept in a quiet room for 1 hour prior to the experiment to acclimate to testing
environment. Measurements were taken blindly by the same experimenter between
9:00 AM and 5:00 PM. The polypropylene pipette tip (10 µL, #T-300, Axygen,
Corning, NY, USA) connected to hand-held force transducer was applied
perpendicularly to the central region of the animals’ hind paw (L5 peripheral
field) and pressure was gradually increased until a paw withdrawal or flinch was
observed. The stimulus was interrupted at this point. The intensity of allodynia
was quantified as the variation of the pressure
Capsaicin (#M2028, Sigma Aldrich) at 10 µg/paw or vehicle (saline) was injected into the central plantar region of the right hind paw of mice (25 µL) or rats (50 µL). The number of flinches and licking were recorded for a period of 5 minutes after injection. The amount of 50 µL of 2.5% formalin (diluted from a 4% of Paraformaldehyde, #30525-89-4, Sigma Aldrich in phosphate-buffered saline, #P4417, Sigma Aldrich) was injected intradermally on the right hind paw of rats. Immediately after injection, we counted the nociception response for one hour, separated into blocks of 5 minutes each. As previously described, the nociceptive response is expressed as the total number of flinches and licks (3 seconds of licking is considered one flinch) [14].
Male rats were euthanized under deep isoflurane anesthesia followed by
decapitation. Thoracic and lumbar DRGs were harvested and transferred to Hank’s
buffered saline solution (H2387, Sigma Aldrich). DRGs were incubated in
0.28 U/mL collagenase (type II; #C6885, Sigma Aldrich) for 50 min,
followed by incubation with 0.25% trypsin (T0303, Sigma Aldrich) for
10 min. Enzymatic dissociation steps were carried out at 36 ℃. Ganglia were
washed three times with DMEM (Dulbecco
DRG cell cultures were loaded with the Ca
Data are expressed as mean
Panx1 peptide blocker (
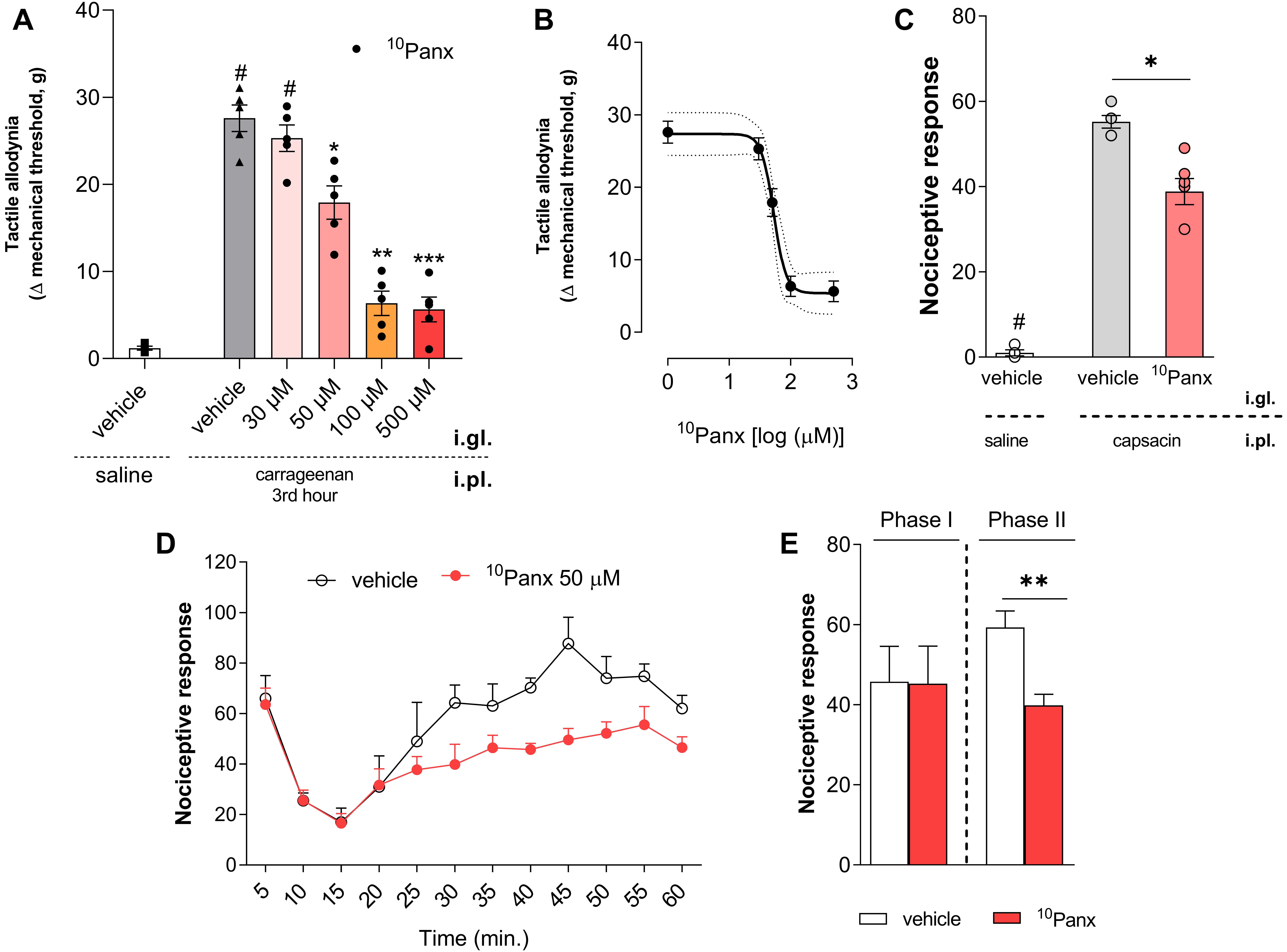 Fig. 1.
Fig. 1.Panx1 blockage in the L5-DRG of rats reduced inflammatory pain
after carrageenan and nociception response after formalin or capsaicin
injection. (A) Tactile allodynia of hind paw at the 3rd hour
after Cg (100 µg/paw); (data expressed as
In vitro capsaicin administration (5 µM) to DRG cell
cultures induced an immediate calcium influx in small diameter DRG neurons, as
indicated by the Fluo-4AM fluorescence intensity increase in these cells (Fig. 2A-I; Fig. 2A-II; Fig. 2B). Pre-incubation with Panx1 channel blocker
(
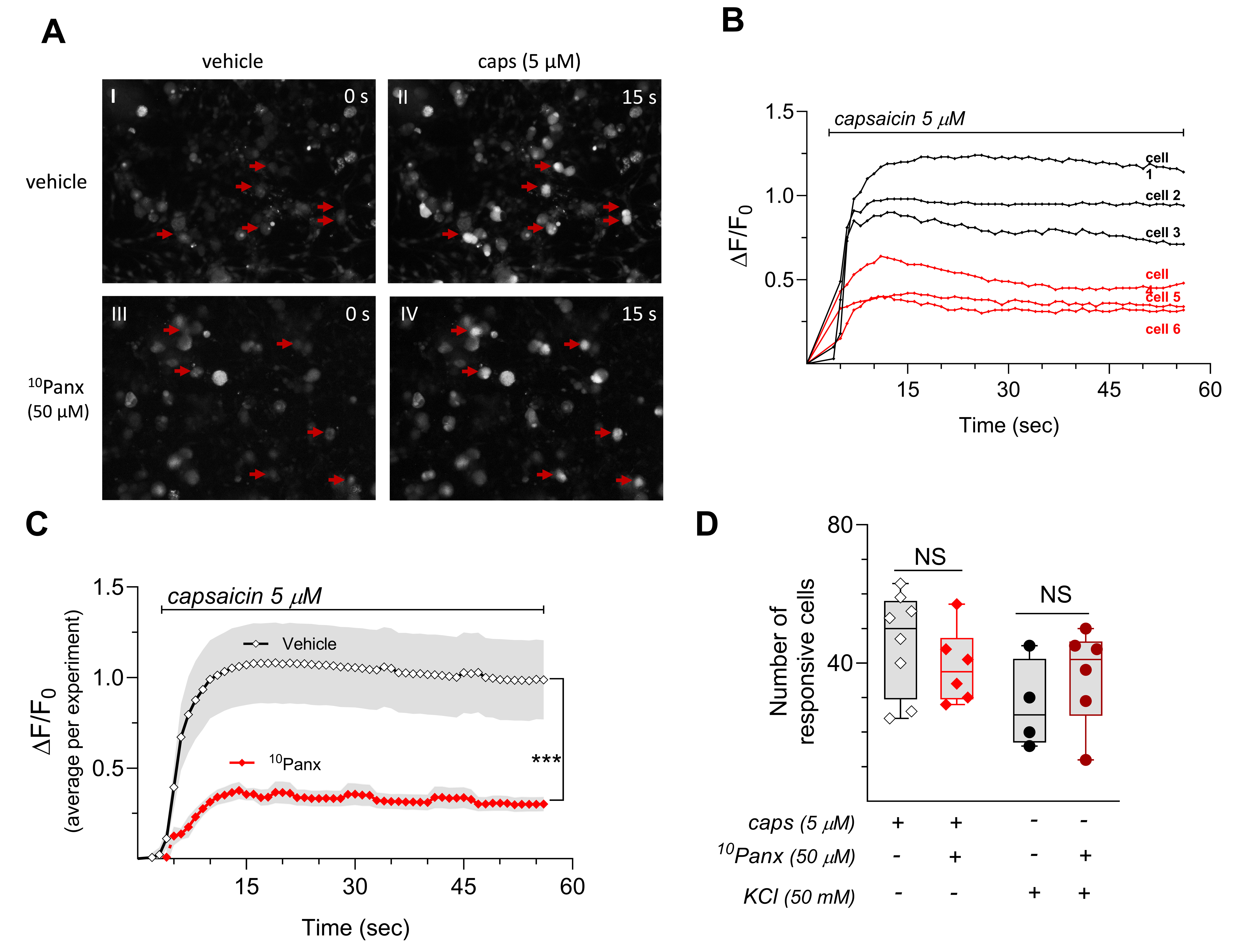 Fig. 2.
Fig. 2.Panx1 inhibition in DRG cell culture decreases calcium influx
induced by capsaicin in neurons. (A) Representative primary DRG cell culture
images before and after capsaicin and in two conditions. (I) Basal Fluo-4AM
fluorescence emission by neurons (red arrows) of a control dish (previously
incubated 30 minutes with Hank’s buffered saline solution). (II) Increased
fluorescence intensity in the same neurons from image (I), indicating increase in
intracellular calcium levels, at 15 seconds following capsaicin (5 µM)
administration. (III) Basal Fluo-4AM fluorescence emission by neurons (red
arrows) in culture pre-treated 30 minutes with
After verifying the role of Panx1 expressed in the primary afferent neuron of rats in inflammatory pain and nociception response, we performed pain behavior assessments in Panx1 global knockout mice (Panx1-KO), aiming to investigate whether non-neuronal cells (immune cells) that express Panx1 can also show a participation at pain phenotypes. Capsaicin (10 µg/paw) was administrated into the peripheral tissue of WT and KO mice via intraplantar injection. The number of flinches was recorded for 5 minutes in both groups. As shown in Fig. 3A, the nociceptive behavior induced by capsaicin in Panx1-KO mice was remarkably lower than observed in WT, with values similar to the control group which did not receive capsaicin.
