1 Department of Systemic Rheumatic Diseases, V.A. Nasonova Research Institute of Rheumatology, 115522 Moscow, Russian Federation
2 Department of Organization and Economics of Pharmacy, Federal State Autonomous Educational Institution of Higher Education I.M. Sechenov First Moscow State Medical University of the Ministry of Health of the Russian Federation (Sechenov University), 119991 Moscow, Russian Federation
Abstract
Systemic sclerosis (SSc) is a rare systemic autoimmune disease of unknown etiology, which is characterized by endothelial dysfunction, pathologic vasculopathy, and increased tissue fibrosis. Traditionally, SSc has been regarded as a prototypical fibrotic disease in the family of systemic autoimmune diseases. Traditionally, emphasis has been placed on the three components of the pathogenesis of SSc: vascular, immune, and mesenchymal. Microvascular lesions, including endothelial dysfunction and smooth muscle cell migration into the intima of vessels in SSc, resemble the atherosclerotic process. Although microvascular disease is a hallmark of SSc, understanding the role of atherosclerotic vascular lesions in patients with SSc remains limited. It is still unknown whether the increased cardiovascular risk in SSc is related to specific cardiac complications (such as myocardial fibrosis) or the accelerated development of atherosclerosis. Different immune cell types appear to be involved in the immunopathogenesis of SSc via the activation of other immune cells, fibrosis, or vascular damage. Macrophages, B cells, T cells, dendritic cells, neutrophils, and endothelial cells have been reported to play the most important role in the pathogenesis of SSc and atherosclerosis. In our article, we reviewed the most significant and recent studies on the pathogenetic links between the development of SSc and the atherosclerotic process.
Keywords
- systemic sclerosis
- inflammation
- atherosclerosis
- macrophages
- B cells
- T cells
- endothelial cells
Chronic inflammation is associated with the uncontrolled activation of innate and acquired immunity and, according to modern concepts, plays a fundamental role in all developmental stages of autoimmune rheumatic diseases (ARDs) and atherosclerosis, which recently have also been attributed to autoimmune diseases. Numerous immune cells and their mediators are involved in the immunopathogenesis of ARDs. Modern researchers consider the imbalance between them as one of the causes of atherosclerosis development.
The review aimed to summarize the new data on cellular mechanisms of inflammation discovered within the last five years and to integrate them into the pathogenesis of the human generalized fibrosis model of systemic sclerosis (SSс) and atherosclerosis. Literature reporting the risk of atherosclerosis in SSс patients was searched for in MEDLINE (through PubMed) and Google Scholar databases using a combination of keywords and medical subject headings (MeSH). The keywords were “systemic sclerosis and atherosclerosis”, “macrophages in systemic sclerosis”, and “immune cells in systemic sclerosis”. Nineteen articles were found that reported the comorbidity of atherosclerosis in SSc patients. Based on the analysis of these articles, we confirmed that SSc is associated with an increased risk of various cardiovascular events.
The study of the proinflammatory response by immune cells and endothelial cells (ECs) associated with the clinical activity of SSc and immunologic markers of inflammation may provide crucial information on the involvement of these cells in the development of ARDs, as well as the participation of the immune inflammatory system in the accelerated development of atherosclerosis in patients with an ARDs.
SSс is a rare ARDs, which is characterized by endothelial dysfunction, increased tissue fibrosis, and hyperproduction of autoantibodies. According to recent reviews, the overall prevalence of SSc is 17.6 (95% confidence interval (CI) 15.1; 20.5) per 100,000 in the population, and the overall incidence of SSc is 1.4 (95% CI 1.1; 1.9) per 100,000 person–years [1]. A wide range of different autoantibodies have been detected in SSс patients. Specifically, these autoantibodies are involved in its pathogenesis; thus, they are included in the SSc classification criteria. There are two main subtypes of the disease: Diffuse, which is associated with anti-topoisomerase I antibodies through evidence of interstitial lung disease (ILD); diffused skin lesions (extremities proximal to the elbows, knees, chest, abdomen, and back), and renal vascular damage; limited, which is associated with anti-centromere antibodies, sclerodactyly and Raynaud’s phenomena, esophageal dysmotility, telangiectasia, calcinosis, limited skin involvement (face, extremities distal to elbows and knees), and high risk of pulmonary arterial hypertension.
Microcirculatory disturbances are one of the earliest signs of SSс. They precede and potentially contribute to widespread fibrosis through tissue ischemia. The pathogenesis of vasculopathy is not fully understood, yet immune reactions to environmental factors, viruses, reperfusion injury, and anti-endothelial antibodies may promote the development of the pathologic process [2]. Endothelial dysfunction plays a central role in the pathogenesis of SSс. Vascular injury contributes to endothelial activation, maintenance of inflammation through innate and adaptive immune responses, vascular remodeling, and fibrotic lesions of visceral organs. Microvascular disorders underlie the pathogenesis of digital ulcers, Raynaud’s phenomenon, pulmonary arterial hypertension, and scleroderma renal crisis [3].
Whether SSc accelerates the development of atherosclerosis remains controversial. A systematic review through a meta-analysis confirmed an increased incidence of coronary atherosclerosis, peripheral vascular disease, and cerebrovascular calcification in patients with SSc compared with healthy individuals [4, 5]. Both subtypes of SSc affect the cardiovascular system. Moreover, the disease duration and renal damage occurring in SSc are independent risk factors for coronary heart disease [6]. While microvascular functional and structural changes are the hallmarks of vasculopathy associated with SSc, macrovascular atherosclerotic disease significantly worsens the disease prognosis and causes mortality in 20–40% of SSc cases [7]. Thickening of the intima–media complex in the common carotid artery is detected in 64–65% of SSc patients [8].
However, even in the absence of intima–media complex thickening in the carotid arteries, atherosclerotic plaques are detected in patients with SSc, with such patients exhibiting a higher relative risk of cardiovascular disease [9]. SSc is associated with an increased risk of myocardial infarction, stroke, and peripheral arterial disease, although the pathogenetic mechanisms of these disorders are not fully understood [1, 10].
