1 Department of Molecular Diagnostics and Therapeutics, Genetic Engineering & Biotechnology Research Institute, University of Sadat City, 32958 Sadat, Egypt
2 Pathology Department, National Cancer Institute, Cairo University, 11796 Giza, Egypt
Abstract
Background and Objective: There is a growing need to comprehend the
potential outcomes of nanoparticles (NPs) on human well-being, including their
potential for detecting and treating leukemia. This study examined the role of
iron folate core–shell and iron oxide nanoparticles in inducing apoptosis and
altering the expression of the B-cell lymphoma 2 (Bcl-2), Bcl-2
associated X-protein (Bax), and Caspase-3 genes in leukemia
cells. Methods: The obtained iron oxide and iron folate core–shell
nanoparticles were analyzed using a variety of analytical techniques, including
ultraviolet-visible (UV-Vis) absorption spectroscopy, Fourier transform infrared
spectroscopy (FTIR), X-ray diffraction (XRD), dynamic light scattering (DLS),
zeta potential, and transmission electron microscopy (TEM). Additionally, FTIR
and UV-Vis were used to characterize doxorubicin. The MTT test was utilized to
investigate the cytotoxicity of iron oxide and iron folate core–shell
nanoparticles. The expression of the apoptotic signaling proteins Bcl-2, Bax, and
Caspase-3 was evaluated using the real-time reverse transcription polymerase
chain reaction (RT-qPCR) method. Additionally, flow cytometry was performed to
gauge the degrees of necrosis and apoptosis. Results: UV-Visible
spectroscopy analysis showed that the generated iron oxide and iron folate
core–shell NPs had a distinctive absorption curve in the 250–300 nm wavelength
range. The XRD peaks were also discovered to index the spherical form with a size
of less than 50 nm, which validated the crystal structure. The FTIR analysis
determined the bonds and functional groups at wavenumbers between 400 and 4000
cm
Keywords
- leukemia
- iron oxide
- iron folate core–shell
- apoptosis
- Bcl-2
- Bax
- Caspase-3
Leukemia is a type of hematological cancer characterized by complex genetic and biological features that promote invasiveness [1, 2]. Leukemia’s high prevalence and low survival rates are a cause of concern for researchers [3]. Leukemia can be treated using various methods, such as radiotherapy, chemotherapy, targeted therapy, immunotherapy, and stem cell transplantation [4, 5]. Doxorubicin (DOX) is a well-known Food and Drug Administration (FDA)-approved anticancer drug. However, despite being an effective anti-tumor medication, DOX can cause serious side effects such as rapid excretion, short retention time, and, most notably, cardiotoxicity that can lead to life-threatening cardiomyopathy and congestive heart failure [6]. Despite researchers’ efforts to improve leukemia treatment strategies, favorable outcomes have not been achieved.
In healthcare, nanotechnology is widely used for tissue healing, diagnosis, and
treatment [7, 8]. Among all the NPs, metal NPs have drawn particular attention
because of their ability to function as various agents. Abdoli et al.
[9] and Xu et al. [10] reported that metal NPs based on gold, silver,
iron, and/or iron oxide have been used as cancer treatments. Iron oxide
(Fe
Folic acid is a popular targeted ligand, particularly in cancer treatment. It is a synthetic version of vitamin B9 or folate. However, folic acid does not occur in nature; it should be noted that both names, folic acid, and folate, are frequently used interchangeably [15]. As it takes part in nucleotide synthesis, amino acid metabolism, and phospholipid production, this essential chemical is required for DNA replication and repair, RNA synthesis, and other biological processes [16]. Folate conjugation consequently offers a substrate for medication delivery that targets Folate receptor (FR). Small compounds, macromolecules, and nanocarriers have been successfully studied using this technique [17, 18]. For instance, a folate-targeted nanosystem focusing on FR expression may improve leukemia treatment’s capacity to target specific cells [19]. Research on the potential cytotoxicity of the folate core–shell, used as the outermost shell of magnetite iron oxide nanoparticles, has yet to be done.
The current study aimed to determine whether the doxorubicin chemotherapeutic drug, magnetite iron oxide nanoparticles, and folate core–shell conjugated with iron oxide nanoparticles was cytotoxic to human acute leukemia cell lines and a control cell line. Analysis of the treated group’s Bax, Bcl2, and Caspase-3 gene expression was performed using real-time reverse transcription polymerase chain reaction (RT-qPCR), and apoptosis was analyzed with flow cytometry.
Iron oxide nanoparticle synthesis was completed as previously described [20]. In a mixture, ferric and ferrous ions were combined at room temperature in a 1:2 molar ratio. Once the pH of the solution reached 10, 1.5 M NaOH was gradually added to the reaction to carry out the co-precipitation. The precipitate was magnetically separated and then washed with distilled water numerous times until the pH reached 7. The cleaned nanoparticles were allowed to air dry at room temperature before being used in further research.
A total of 0.1 g folic acid was added to 100 mL of deionized water, agitated for 30 minutes at 60 °C, and then exposed to ultra-sonication for two hours at 0.9 cycles and 90% amplitude. After adding 50 mL of previously prepared folic acid, 0.1 g of magnetite nanoparticles were added to 50 mL of doubled deionized water and stirred for 0.5 hours with a mechanical stirrer. The mixture was then sonicated for 6 hours (0.6 cycles and 50% amplitude).
This study characterized the optical absorption characteristics of materials using ultraviolet-visible (UV-Vis) spectroscopy. All absorbance measurements and data manipulation were performed on a computer running Shimadzu UV probe software, and a UV/Vis spectrophotometer from the Shimadzu UV 2450 PC series (Tokyo, Japan) was used to capture the spectra. The spectrophotometer was equipped with two matching 1 cm quartz cells and had the following spectral settings: one quick scan mode and a slit width of 2 nm. The development of iron oxide nanoparticles, iron folate core–shell nanoparticles, and doxorubicin was monitored using UV-Vis spectra [21].