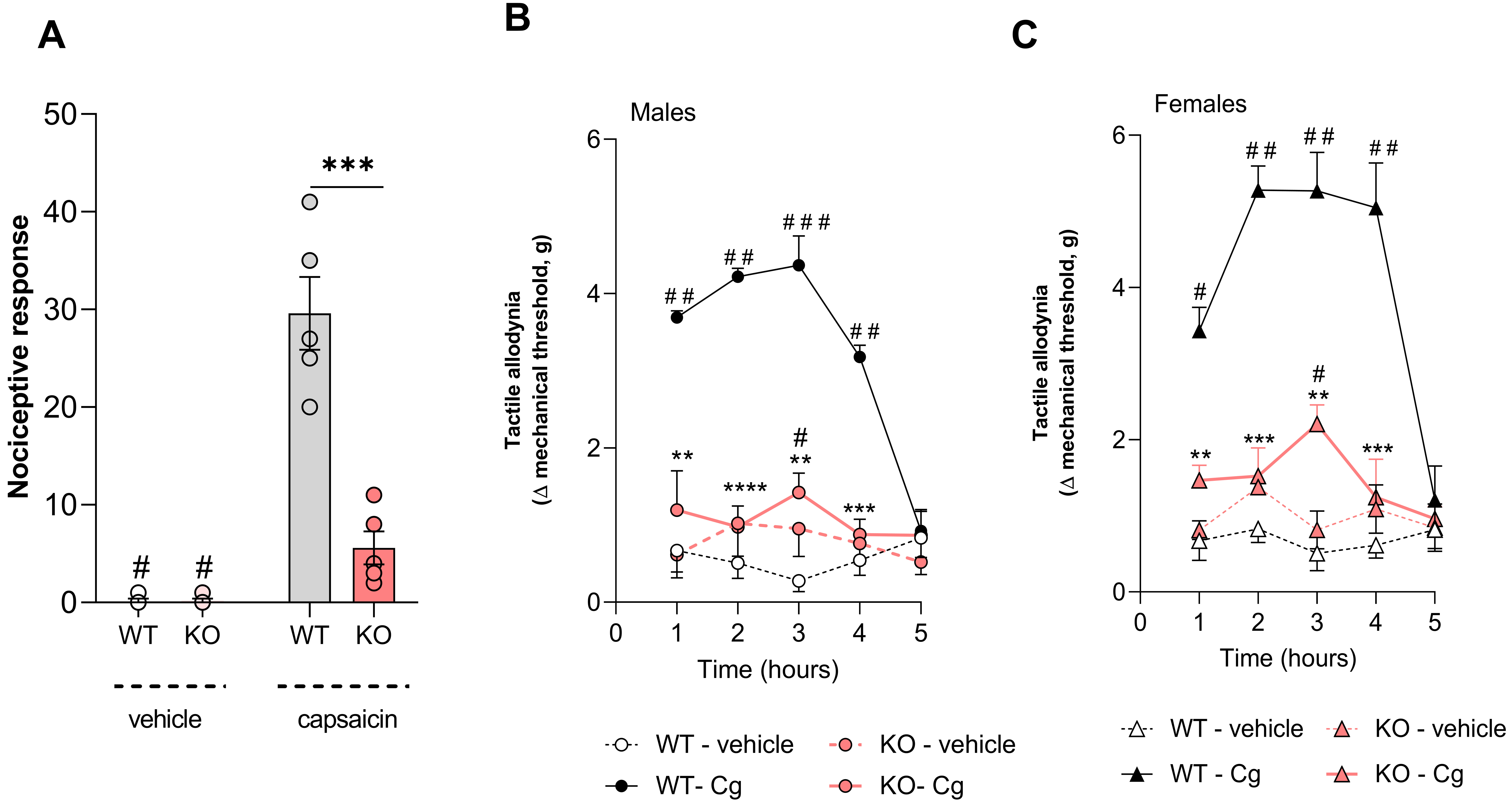 Fig. 3.
Fig. 3.Panx1-KO mice showed lower nociceptive response to capsaicin and
reduced Cg-induced mechanical allodynia. (A) Nociceptive response to
intraplantar injection of capsaicin (10 µg/paw) by WT and Panx1-KO mice.