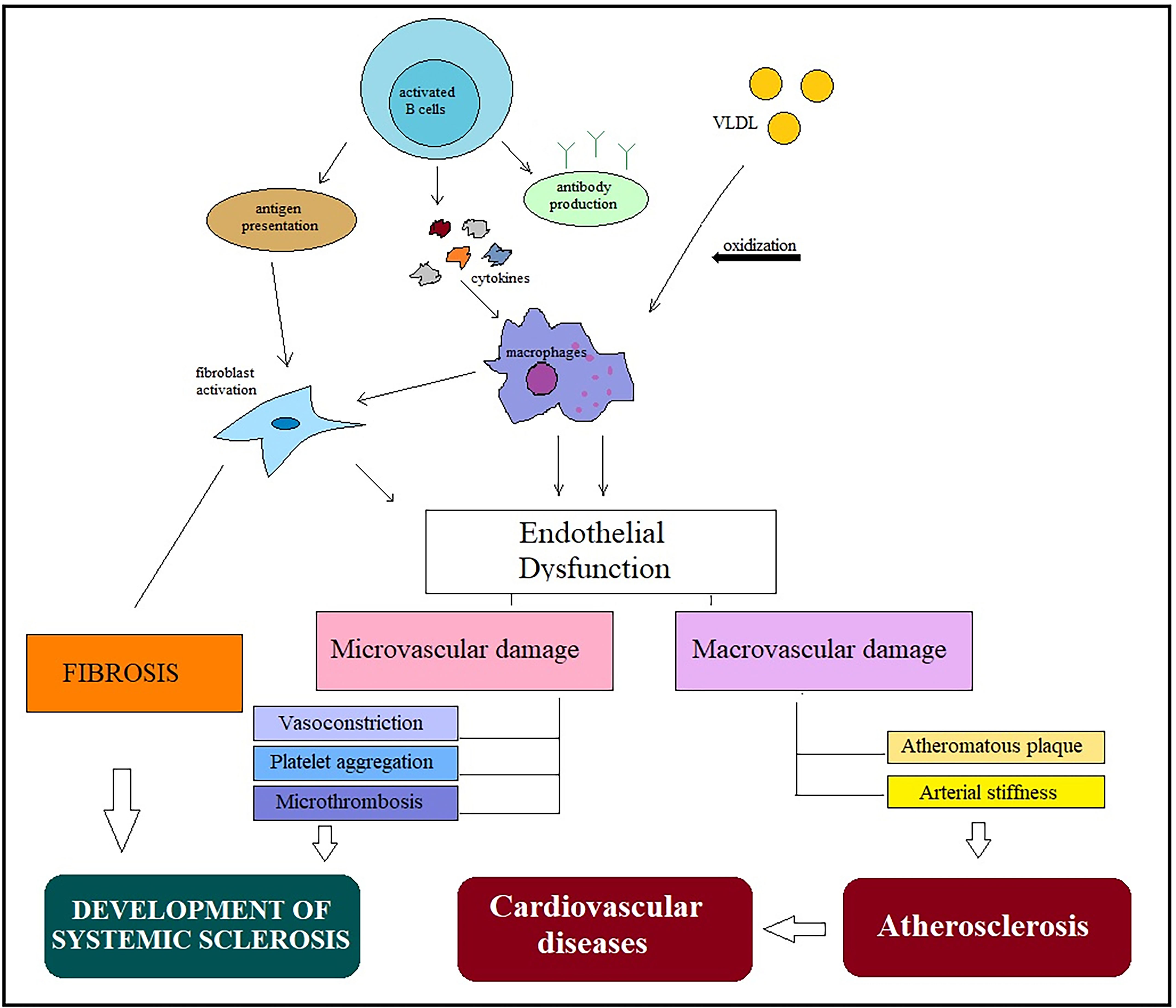
Defects in the coronary microcirculation are pathognomonic in patients with SSc and are aggravated by vasospasm and fibrotic changes. In addition to the high incidence of atherosclerotic lesions in coronary arteries, patients with SSc may develop coronary spasms (Raynaud’s cardiac syndrome); these are particularly common in SSc patients with coronary thrombosis. Microvascular lesions occurring in SSc, including endothelial damage and smooth muscle cell migration into the intima of vessels, have some similarities with the atherosclerotic process [11]. Endothelial dysfunction plays a key role in both SSc and atherosclerosis (Fig. 1).