The size and shape of iron oxide and iron folate core–shell nanoparticles were determined using the transmission electronic microscope (TEM). The microscope (JEOL -Tokyo -Japan- JEM.1400 electron microscope) was used for the TEM characterization of nanoparticles. A drop of the nanoparticle solution was placed on a carbon grid and let dry in a descriptor before being spotted with TEM.
Using spectroscopic-grade potassium bromide (KBr) that was dried at 100
°C and chilled in a vacuum desiccator to minimize moisture absorption,
functional groups on compounds were analyzed using FTIR (FT/IR-4X, Jasco, Japan)
in the intermediate wavenumber (450–4000 cm
The size and size distribution of NPs were measured using the dynamic light scattering (DLS) method with the Zetasizer Nano ZS (Malvern Pananalytical Ltd, Malvern, UK). The sample was put within a cuvette made of quartz. Following equipment calibration, each sample was subjected to three measurements. Size distribution was calculated using the collected data for iron oxide and iron folate core–shell nanoparticles.
Utilizing a Philips1820 advance diffractometer with Ni-filtered Cu K
The laser Doppler electrophoresis technique was employed to quantify the particle electrostatic charge, and the findings are represented as zeta potential using the Zetasizer-nano instrument (Malvern, UK). A total of 100 µL of the particle solution was diluted with 1.5 mL of water and added into a cuvette to determine the particle electrostatic charge (ZP) of iron oxide and iron folate core–shell nanoparticles.
The American Type Culture Collection (ATCC) was used to obtain the human acute lymphocytic leukemia (ALL) cell lines (CCRF-CEM, RS4; 11™), human acute myelogenous leukemia (AML) (THP1 (TIB-202™), Kasumi-1 (CRL-2724™), and normal cell line (CRL-1980™) (ATCC, Gaithersburg, MD, USA). The acute leukemia cells were typically grown in a DMEM medium. We added 2 mM of L-glutamine to the media, 100 units of penicillin G sodium, 250 ng of amphotericin B, 100 units of streptomycin sulfate, and 10% fetal bovine serum (FBS). At sub-confluence, cells were kept at 37 degrees Celsius in humidified air containing 5% carbon dioxide. At 75% confluence, cells were collected using trypsin/EDTA. Gibco®/Invitrogen, USA, was the source of all cell culture supplies. Unless otherwise specified, all compounds were purchased from Sigma-Aldrich in the United States.
As performed by ATCC, short-tandem repeat STR profiling uses multiplex PCR to simultaneously amplify the amelogenin gene and seventeen polymorphic markers, including the most informative polymorphic markers in the human genome. ATCC uses the Promega PowerPlex 18D system and the ThermoFisher Scientific GeneMapper ID-X v1.2 software to analyze the amplicons.
The Mycoplasma test was performed in all cell lines using Hoechst 33258 (Sigma-Aldrich), as recommended by ATCC (Fluorescent Hoechst staining at 500X magnification). Authentication of cell lines was performed by the authors. Mycoplasma testing was performed using the Lookout one-step Mycoplasma PCR detection kit (MP0050, Sigma-Aldrich).
The cytotoxic effect of IONPs, iron folate core–shell NPs, and Doxorubicin
against acute leukemia cells and CRL-1980 was estimated by MTT
(3-[4,5-Dimethylthiazol]-2,5-Diphenyltetrazolium bromide) assay. During the
metabolic activity, mitochondrial dehydrogenases convert the yellow tetrazolium
salt of MTT to generate insoluble purple formazan crystals, which may be
dissolved with detergent (acidic isopropanol). Before subjecting them to an MTT
experiment, cells (5104 cells/well) were treated with a serial concentration of
nanoparticles and doxorubicin (12.5, 25, 50, and 100 g/mL) at 37 °C in
an FBS-free mixture for 24 hours. A 570 nm absorbance reading was taken (FLUOstar
OPTIMA; BMG Labtech GmbH, Offenburg, Germany). The percentage of MTT transformed
into the insoluble formazan salt represented the relative cell viability.
Information was reported as the average percentage of still-alive cells in each
treatment group vs. the corresponding solvent-treated control groups. The
dose–response line equation determined each chemical’s half-maximal growth
inhibitory concentration (IC
Following the manufacturer’s instructions, total RNA was isolated from the cell
lines using the Qiagen RNA extraction kit (Qiagen Inc., Valencia, CA, USA). The
Q5000 137 nanodrop (Quawell Technology, Inc., San Jose, CA, USA) was used to
measure the purity and amount of RNA. The complementary DNA (cDNA) was produced
by reverse transcription using a kit from Qiagen in Valencia, California, USA
(Cat. No. 218161). The total reaction volume contained 100 ng of total RNA and
was 20 µL. The reference housekeeping gene used in the qPCR for apoptotic
genes (Bax, Bcl2, Caspase-3) was actin. Thermo
Scientific’s Maxima SYBR Green/ROXqPCR Master Mix and specific primers were
created (Table 1). The following conditions were used to carry out the qPCR:
initial denaturation at 95 °C for 5 min, followed by 40 cycles of
denaturation at 95 °C for 15 seconds, 64 °C for 40 seconds, and
strand extension at 72 °C for 1 min. Target gene critical threshold (Ct)
values were standardized using the equation x = 2
| Primer name | Forward (5 |
Reverse (5 |
| CTGTCTGGCGGCACCACCAT | GCAACTAAGTCATAGTCCGC | |
| Bax | AAGCTGAGCGAGTGTCTCAAGCGC | TCCCGCCACAAAGATGGTCACG |
| Bcl-2 | AGATGTCCAGCCAGCTGCACCTGAC | AGATAGGCACCCAGGGTGATGCAAGCT |
| Caspase-3 | TTGATGCGTGATGTTTCTA | CAATGCCACAGTCCAGTTC |
Bcl-2, B-cell lymphoma 2; Bax, Bcl-2 associated X-protein.
The impact of iron oxide and iron folate core–shell nanoparticles at 100
µg/mL on cell cycle arrest was compared to the control samples. Cell cycle
distribution was analyzed using the DNA PREP Kit from Beckman Coulter USA, Inc.