(B) Tactile allodynia (data expressed as
The single dose of Cg (100 µg/paw) in WT mice induced mechanical allodynia in either male or females when compared to controls (saline) (Fig. 3B,C). The sensitization lasted up to 4 hours, returning to the baseline threshold 5 hours after injection. Male or female Panx1-KO mice Cg group (100 µg/paw) presented mechanical sensitivity only at the 3rd hour after injection, and in all other time points, no significant differences were detected between KO Cg-injected and its control group. Even though the KO-treated group showed significant allodynia at the 3rd hour, the intensity was significantly lower than the WT-treated group (Fig. 3B,C). Regarding the baseline tactile sensitivity between the strains, no significant difference was detected on von Frey between WT and Panx1-KO mice and between male and female at naïve conditions (data not shown).
Subsequently, we investigated the participation of Panx1 channels in
chemotherapy-induced peripheral neuropathy (CIPN). A single systemic injection of
paclitaxel (2 mg/kg; i.p.; Fig. 4) induced short-lasting mechanical allodynia in
male and female WT mice that resolved 7 hours after the injection (Fig. 4B,C).
WT-female mice presented higher intensity of allodynia than WT-male mice at the
five-hour time point (male mean: 2.265; female mean: 5.065, *p
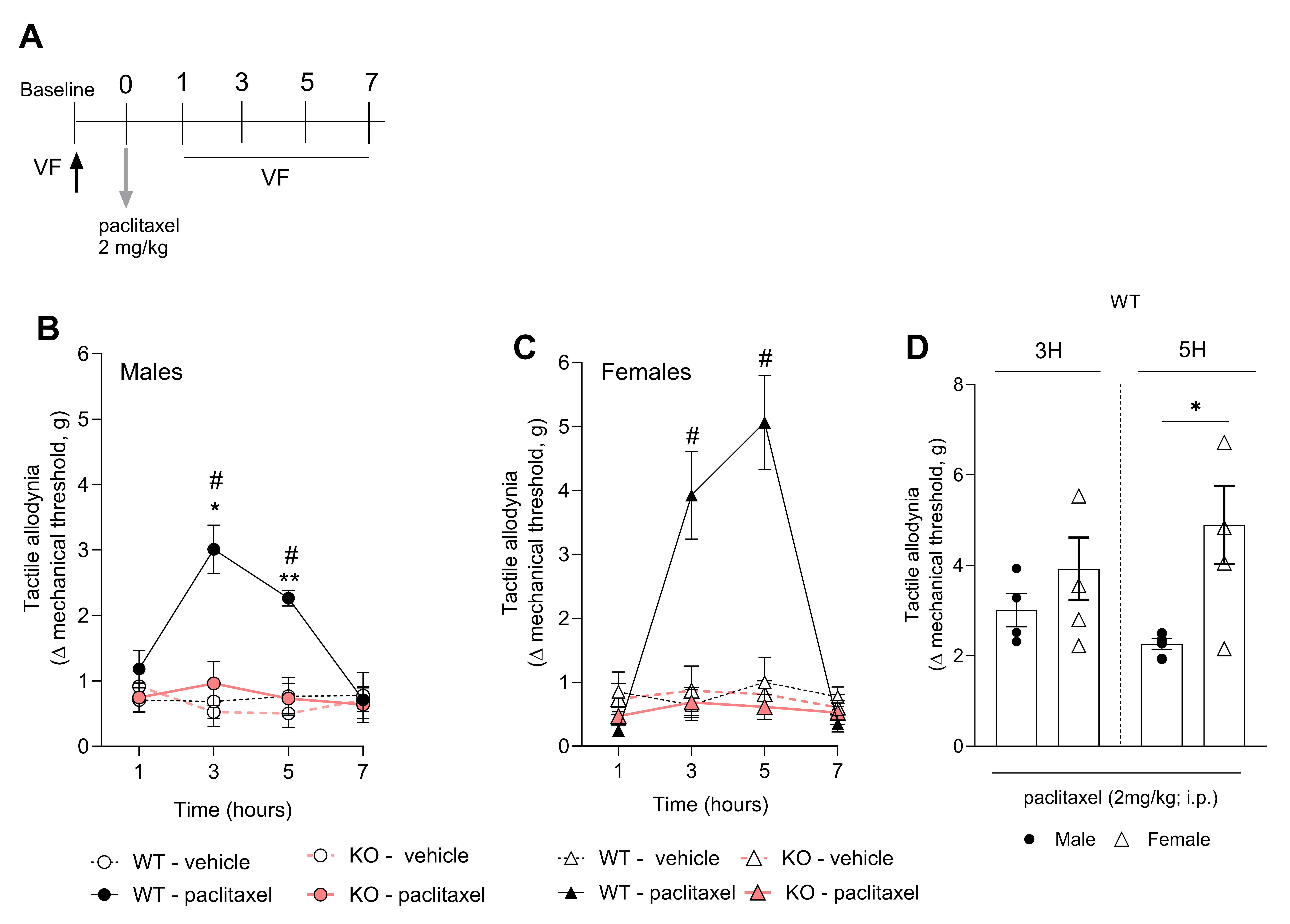 Fig. 4.