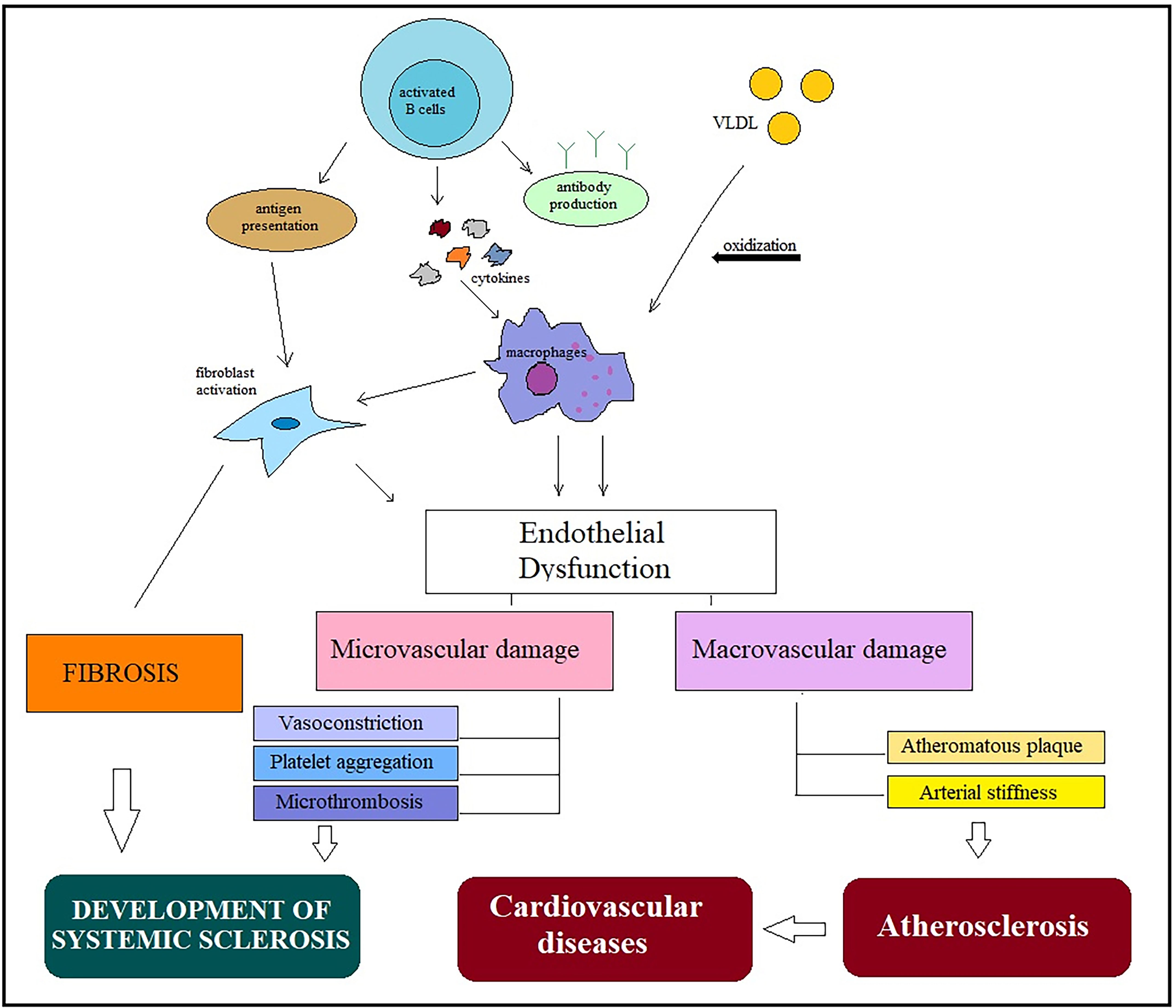 Fig. 1.
Fig. 1.General mechanisms in the development of systemic scleroderma and atherosclerosis. Activation of immune cells in different pathways leads to endothelial dysfunction and low-grade inflammation. Macrophage activation plays a key role in the pathogenesis of both diseases and is involved in the occurrence of micro- and macrovascular damage. Microvascular abnormalities, including vasoconstriction, platelet aggregation, and microthrombosis, are involved in SSc development, while macrovascular damage leads to increased arterial stiffness and progression of atherosclerotic plaques, accelerating the development of cardiovascular disease. Abbreviations: VLDL, very-low-density lipoprotein; SSc, systemic sclerosis.
The increased prevalence of macrovascular disease and the presence of early atherosclerosis in patients with SSс have been demonstrated in several studies [10, 12]. In a recent prospective study, C. Cassius et al. [12]reported that 76% and 28% of patients with SSc had hemodynamic abnormalities and increased vascular stiffness of lower limb arteries, respectively.
Abnormal activation of immune cells indicates the autoimmune nature of the disease [13].