(Beckman Coulter 250 S. Kraemer Blvd. Brea, CA, USA). Monolayer cells from the
CCRF-CEM and THP1 cell lines were treated with the IC
All data have been computed as mean and standard deviation (SD). One-way
variance analysis (ANOVA) was utilized to compare the data with the control
group. p
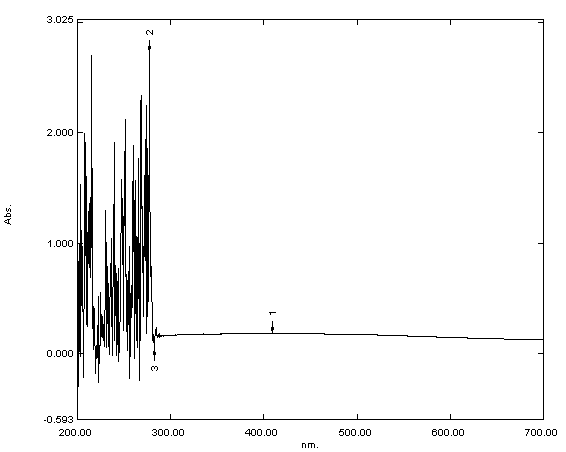
The UV-Vis analysis confirmed the preparation of iron oxide, iron folate
core–shell nanoparticles, and doxorubicin. The absorption spectra dispersed in
deionized water displayed abroad absorption peak at about 250–300 nm. The mean
absorption for doxorubicin, iron oxide, and iron folate core–shell was 0.262
 Fig. 1.
Fig. 1.
Ultraviolet-visible (UV-Vis) spectroscopy analysis of iron oxide
(Fe
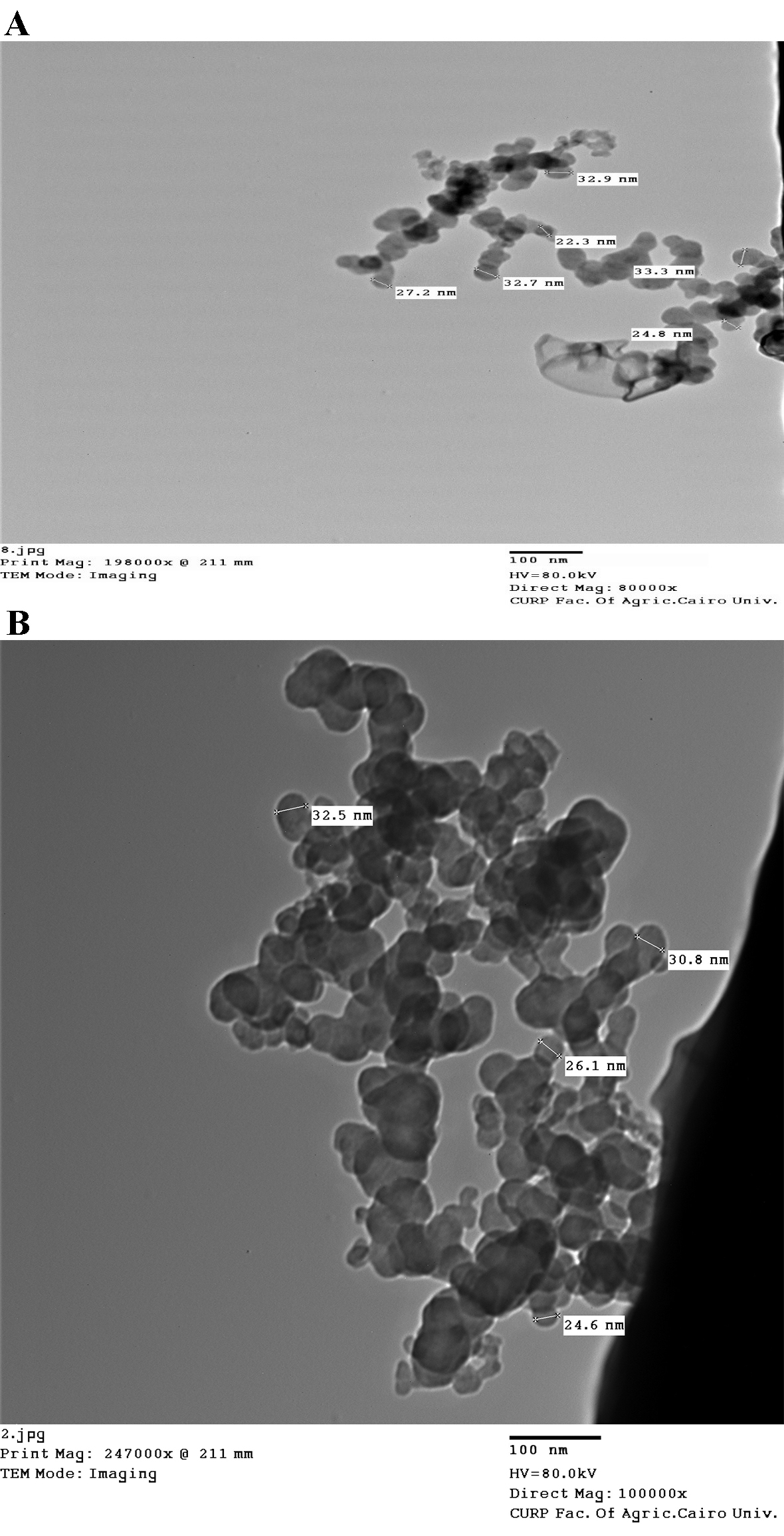
According to the size distribution, the spherical micelles observed in the TEM image (Fig. 2) were nanocapsules with sizes between 7 and 42 nm.
 Fig. 2.