Fig. 4.A single dose of paclitaxel induced acute pain in WT mice. (A)
Experimental design of behavior assessment in mice, VF means von Frey test. (B)
Tactile allodynia (data expressed as
Knowing that paclitaxel has been applied for cancer patients’ treatment in more than one dose and frequently in women with ovary cancer [24], we performed four systemic administrations of paclitaxel in female WT and Panx1-KO mice (Fig. 5A). The injections of paclitaxel (2 mg/kg/i.p./injection; every other day) promoted chronic mechanical pain in both genotypes that lasted more than 21 days (Fig. 5B). After the first dose of paclitaxel, KO mice did not show mechanical allodynia; however, chronic pain was indeed developed from the second and onward doses. Even so, the intensity of allodynia until the 8th day was significantly lower in the Panx1-KO group compared to the WT-treated group (Fig. 5B), suggesting a partial role for Panx1 in mechanisms underlying the initial peripheral neuronal sensitization.
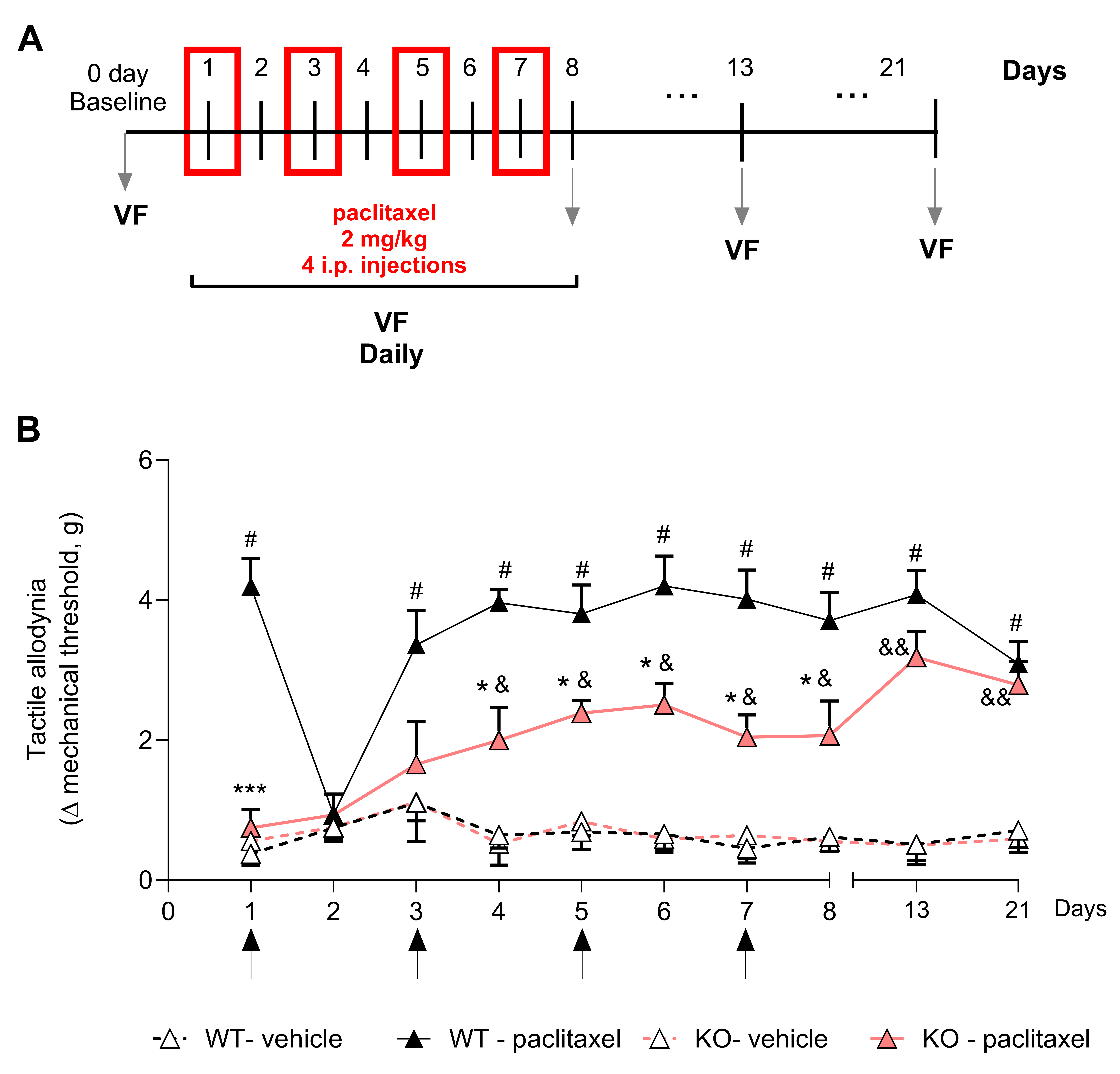 Fig. 5.
Fig. 5.Panx1-KO mice developed chronic Paclitaxel-induced Neuropathy
although with lower intensity in the initial phase. (A) Experimental design and
timeline: paclitaxel intraperitoneal injection were performed on days 1, 2, 5 and 7
and von Frey test on baseline and day 8 until 21, daily. (B) Tactile allodynia of
female mice on right paw along 21 days. From day 4 to day 8, KO-paclitaxel group was significant diffrent to WT-paclitaxel group (
The current study demonstrates that Panx1 in the DRG plays a critical role in
mechanisms of peripheral inflammatory pain and nociception transmission involving
activation of TRPV1
The mechanism(s) by which Panx1 acts on the peripheral nervous system to change
the nociceptor threshold directly or indirectly is still poorly understood. In
the periphery, these channels are present in the sensory ganglia and in different
compartments of nerves, such as axons and myelin [25]. Following nerve injury, an
upregulation of Panx1 protein expression in the DRG and Schwann cells was observed, which
was accompanied by mechanical and thermal sensitivity [11, 12]. The inflammatory
orofacial pain model induced by Complete Freund’s Adjuvant (CFA) injection into
the submandibular skin promoted an increase of Panx1 levels in trigeminal ganglia
that was responsible for the maintenance of a hyper-excitable state [7]. Here, we
performed subcutaneous administration of an inflammatory agent, carrageenan, in
rat and mouse paws to induce peripheral inflammation. Cg injection promotes local
interleukin 1 beta (IL-1
The formalin test presents a stereotyped behavior with two well-defined phases.