The development of microvascular lesions in SSс is based on endothelial damage and migration of smooth muscle cells into the intima of vessels, which has certain similarities with the atherosclerotic process [14].
According to modern concepts, uncontrolled activation of innate and acquired immunity leads to the development and maintenance of chronic inflammation, which plays a vital role in all stages of SSc and atherosclerosis and was recently classified as an autoimmune disease.
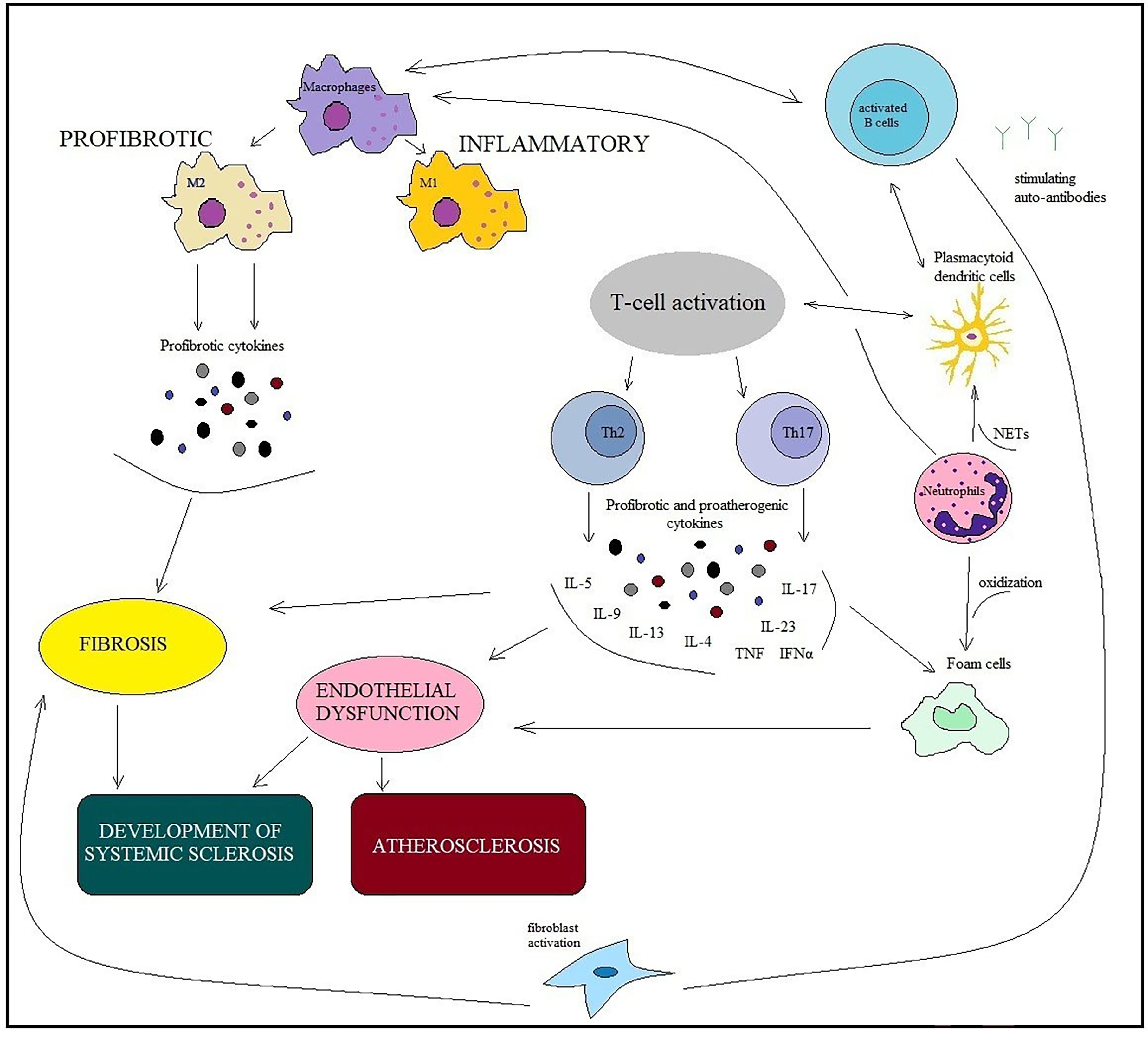
Immune cells and inflammation have been found to play a crucial role in the pathogenesis of SSс and atherosclerosis [15, 16]. Inflammation releases many cytokines, which interfere with the action of nitric oxide and lead to vasoconstriction. Immune disorders in SSс are characterized by the activation and recruitment of immune cells, the production of autoantibodies, and profibrotic cytokines. The appearance of immunocytes and their production of proinflammatory cytokines and chemokines can enhance plaque progression and promote plaque rupturing [17, 18] (Fig. 2).
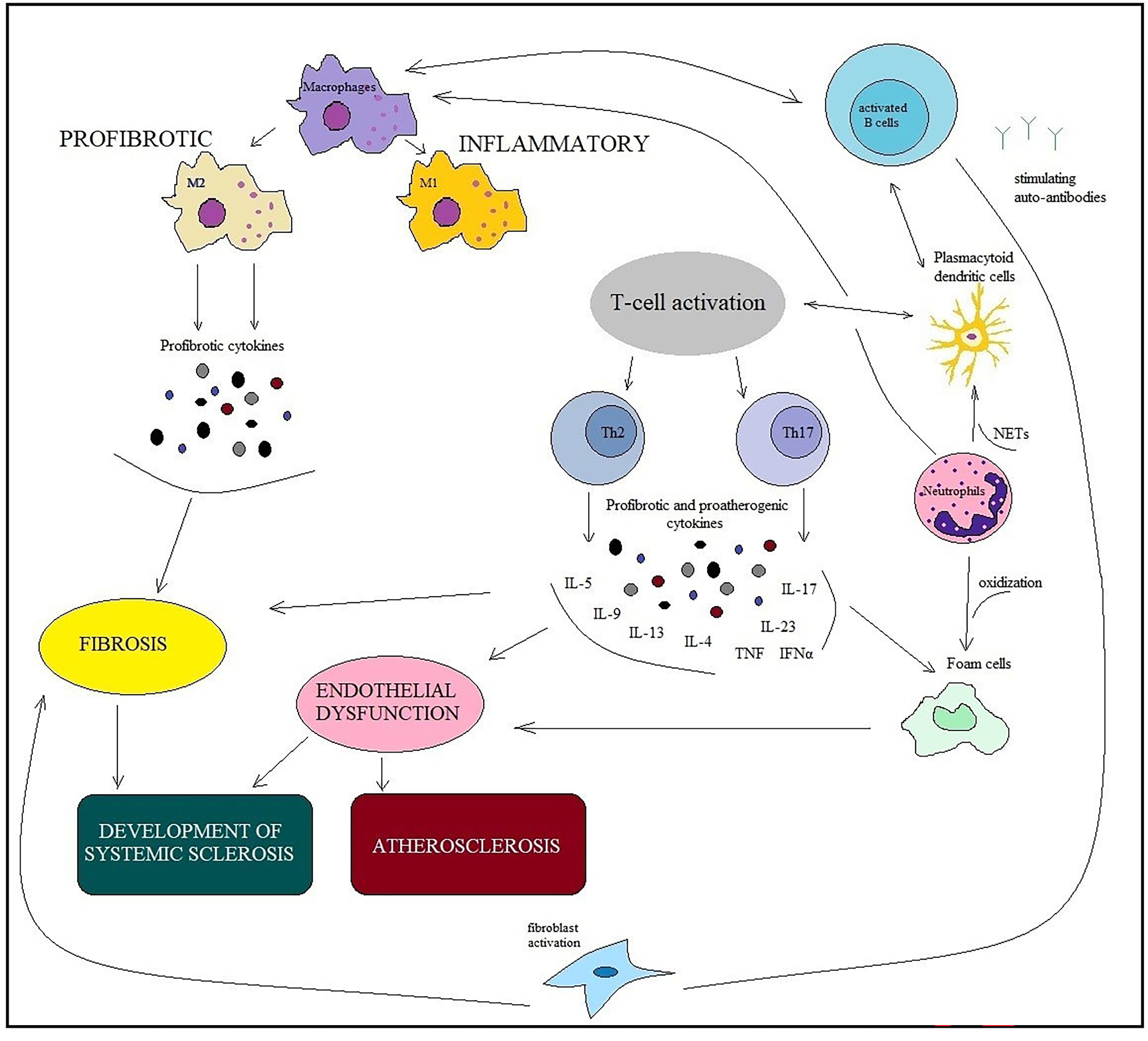 Fig. 2.