Fig. 2.Transmission electron microscopy (TEM) images of (A) iron oxide and
(B) iron folate core–shell nanoparticles. The size of nanoparticles depends mainly on the concentration of precursor used in the synthesis of hematite. which proved that the particle size increased with the raise of the precursor concentration (FeCl
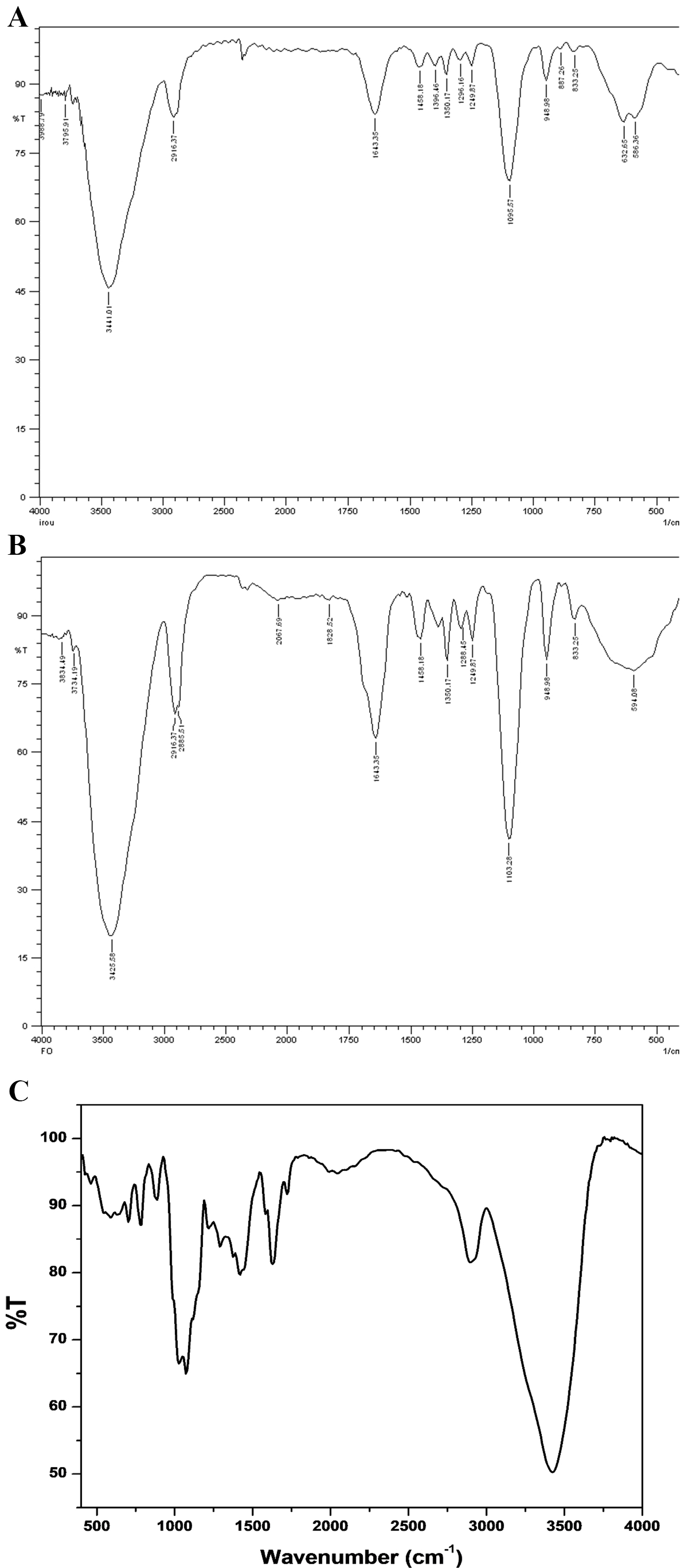
The FTIR spectra of iron oxide and iron folate core–shell nanoparticles and doxorubicin are shown in Fig. 3. Peaks and troughs in the spectra indicate absorption and transmission of infrared light, revealing details about functional groups, chemical bonds, and molecular structures. Interpretation involves correlating peaks with known vibrational modes of specific functional groups.
 Fig. 3.
Fig. 3.Fourier transform infrared spectroscopy (FTIR) spectra of (A) iron oxide, (B) iron folate core–shell, and (C) doxorubicin, respectively.
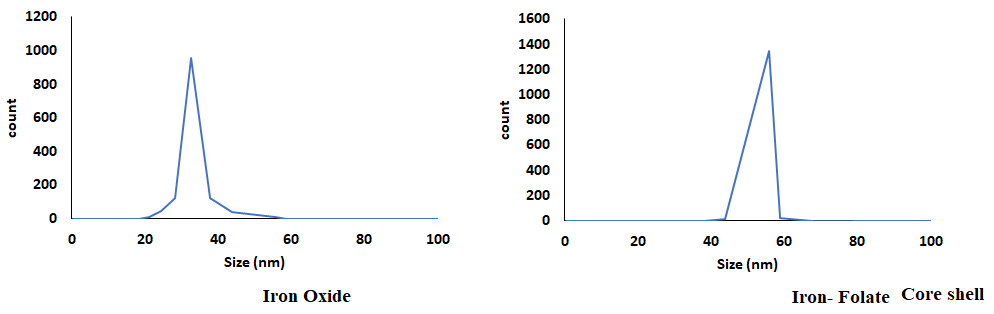
The DLS analysis confirmed the size of magnitude of Iron oxide and folic core–shell nanoparticles (Fig. 4). The size of the iron oxide was 33 nm, and the iron folate core–shell was 55 nm.
 Fig. 4.
Fig. 4.The dynamic light scattering (DLS) analysis on the nanoparticle size.