Phase 1 lasts
Studies that we conducted in vivo and in vitro using capsaicin
disclosed a potential functional interaction between Panx1 and TRPV1 channels in
peripheral mechanisms of pain modulation. Capsaicin stimulates nociceptive
neurons by activating the TRPV1 channel, a cationic channel selectively expressed
in nociceptive c-fiber [34]. Activation of TRPV1 induces acute nociception
initially by mediating Na
Different Panx1 blockers, such as Carbenoxolone, Probenecid, Mefloquine, and
Brilliant Blue dye, were previously used in other pain studies; however,
according to recent works, some do not show selectivity; Carbenoxolone can also
block gap junctions. For this reason,
In the CIPN model, we observed that Panx1 channels are not required for the chronic establishment of paclitaxel-induced neuropathic pain. However, Panx1-KO-treated mice displayed a two-day delay in the development and reduced mechanical allodynia up to 8 days post-treatment compared to WT-treated mice. Strikingly, one single injection of paclitaxel did not induce acute mechanical pain in Panx1-KO mice. These results suggest that Panx1 plays a partial role in the initial phase of neuronal sensitization in the paclitaxel model. However, other elements begin to emerge and contribute to establishing chronic pain. Nevertheless, in the spared nerve injury (SNI) model, global Panx1-KO mice did not develop chronic pain [36] and intra-sciatic injection of Panx1 blocker ten days after chronic constriction injury (CCI) effectively reduced mechanical and heat hypersensitivity [12]. Although it seems contradictory to our results, the neuronal sensitization process caused by chemotherapy drugs might involve different mechanisms compared to a surgical model. Previous studies revealed that paclitaxel causes deficits in mitochondrial bioenergetics, macrophage infiltration, and increased sodium channels in DRG [21, 37, 38]. More recently, there have been reports of increased Toll-like receptor 4 (TLR4), lipid rafts, and TRPV1 protein expression in the DRG after treating male mice with paclitaxel [39]. All these factors may explain why the absence of functional Panx1 receptor expression did not prevent chronic neuropathic pain induced by paclitaxel. Yet, a group of researchers observed an increase of glutamate release in the cerebrocortical synaptosomes of Oxaliplatin-treated rats which was mediated by functional recruitment of Panx1-P2X7R. By using Panx1 inhibitors they demonstrated reversal on neuropathic pain induced by oxaliplatin [40].
Another aspect that can be involved in Paclitaxel-induced neuropathy is the type of nociceptors. A study revealed that Paclitaxel induces A-fiber hypersensitization, contributing to mechanical allodynia [41]. In our data, Panx1 is shown to be associated with TRPV1 receptors, which are expressed in c-fibers neurons. This can be the reason that Panx-1 KO mice stayed allodynic after four injections of Paclitaxel, as WT mice.
It is important to mention that studies involving genetically modified animals can present some limitations. In Panx1-KO mice, the mRNA expression that leads to Panx1 protein translation is mostly but not completely abolished in different cell types and tissue. Thus, they are considered functional knockout [18]. Also, there might be compensatory effects on the expression and/or function of non-depleted channels, which can result in modifications of animal physiology. The sensory system is not excluded from these changes. In animals with genetic deletion for Panx1, it was seen an auditory sensory loss [42] and an increase in the expression of Panx3channels in sensory nerve fibers of the nasal epithelial layer [43]. However, no study has yet shown changes in receptor expression in nociceptive neurons of Panx1-KO mice. Besides that, we observed here a deficient spontaneous pain response (acute nociception) in Panx1-KO mice at the capsaicin test compared to WT group, indicating a potential modification of TRPV1 protein expression or activity in these genetically modified mice.
The data presented in this work brings attention to the critical role of Panx1 channels in developing peripheral inflammatory pain and acute nociception involving TRPV1 activation and the lack of involvement of these channels in CIPN induced by paclitaxel. Considering the DRG is located outside of the blood-brain barrier, targeting Panx1 channels expressed in the DRG may provide a novel and exciting approach to inflammatory pain treatment.
All data points generated or analyzed during this study are included in this article and there are no further underlying data necessary to reproduce the results
Conceived and designed the experiments: JBPL, CAP, CMdCL, SOS. Performed the experiments: JBPL, KFM, NSC, AFN, MU-M, CMN. Analyzed the data: JBPL, KFM, AFN, CCF, PRGK. Wrote the paper: JBPL, KFM, CCF. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
All experimental protocols were approved by the Albert Einstein College of Medicine Animal Care and Use Committee (IACUC) (protocol number 00001131) and by the Committee on Animal Research CEUA-UNICAMP (protocol number 4566-1/2017, 4566-1(A)/2017).
Not applicable.
This study was supported by São Paulo Research Foundation (FAPESP) grant #2017/19105-8; #2019/15255-0 and by the Coordination for the Improvement of Higher Education Personnel (CAPES – Brazil), Finance Code 001.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.