Fig. 2.Similarities of immunopathogenesis mechanisms in ARDs and
chronic low-grade inflammation in atherosclerosis. Involvement of immune cells
(macrophages, B and T cells, dendritic cells, neutrophils, fibroblasts, and foam
cells) in developing SSc and atherosclerosis. The main point is macrophage
dysfunction, which is inextricably linked with the dysfunction of other cells and
is involved in the pathological processes of both SSс and
atherosclerosis. Macrophages are affected by cytokines produced by B cells, T
cells, neutrophils, and other innate immune cells. M1 macrophages support
inflammation, whereas M2 macrophages produce profibrotic cytokines. The
pathogeneses of SSc and atherosclerosis involve the same cytokines: IL 4, IL 6,
IL 13, TGF-
Among the causes of abnormal immune cell functions, the role of mitochondria has been discussed. Evidence is accumulating that mitochondria are a key link in the development of inflammation and cell destruction [19]. Mitochondrial dysfunction in immunocompetent cells due to mitochondrial deoxyribonucleic acid (DNA) mutations can lead to the non-stop activation of monocytes.
The produced macrophage monocytes and cytokines deserve special attention among many immune cells and mediators involved in the pathogenesis of autoimmune diseases. Macrophages are one of the leading links in the pathophysiology of atherosclerosis.
In patients with atherosclerosis and animal models, the number of circulating monocytes is related to the stage and size of the atherosclerotic plaque [20, 21]. Monocytes can further differentiate into macrophages, which, after lipid accumulation, become foamy cells [14].
An imbalance between macrophage phenotypes is considered to be a reason that SSc develops. Macrophages are important in the immunopathogenesis of systemic lupus erythematosus (SLE) since they produce cytokines that support inflammation by recruiting new immune cells (monocytes, neutrophils), polarizing T cells, and activating fibroblasts [16].
Activated macrophages are classified as M1 and M2 depending on the nature of the inflammatory response. M1 macrophages contribute to the development of inflammation, while M2 macrophages promote tissue repair by producing profibrotic cytokines [22].
There is strong evidence that abnormal macrophage activation is involved in developing SSс [23]. Since the 1990s, many studies have established the presence of monocyte/macrophage activation in SSс. High numbers of macrophages were observed in the skin of SSс patients. At the same time, cells positive for cluster of differentiation 163 (CD163), a possible M2 macrophage marker, were found in the serum of SSc patients [24]. A recent study showed that the level of interleukin (IL)-34 in serum is elevated in SSc [25].
IL-34 promotes the survival and proliferation of monocytes and their
differentiation into profibrotic macrophages [26]. A number of studies have shown
increased expression of monocyte/macrophage-related genes (e.g., IL-8, vascular
endothelial growth factor (VEGF), and epiregulin) in mononuclear cells in
patients with SSc [24]. Macrophages are also a major source of transforming
growth factor-beta (TGF-
Sequencing of the skin transcriptome has shown that M2-type macrophages are
involved in molecular processes in the skin of SSc patients, leading to the
activation of IFN, adaptive immunity, extracellular matrix remodeling, and cell
proliferation [30]. The cytokines IL-4 and IL-13 are involved and essential in
the pathogenesis of fibrotic disorders [31]. In unchanged fibroblasts, IL-4
promotes proliferation, chemotaxis, and collagen synthesis, increasing
TGF-
Previous studies have shown that profibrotic phenotypic M2 macrophages are found in the skin and peripheral blood of SSc patients and contribute to developing ILD in SSc [25, 33, 34].
Interestingly, the gene expression profile of profibrotic macrophages differed between skin and lung. This suggests that although the role of macrophages in the immunopathogenesis of fibrosis in both skin and lung in SSc seems similar, there may still be differences [35].
Recent studies in a mouse model have found that skin fibrosis with knockout of
the conditional regulatory factor IFN 8 (specific for myeloid cells) leads to
increased mRNA levels of extracellular matrix components and enhanced
bleomycin-induced skin fibrosis [36]. Higher circulating mixed M1/M2
monocytes/macrophages levels are associated with ILD, systolic pressure in the
pulmonary artery, and positive antibodies for topoisomerase I [32]. Notably,
single-cell ribonucleic acid sequencing identified the presence of secreted
phosphoprotein 1+ (SPP1+), pulmonary macrophages, or Fc
D. Toledo and P. Pioli [38] presented an important model of SSс
pathogenesis involving macrophages. Here, activation of the Wnt, JAK/STAT, and
Sonic hedgehog (SH) cell differentiation signaling pathways under the influence
of genetic and environmental factors led to the increased expression of fibrosis
mediators, including TGF-
Recently, several studies have focused on the possible activation of the coagulation pathway in SSc and its influence on the inflammatory and fibrotic response through the involvement of PDGF and protease-activated receptor 1 (PAR-1) [39, 40, 41]. PDGF is a well-described mediator of fibrosis, whose individual epitopes, stimulated by autoantibodies in SSc, cause activation of intracellular signaling and collagen gene overexpression [40]. The IgG antibody fraction detected in patients with SSc modulates signaling through PAR-1 and affects IL-6 secretion by human endothelial cells (ECs) [41].
B- and T- cells interactions are essential in adaptive immune responses and
significantly impact the physiopathology of autoimmune diseases. Cellular
immunity requires the activation of B cells, which occurs due to the increased
CD19
Various cells with innate or adaptive immunity, which have been infiltrated by B- and plasma cells, have been identified in the skin, lungs, and gastrointestinal tract of SSс patients [42, 43, 44, 45, 46]. B cells are involved in various regulatory immune processes in SSс, such as antigen presentation, cytokine synthesis, T cell development and differentiation, and structural disorganization of lymphoid organs [46]. The upregulation of costimulatory molecules CD80/86–CD28 contributes to the activation of autoreactive T cells and the increased secretion of profibrotic cytokines in SSс [47].