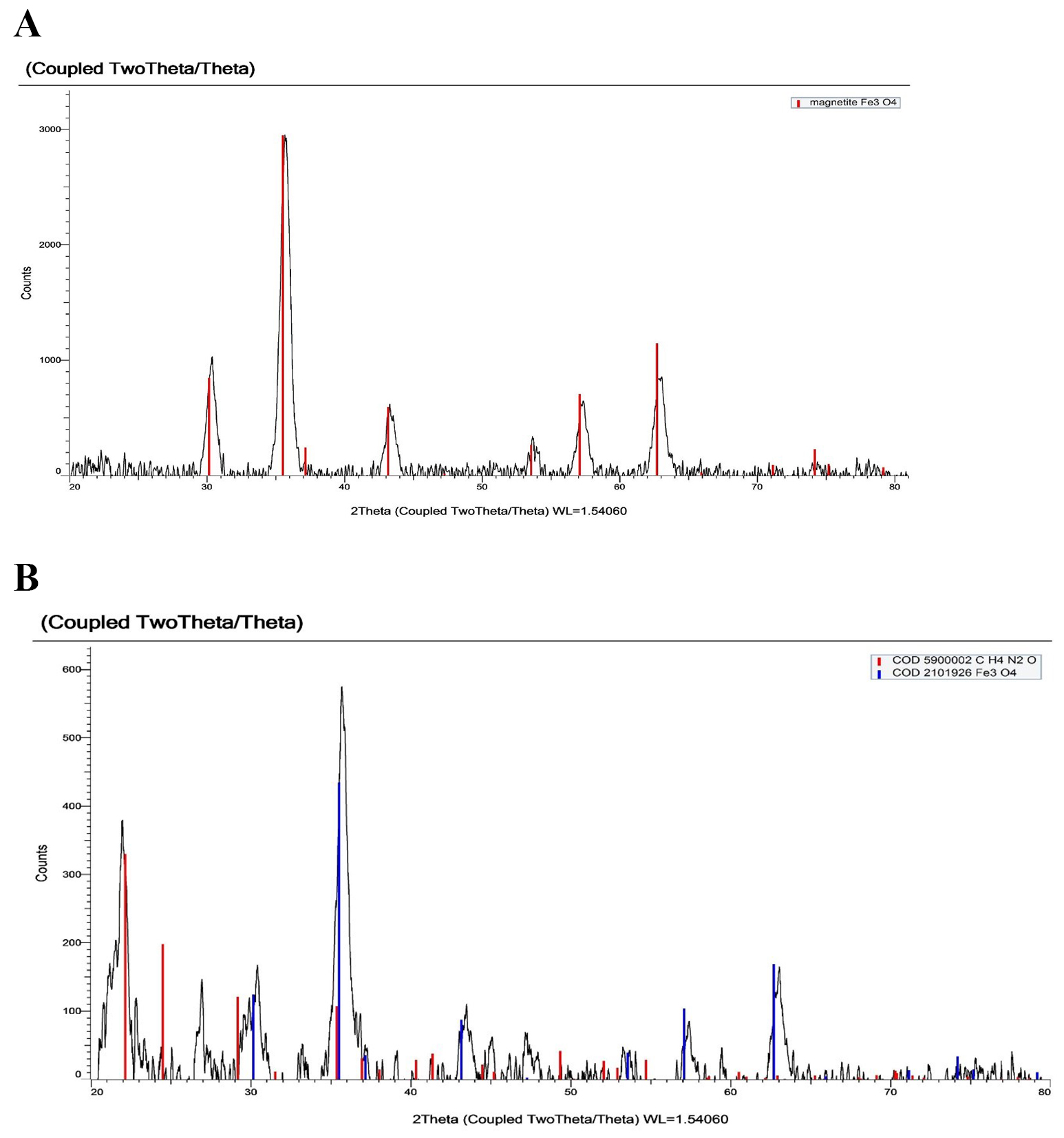
The structure and crystallinity of the produced nanoparticles were evaluated by
X-ray diffraction (XRD) crystallography. Iron oxide chemical makeup determines
their color. XRD depicts the diffraction of X-rays by a crystal lattice. Peaks in the pattern correspond to constructive interference of X-rays scattered by crystal planes. The position and intensity of these peaks provide information about the crystal structure, including lattice spacing and orientation, helping identify crystalline phases in a sample. Due to the magnetite (Fe
 Fig. 5.
Fig. 5.X-ray diffraction (XRD) pattern of (A) iron oxide and (B) iron folate core–shell depict the diffraction of X-rays by a crystal lattice.
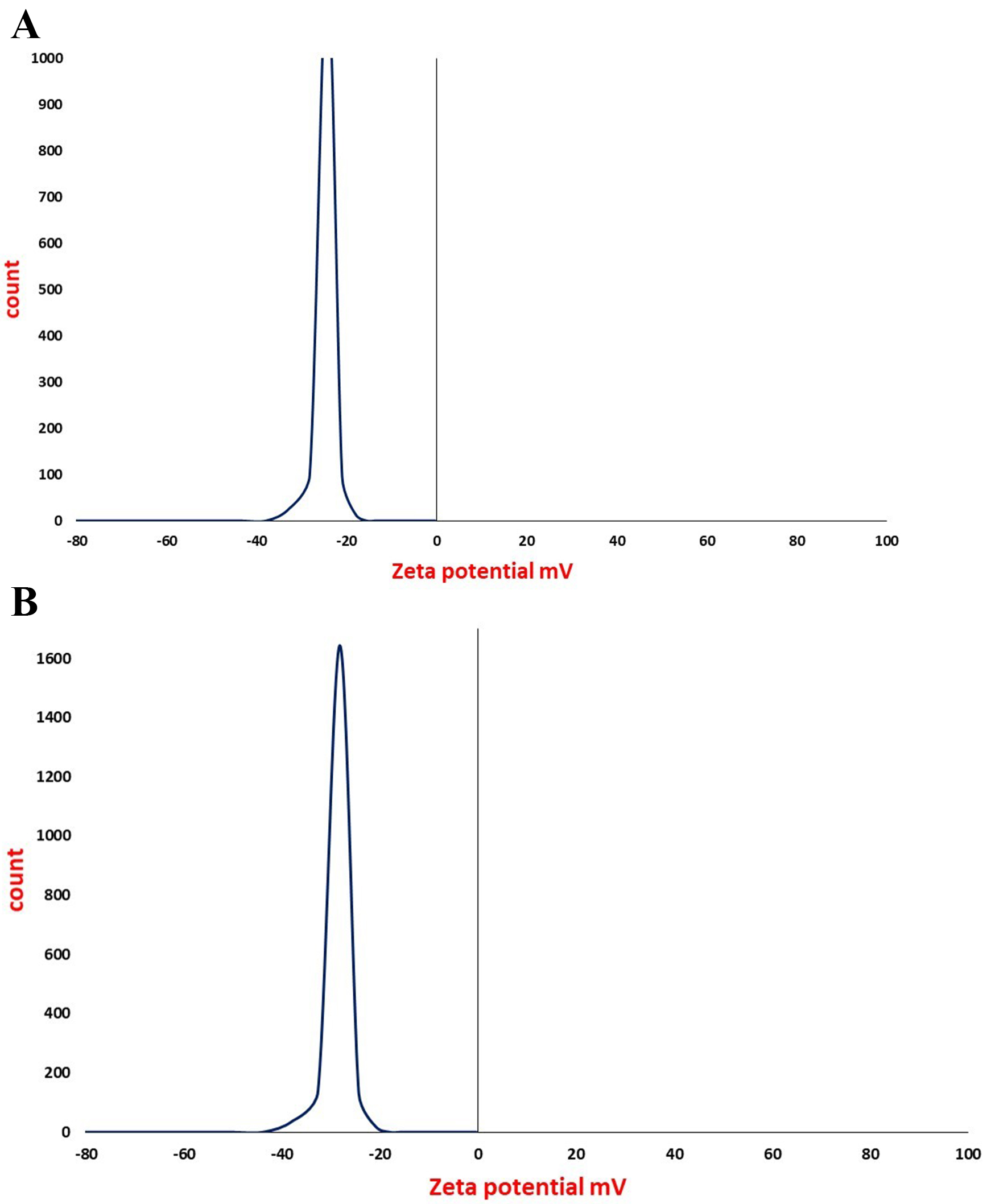
The stability of the particles was good as evidenced by the zeta potential,
which existed in the –24
 Fig. 6.
Fig. 6.Zeta potential analysis of (A) iron oxide and (B) iron folate core–shell nanoparticle which suggest that the surface of the particles in a colloidal system is negatively charged.
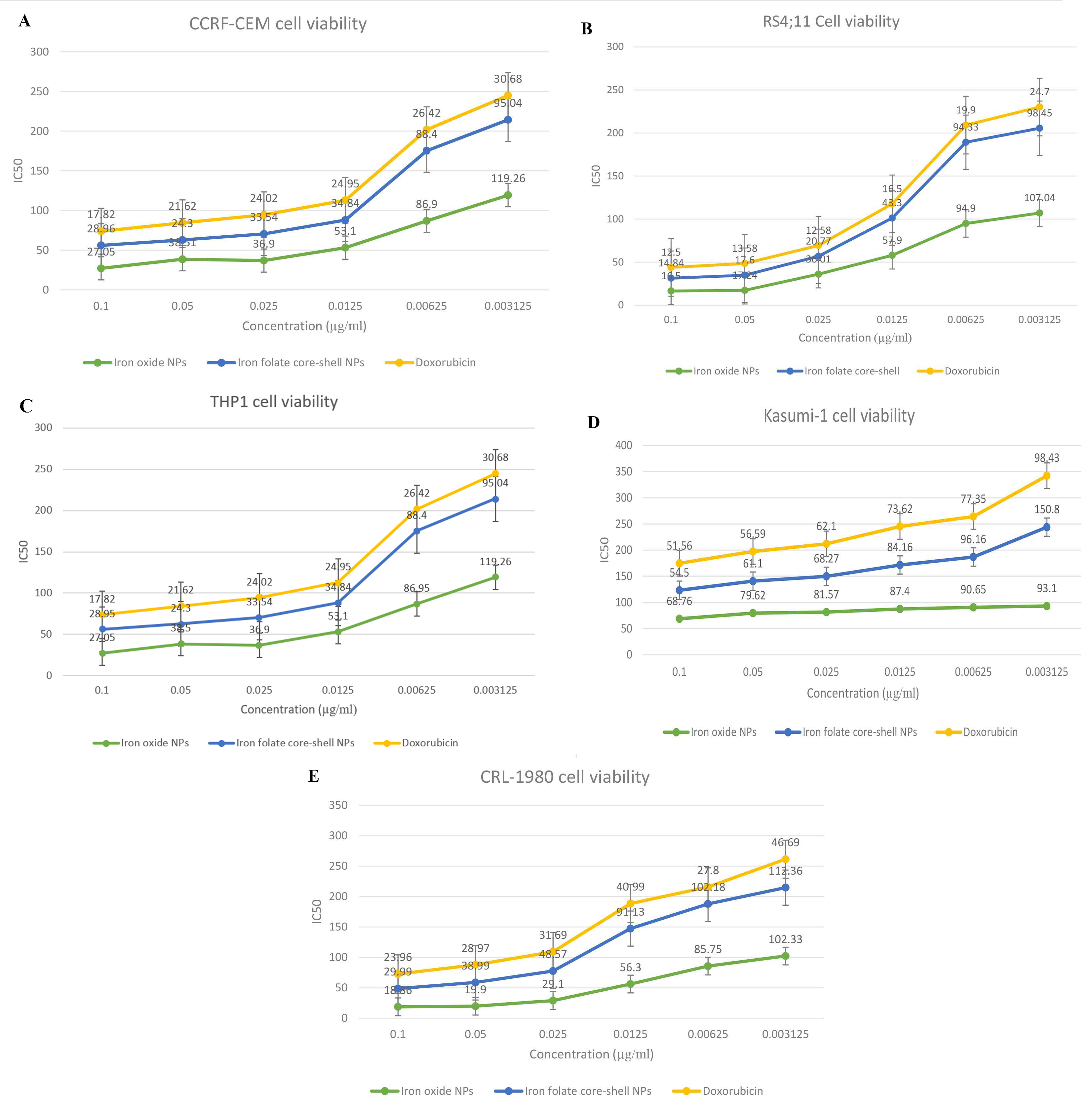
In this study, the cells treated with various concentrations of iron oxide, iron folate core–shell nanocomposite, or doxorubicin were examined using the MTT test for the cytotoxicity of leukemia ALL (CCRF-CEM; RS4; 11) and AML (THP1; Kasumi-1), and normal (CRL-1980) cell lines (Table 2). The results showed that the presence of iron folate core–shell nanocomposite reduced the viability of CCRF-CEM, RS4;11, and THP1 cells dose-dependently, while the cytotoxicity effect increased in Kasumi-1 and CRL-1980 cell lines (Fig. 7).
| Cell Lines | IC |
p-value | |||
| Iron | Iron folate core–shell | Doxorubicin | |||
| ALL | CCRF-CEM | 46.8 |
42.65 |
14.8 |
p |
| RS4;11 | 54.94 |
48.2 |
19.38 |
p | |
| AML | THP1 | 60.29 |
50.8 |
24.24 |
p |
| Kasumi-1 | 83.5 |
85.83 |
64.94 |
p | |
| Normal | COLO 829BL-CRL-1980 | 52.04 |
70.54 |
43.52 |
p |
p
*: Statistically significant at p
 Fig. 7.