At the same time, the possibility of B cells generating IL-10, which is involved in the suppression of regulatory B cells (Breg), changes [48]. B cells express many molecules on their surfaces, which are involved in the activation or surviving pathways while also reducing levels of co-receptor inhibitors. They also express the co-receptors necessary for interference with T cells. B cells react with different immune cells (macrophages, fibroblasts, and ECs) through direct and indirect exposure. Moreover, they all participate in profibrotic and proinflammatory processes, vascular remodeling, and impaired immune regulation [46].
B effector cells stimulate macrophages by synthesizing the
granulocyte–macrophage colony-stimulating factor (GM-CSF), causing inflammatory
and fibrotic lesions. These cells belong to the memory B cell subgroup and
actively produce IL-6 and tumor necrosis factor-alpha (TNF-
T cells are apparently able to influence the autoimmune response through the
co-stimulatory activity of B cells in the interaction of B–T cells [51]. The
importance of CD4
CD8
An important pathologic aspect of IL-34-induced macrophages is their participation in transforming memory T cells into Th17 cells [26]. Indeed, increased amounts are found in the skin and peripheral blood of patients with high SSс activity [55]. In addition, Th17 cytokine levels of IL-17 and IL-23 in the peripheral blood and exhaled air condensate are associated with ILD severity in SSc patients [56]. At the same time, Th1 and Th17 cells have a stimulatory effect on the formation of atherosclerosis. However, Treg also performs a protective function in the process of atherosclerosis. Thus, the ratio of helper Th17 cells to Treg cells plays an important role in the formation of atherosclerosis.
Lipid deposition in the arterial wall is the initiating event in atherosclerosis. Lipids, under the action of oxidative enzymes produced by cells in the vascular wall, subsequently become oxidation-specific epitopes (OSEs), such as ECs and smooth muscle cells. OSEs can activate vascular cells and produce adhesion molecules, cytokines, and chemokines, which subsequently attract circulating monocytes and T cells to the vessel wall [18]. More recent studies have shown that antibodies to OSEs can inhibit the uptake of oxidized-low-density lipoprotein (OxLDL), which supports the hypothesis that the B-cell component has a functional role in the development of atherosclerosis [57]. Neoantigens, including OSEs, can interact with Toll-like receptors (TLRs), further enhancing the inflammatory response in macrophages and T cells [58].
The importance of the B cell response in atherogenesis is emphasized by genome-wide association and transcriptomic data, indicating the status of B cell activation and proliferation as significant risk factors for cardiovascular disease [59]. Antigen-presenting B cells enter into antigen-specific interactions with T cells through signals transmitted by co-stimulatory molecules such as CD40, CD80, and CD86, which are recognized by peptides in the major histocompatibility complex (MHC). Therefore, T cell activation occurs through an interaction with the T cell receptor, whereby the antigen-presenting cell presents the MHC class II and antigen.
In recent years, an important role for B cells has been shown in atherosclerosis
[60]. Available studies emphasize the strong specific effects of B1, follicular,
and marginal zone B cells, Breg, and innate response activator B cells [42].
Inflammation further stimulates the recruitment of B cells to atherosclerotic
plaques and leads to the subsequent formation in vessels of arterial tertiary
lymphoid tissues. A recent study showed that CD19
Different types of chemokines and chemokine receptors, such as C-X-C motif ligand (CXCL)13/C-X-C motif receptor (CXCR)5, C-C motif ligand (CCL)19/CCL21/C-C motif receptor (CCR)7, allow for the direct migration of B cells into lymphoid and non-lymphoid tissues, supporting the return of B cells to lymphoid structures. CXCL13 and CCL21 have been identified as chemokines, which are critical for the presence of B cells in adventitial tertiary lymphoid organs [62]. Recently, an additional role of CCR6 was discovered in the recruitment of B cells to the atherosclerosis-prone aorta under the control inhibitor of differentiation-3 [63].
Dendritic cells (DCs) are predominantly involved in T cell activation. Thus,
they are closely involved in the low-grade inflammation in both SSс
and atherosclerosis. DCs are involved in major events in the pathogenesis of SSc.
The maturation of DCs is induced by activated B cells, CD83, CD80, CD86, CD40,
and HLA-DR, via the B cell receptor and B cell activation factor receptor (BAFF).
B cell-matured DCs have a secondary effect on the polarization and activation of
naive CD4
There are two major types of DCs: Conventional and plasmacytoid. Plasmacytoid
DCs (pDCs) are specialized cells whose activation increases IFN production. A
recent study showed that activation of pDCs, which infiltrates the skin of SSc
patients, is accompanied by the production of large amounts of IFN-
Much earlier, the role of pDCs was studied in atherosclerotic vascular lesions.
It is known that the function of pDC is impaired in atherosclerosis. Moreover,
higher expression of CD83, a marker of DCs activation, has been demonstrated in
plaque tissue from patients with ischemic complications [70]. In humans,
pDC-mediated IFN-
In the last decade of research, neutrophils have been shown to accelerate atherosclerosis development and to be actively involved in inflammation and cardiovascular repair [72]. Dysregulation of cholesterol and glucose metabolism in cardiometabolic diseases determines the chronic nature of inflammation, while neutrophils activate inflammatory pathways.
Accumulation of immune cells and lipoproteins in the arterial intima promotes resorption and instability of atherosclerotic plaques. Disrupting the efflux of cholesterol into neutrophils can also enhance inflammatory activation and promote neutrophil infiltration and the release of neutrophil extracellular traps (NETs) in atherosclerotic lesions, ultimately, accelerating atherosclerotic lesion formation [73]. NETs are net-like structures composed of DNA–histone complexes and proteins (histones, enzymes, and other antimicrobial proteins) released by activated neutrophils. Activated neutrophils release granular proteins, including cathelicidin and cathepsin G, which directly or indirectly promote myeloid cell recruitment. In addition to their key role in the neutrophil innate immune response, NETs are also involved in the development of autoimmune diseases.