Fig. 7.Cell viability in ALL, AML, and normal cell lines treated with
ligands at various concentrations was determined using MTT assay to assess the
anticancer activity of iron oxide, iron folate core–shell NPs, and doxorubicin
on CCRF-CEM, RS4;11, THP1, Kasumi-1, and COLO 829BL-CRL-1980 cells. Each cell
line was subjected to a dose of a different treatment (0.003–0.1 µg/ mL)
for 24 h. Each data point shown is the mean
By analyzing Bax, Bacl2, and Caspase-3 activity in relation to different
quantities of protein content for cells treated with the IC
| Apoptosis | Necrosis | ||||
| Total | Early | Late | |||
| 1 | Iron/CCRF | 12.4 |
5.1 |
4.6 |
2.7 |
| 2 | Iron/THP | 10.2 |
2. 3 |
5.8 |
2.1 |
| 3 | Iron folate core–shell/CCRF | 16. 0 |
5.3 |
7.6 |
3.1 |
| 4 | Iron folate core–shell/THP | 12. 9 |
4.3 |
6.5 |
2.1 |
| 5 | cont. CCRF | 2.3 |
1. 4 |
0.3 |
0. 6 |
| 6 | cont. THP | 2.2 |
1.3 |
0.4 |
0.5 |
| p |
p |
p |
p | ||
p
*: Statistically significant at p
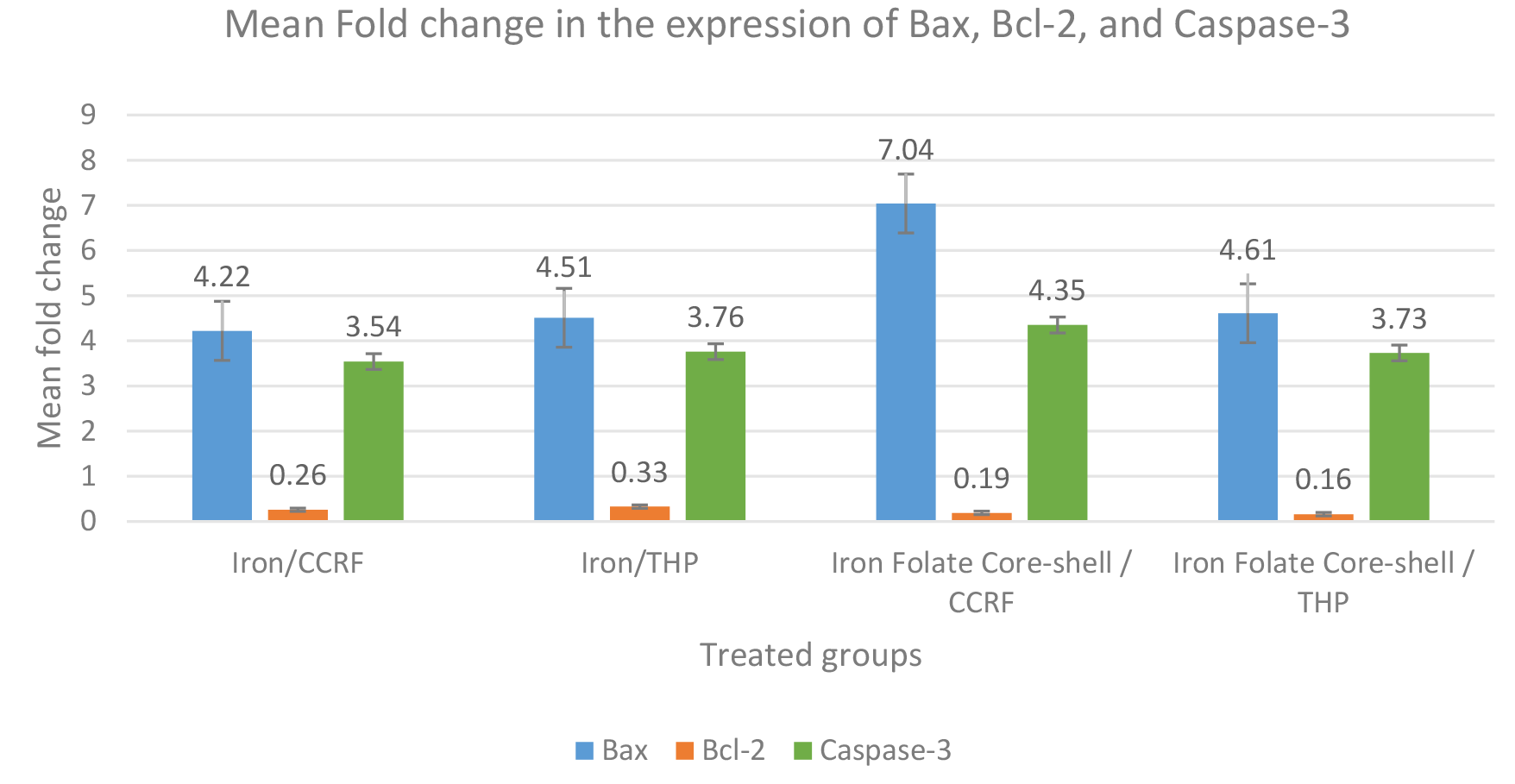
Our data demonstrate a high Bax and Casepase-3 expression level in the CCRF-CEM cell line treated with an iron folate core–shell compared with the THP cell line, while Bax and Caspase-3 expression in the CCRF-CEM and THP cell line treated with iron were slightly similar. On the other hand, Bcl-2 showed high expression in the THP cell line treated with iron compared with the CCRF-CEM cell line, while Bcl-2 expression in the CCRF-CEM and THP cell line treated with iron folate core-shell were slightly similar (Table 4, Fig. 8).
| Treated Groups | Bax | Bcl-2 | Caspase-3 |
| Iron/CCRF | 4.22 |
0.26 |
3.54 |
| Iron/THP | 4.51 |
0.33 |
3.76 |
| Iron folate coreshell/CCRF | 7.04 |
0.19 |
4.35 |
| Iron folate coreshell/THP | 4.61 |
0.16 |
3.73 |
| p |
p |
p |
p
*: Statistically significant at
p
 Fig. 8.
Fig. 8.Expression changes of three apoptosis-associated proteins (Bax,
Bcl-2, and Caspase-3) in the treated cells. The data represented by the means of
different groups differ significantly (p
UV-Vis spectroscopy was used to validate the production of nanoparticles. Fig. 1 displays the UV-Visible spectrum of iron oxide nanoparticles—iron oxide nanoparticles were present, as shown by the strong 250–300 nm peak. Bonvin et al. [22] and Niraimathee et al. [23] reported similar results. The created iron oxide nanoparticles were evenly distributed and nearly spherical, as seen in the TEM image in Fig. 2. The size range of the core–shell nanoparticles for iron oxide and iron folate was 7–42 nm. This finding is consistent with Guo et al. [24].
The FTIR examination of the synthetic iron oxide and iron folate core–shell
nanoparticles yielded results in the 400–4000 cm
The type of solvent used, as well as the duration of the chemical reaction,
affected the particle size of the Fe
In general, zeta potential measurements of the iron oxide and iron folate
core–shell nanoparticles revealed negative surface charges. Surface
modification, however, could produce highly positively charged NPs for improved
colloidal stabilization [28]. The zeta potential values were approximately –24
Various clinical results for identifying multiple efficacy-affecting components
of nanomedicine are essential to address the intricacy surrounding disease
progression, therapy, care, and recurrence risk. It is essential to balance
effectiveness against the impact on healthy cells to minimize the adverse effects
on the quality of life in this situation [32]. Various nanocarriers are described
in the literature for their drug delivery systems to circumvent these
restrictions in cancer treatment [33]. To assess the inhibitory effects of the
free and conjugated nanoforms on cell growth in leukemia cell lines, we devised
the conjugation between iron oxide and iron folate core–shell as a carrier in
this study. Initially, the cell viability of leukemia ALL (CCRF-CEM; RS4; 11) and
AML (THP1; Kasumi-1) and normal (CRL-1980) cell lines were evaluated using the
MTT method. The results showed the viability of CCRF-CEM; RS4; 11 and THP1 cells
reduced dose-dependently in the presence of iron folate core–shell
nanocomposite, while the cytotoxicity effect increased in Kasumi-1 and CRL-1980
cell lines (Fig. 7). The cytotoxicity effect of iron conjugated to folate
core–shell was lower than iron oxide in a free nanoform. The IC
The assessment of the number of apoptotic cells using flow cytometric analysis with the AV/PI double labeling provided additional evidence of the apoptotic impact of the induction of iron oxide and iron folate core–shell NPs. Phosphatidylserine is often translocated from the inner to the outer section of the plasma membrane to initiate an early apoptotic event. Green fluorescence is produced when annexin V binds to phosphatidylserines in the presence of calcium ions. These findings showed that late apoptosis or necrosis enhanced membrane permeability, allowing PI to enter cells. PI binds to cellular DNA and dyes the nucleus red. The findings showed that iron folate core–shell may cause apoptosis in CCRF-CEM and THP and that early apoptosis and necrosis could be observed in CCRF-CEM treated with iron and iron folate core–shell, respectively. Results of our study revealed a rise in the percentage of annexin V-FITC and PI-positive cells (upper right quadrant) in a time-dependent manner, indicating late apoptosis (Table 3), with a significant difference between treatment groups with iron oxide NPs, iron folate core–shell NPs, and doxorubicin (p = 0.007).
Currently, apoptosis is thought to be the primary cause of cell death. The antiapoptotic protein B-cell lymphoma-2 (Bcl-2) has an antiapoptotic function. The pro-apoptotic protein known as Bcl-2 associated X protein (Bax) can accelerate cell death. When Bcl-2 is overexpressed, it exerts an antiapoptotic impact because Bax has an antagonistic effect on the protein. Contrarily, when Bax is dominant, cells are more prone to apoptosis [34]. Both CRRF-CEM and THP cells were treated, which led to a reduction in Bcl-2 and an increase in Bax gene expression. This data indicated that nanoparticles predisposed these chemo-resistant malignant cells to induce apoptosis by altering gene expression. The most thoroughly researched effector, caspase, is caspase-3, which is activated in the perinuclear region of the cell and linked to the release of mitochondrial cytochrome c, which may be inhibited by upregulating Bcl-2 proteins [35]. Our investigation demonstrated that the combination of the folate core–shell and iron oxide NPs significantly increased the transcription and expression of caspase-3 in CCRF CEM cells. This finding confirms that IONPs enhance the folate core–shell-mediated apoptosis and this is linked to the gene and protein expression level. In cancerous cells, apoptosis can be initiated by activating molecules upstream of the signaling pathway or inhibiting antiapoptotic factors.
Our study shows that the combination of iron oxide and folate core–shell iron was an effective treatment for leukemia. Iron oxide and iron folate core–shell NPs were characterized by FTIR, XRD, TEM, and UV-Visible spectroscopy. The produced nanoparticles ranged in size from 7 to 42 nm, had appropriate monodispersity, and did not aggregate. With no toxicity and compatibility with normal cell lines, iron oxide and iron folate core–shell nanoparticles exhibited the right amount of cytotoxicity against acute leukemia malignancy. Therefore, synthetic iron oxide with a folate core–shell could be considered as a possible anticancer drug.
The data supporting the findings and conclusions are available and can be found in the article’s manuscript; any additional data can be supplemented upon request.
GMN & MM designed the study. GMN, OMT & MM contributed to the practical work, study methodology, and resources. GMN, MOE & RMT already feed the research by updated studies and review the writing and editing. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.