The occurrence of oxidative stress is associated with the pathogenesis of SSс. Polymorphonuclear neutrophils (PNN) generate a significant amount of reactive oxygen species (ROS) during the development of the disease [74]. Neutrophils from SSс patients lack several effector molecules and effector properties, including key chemokine receptors, the formation of NETs, and phagocytosis of bacterial particles [75]. Neutrophil deficiency in SSс patients may be accompanied by impaired tissue remodeling, inflammation, and neutrophil antimicrobial defenses [75]. Neutrophils are characterized by many phenotypic changes and functional impairments. Neutrophils in SSс patients are characterized by increased phosphorylation of signal transducer and activator of transcription 3 (STAT3) and STAT6 proteins, decreased expression of CD16 and CD62L on their surface, and a number of functional abnormalities, including the absence of typical chemokine receptors CXCR1 and CXCR2, which regulate migration to the strong neutrophil chemoattractant CXCL8 [75].
Markers of neutrophil activation, such as increased levels of calprotectin in
bronchoalveolar lavage fluid [76] and serum [77], are associated with increased
pulmonary fibrosis and positive antitopoisomerase I tests in patients with SSc.
R. Kuley et al. [78] showed increased calprotectin levels in SSc
patients with vascular-related manifestations, such as cutaneous pitting scars.
However, this study found no association between autoantibodies, intracellular
antigens, and circulating NET levels. At the same time, neutrophil activation
upon stimulation with blood plasma of SSc patients was terminated by blocking the
binding sites of immune complexes on Fc
During the progression of atherosclerosis, the neutrophil granular proteins
cathelicidin and
Damage to ECs and apoptosis in the early stages of SSc can lead to perivascular inflammation, oxidative stress, and tissue hypoxia. Several clinical symptoms that are characteristic of the disease (Raynaud’s phenomenon, hand edema, digital ulcers, pulmonary hypertension, erectile dysfunction, scleroderma renal crisis, and cardiac damage) are primarily related to EC dysfunction [3, 80]. Activation of ECs develops under the influence of cell adhesion molecules: E-selectin, vascular cell adhesion molecule 1 (VCAM-1), and intercellular adhesion molecule 1 (ICAM-1). Increased expression of these molecules on the endothelium may lead to their transfer into the bloodstream. Moreover, increased levels of circulating soluble adhesion molecules are associated with peripheral microvascular abnormalities in SSс [81, 82] and the development of atherosclerotic plaques from neovascularization to fibrotic plaques in the general population [83, 84].
The angiopoietin (Ang)–Tie system is essential for the embryonic development of the cardiovascular and lymphatic systems and regulates postnatal angiogenesis, vascular remodeling, and vascular permeability. The system is involved in the development of the vascular network pathology in SSс [85, 86] and atherosclerosis [87, 88, 89].
There is evidence that indicates that damage to the ECs results in decreased activity of angiotensin-converting enzyme (ACE)-2, angiotensin (AT) 1–7, and anti-inflammatory cytokines, and increased activity of AT II and proinflammatory cytokines [90]. A decrease in ACE-2 levels and an increase in AT-II can lead to endothelial dysfunction, damage to the arterial intima, and increased vascular permeability.
Molecules in the renin–angiotensin–aldosterone system (RAAS) can be produced by inflammatory cells: Cathepsin G by neutrophils and AT II and ACE by macrophages. The AT II type 1 receptor (AT1R) is expressed on the surface of immune cells, vascular smooth cells, endothelial cells, and fibroblasts. Antibodies against the AT II type 1 receptor (anti-AT 1R) and endothelin-1 type A receptor may participate in the pathophysiology of SSс through vasoconstrictor, pro-inflammatory, and profibrotic effects [91]. Anti-AT 1R is a potential marker of high-risk plaques and atherosclerosis progression [92].
Contradictory data have been obtained on the association of anti-endothelial cell antibodies (AECA) following the development of atherosclerosis [93, 94]. According to recent data, high AECA levels are associated with less coronary atherosclerosis by angiogram, calcified lesions, and lower cardiovascular mortality [94].
Chronic endothelial damage is also caused by ischemia and reperfusion, which lead to dysfunction, loss of cellular integrity, and tissue damage. In SSс, tissue hypoxia, and chronic decreased blood flow are associated with microvascular abnormalities as a stimulus for increased VEGF expression and angiogenesis [95, 96, 97]. Consequently, dysregulation of angiogenesis leads to chronic and progressive vascular damage, while suppression of VEGF gene transcription enhances vascular damage and the development of atherosclerosis [98]. Increased serum endostatin levels are associated with carotid atherosclerosis in healthy residents [99].
Thrombospondin-1 (TSP-1) is an extracellular matrix glycoprotein that can positively or negatively regulate adhesion, motility, proliferation, and survival in various cell types, including immune cells. This factor is a potent inhibitor of angiogenesis [100, 101]. Endostatin and VEGF also contribute to the development of fibrosis [95, 97] and TSP-1–brachiocervical inflammatory myopathy [102].
Endothelin-1 is a peptide of endothelial origin with powerful vasoconstrictor and mitogenic effects.
Activated endothelium secretes other molecules (von Willebrand factor, soluble thrombomodulin (TM), and tissue plasminogen) that may be markers of procoagulant activity [103, 104]. A recent study showed that serum TM levels are elevated in SSc and associated with atherosclerotic carotid disease risk [105].
The biomarkers associated with endothelial activation or vascular damage in SLE are summarized in Table 1 (Ref. [81, 82, 83, 84, 85, 86, 87, 88, 89, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 106, 107, 108, 109]). Functional endothelial dysfunction may lead to increased levels of proinflammatory, proatherosclerotic and prothrombotic factors and, consequently, to an increased prevalence of cardiovascular disease.
| System/biomarkers | Class/function | Clinical associations | References |
| Adhesion molecules (E-selectin, VCAM-1ICAM-1) | Cell–cell interactions, cell recognition, signal transduction, proliferation, differentiation, and activation cells. Recruitment of leukocytes into subendothelial space | SSc: Raynaud’s phenomenon Erectile dysfunction Pulmonary hypertension Disease activity |
[81, 82] |
| Atherosclerosis: Early induction of inflammation Development of atherosclerotic plaques |
[83, 84] | ||
| Angiopoietin (Ang) system/ANG–Tie | Protein growth factors are expressed in the myocardial wall and mesenchymal cells in the surrounding blood vessels. Vascular destabilization and remodeling. Neoangiogenesis | SSc: Angiopathy Proliferative vasculopathy digital ulcers Skin sclerosis Disease activity |
[85, 86] |
| Atherosclerosis: | [87, 88, 89] | ||
| Postnatal angiogenesis | |||
| Vessel remodeling | |||
| Vascular permeability | |||
| Atheroma formation | |||
| Renin–angiotensin–aldosterone system (RAAS)/Anti-AT 1R, antibodies against endothelin-1 type | Endothelial dysfunction Production of cytokines Oxidative stress Production of ROS | SSc: High risk of diffusion in SSc Pulmonary hypertension Lung fibrosis Digital ulcers Predict SSc-related mortality |
[91] |
| Atherosclerosis: | [92] | ||
| Angiotensin-II induces plaque formation at early stages | |||
| Anti-AT 1R could be a marker of high-risk plaques and atherosclerosis progression | |||
| Stent restenosis through the induction of VSMCs and neointima hyperplasia | |||
| Anti-endothelial cell antibodies (AECA) | Autoantibodies | SSc: Pulmonary fibrosis |
[106] |
| Atherosclerosis: | [93, 94] | ||
| AECA was higher in unstable angina than in effort angina | |||
| The relationship of AECA and angiographically documented clinical recurrences | |||
| High rate of clinical recurrences after percutaneous transluminal coronary angioplasty | |||
| Possible atheroprotective effect | |||
| Endostatin | Angiogenesis | SSc: | [95] |
| Digital vascular damage | |||
| Skin and pulmonary fibrosis | |||
| Atherosclerosis: | [99] | ||
| Association with carotid atherosclerosis | |||
| Endothelin-1 | Vasoconstrictor molecule | SSc: | [107] |
| Interstitial lung disease | |||
| Right ventricle dysfunction | |||
| Atherosclerosis: | |||
| Endotelin-1 expression enhances the progression of atherosclerosis in type 1 diabetes, perivascular oxidative stress, and inflammation | [108, 109] | ||
| Thrombomodulin (TM) | Coagulation disruption of protein C activation with the formation of a prothrombotic surface and facilitates the influx of leukocytes into the arterial wall | SSc: Pulmonary hypertension |
[100] |
| Atherosclerosis: | [101] | ||
| Soluble TM is associated with the severity of endothelial damage in patients with established atherosclerosis | |||
| TM expression was reduced in endothelial cells associated with severe coronary atherosclerosis | |||
| Thrombospondin-1 (TSP-1) | Antiangiogenic glycoprotein | SSc: | [102] |
| Brachio-cervical inflammatory myopathy | |||
| Atherosclerosis: | [99, 100] | ||
| TSP-1-dependent mechanism for the development of leptin-stimulated atherosclerosis | |||
| TSP1 inhibits lymphangiogenesis by activating CD47 in lymphatic ECs, which attenuates the formation of atherosclerotic lesions | |||
| Vascular endothelial cell growth (VEGF) | Angiogenesis | SSc: Diffuse skin subset Interstitial lung involvement Nailfold capillary loss Pulmonary hypertension |
[96, 97] |
| Atherosclerosis: | [98] | ||
| Potential indicator of atherosclerotic cardiovascular disease severity |
ROS, reactive oxygen species; VSMCs, vascular smooth muscle cells; TM, thrombomodulin; ECs, endothelial cells; anti-AT 1R, against the AT II type 1 receptor.
SSc and atherosclerosis are potentially caused by the activation of abnormal immune cells, which may lead to endothelial dysfunction, dysregulation of angiogenesis, autoimmune inflammation, and ischemic lesions. Abnormal activation of immune cells (proinflammatory reaction of monocytes and hyperactivation of B- and T cells) indicates that they are involved in the development of autoimmune disease, and the immune system is involved in the accelerated development of atherosclerosis in SSc.
Functional endothelial dysfunctions may cause the amplification of proinflammatory, pro-atherosclerotic, and prothrombotic factors, thereby increasing the risk factor of cardiovascular disease.
Reliable biomarkers of ischemia–reperfusion injury and vascular damage severity in SSc need to be developed. Differentiating between the mechanisms of vascular lesions and distinguishing atherosclerotic components in SSc will enable the development of new pathogenetic treatment approaches for SSc and atherosclerosis.
The plasticity and large pool of profibrotic cytokines of immune cell and ECs mediators make them an attractive target for therapeutic interventions in SSс and atherosclerosis. Cell therapy has great potential and can be expanded and refined by future researchers. In addition to reducing inflammation, future strategies should focus on the complete repair of damaged vessels. Overall, this indicates the need for further investigation and research into the molecular and cellular mechanisms involved in chronic inflammation.
EVG: Conceptualization, Substantial contributions to the conception or design of the work; Drafting the work; Writing – review & editing; RUS: The acquisition and analysis of data for the work; Drafting the work; Writing – original draf; DAG: The acquisition of data for the work, Writing – review; TVP: The interpretation of data for the work, provided assistance and advice on the outline of the manuscript, advice on the critically for important intellectual content; LPA: Drafting the work; The interpretation of data for the work. All authors contributed to final approval of the version to be published. All authors agreed to be held accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors contributed to editorial changes in the manuscript.
Not applicable.
Not applicable.
This work was supported by the Russian Science Foundation (Grant # 22-25-00358).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.