1 Laboratory for Oxidative Stress, Division of Molecular Medicine, Rudjer Boskovic Institute, 10000 Zagreb, Croatia
2 Department of Analytical Chemistry, Medical University of Białystok, 15-222 Białystok, Poland
3 Division of Pathology, School of Medicine, University of Zagreb, University Hospital Centre Zagreb, 10000 Zagreb, Croatia
Abstract
Oxidative stress often affects the structure and metabolism of lipids, which in the case of polyunsaturated free fatty acids (PUFAs) leads to a self-catalysed chain reaction of lipid peroxidation (LPO). The LPO of PUFAs leads to the formation of various aldehydes, such as malondialdehyde, 4-hydroxynonenal (4-HNE), 4-hydroxyhexenal, and 4-oxo-2-nonenal. Among the reactive aldehydes, 4-HNE is the major bioactive product of LPO, which has a high affinity for binding to proteins. This review briefly discusses the available information on the applicability of assessment options for 4-HNE and its protein adducts determined by immunosorbent assay (the 4-HNE-ELISA) in patients with various diseases known to be associated with oxidative stress, LPO, and 4-HNE. Despite the differences in the protocols applied and the antibodies used, all studies confirmed the usefulness of the 4-HNE-ELISA for research purposes. Since different protocols and the antibodies used could give different values when applied to the same samples, the 4-HNE-ELISA should be combined with other complementary analytical methods to allow comparisons between the values obtained in patients and in healthy individuals. Despite large variations, the studies reviewed in this paper have mostly shown significantly increased levels of 4-HNE-protein adducts in the samples obtained from patients when compared to healthy individuals. As with any other biomarker studied in patients, it is preferred to perform not only a single-time analysis but measurements at multiple time points to monitor the dynamics of the occurrence of oxidative stress and the systemic response to the disease causing it. This is especially important for acute diseases, as individual levels of 4-HNE-protein adducts in blood can fluctuate more than threefold within a few days depending on the state of health, as was shown for the COVID-19 patients.
Keywords
- oxidative stress
- lipid peroxidation
- 4-hydroxynonenal (4-HNE)
- protein modifications
- cancer
- COVID-19
- inflammatory diseases
- inflammation
- autoimmune diseases
- immunochemistry
Oxidative stress, i.e., excessive production of reactive oxygen species (ROS), is often associated with the oxidation of lipids, which, in the case of polyunsaturated free fatty acids (PUFAs), can lead to a self-catalyzed chain reaction of lipid peroxidation (LPO), resulting in the destruction of biomembranes and the formation of reactive aldehydes. LPO of PUFAs yields the formation of various aldehydes, such as malondialdehyde, 4-hydroxynonenal (4-HNE), 4-hydroxyhexenal, and 4-oxo-2-nonenal, among which 4-HNE is the major bioactive product and is therefore the focus of this review [1]. The 4-HNE regulates various cellular processes, and its effects are concentration- and cell-type-dependent. At lower concentrations, it acts as a signaling molecule that regulates proliferation, differentiation, antioxidant capacity, and apoptosis in various (patho)physiological processes, while at high concentrations, it can cause irreparable damage to cells, leading to necrosis [2, 3, 4]. Accordingly, 4-HNE and similar aldehydes are considered second messengers of free radicals that exert their effects even without excessive production of reactive oxygen species (ROS), mainly due to their strong affinity for binding to proteins.
There are two well-known types of chemical reactions that characterize the interactions of 4-HNE with proteins, in particular with the amino acid residues histidine, cysteine, lysine, and arginine, forming Michael adducts or Schiff’s bases [5]. On the other hand, at least in the case of lysozyme and bovine serum albumin, 4-HNE can form Michael adducts on threonine and Schiff’s base on tryptophan, as well as pyrrole-type adducts with histidine and lysine residues. The modification of proteins and peptides by 4-HNE can alter their structure and function, which appears to be the key mode of action of 4-HNE as a bioactive factor. The list of known target proteins of 4-HNE and the biological consequences of such modification can be found in a recently published review [6]. The list will certainly be extended by further research based on immunochemical studies using mostly monoclonal antibodies and/or mass spectrometry/chromatography analyses of the 4-HNE-modified proteins.
Since LPO occurs mostly in the lipid layer of the biomembranes, 4-HNE is usually found within or near the biomembranes bound to the proteins, even when administered exogenously [7]. In the case of the protein moiety of low-density lipoproteins (LDL), the apolipoprotein B (ApoB), the 4-HNE produced by LPO mainly forms adducts with histidine [8]. Such modifications of ApoB were the first recognized pathogenic mechanism involving 4-HNE in addition to its mutagenic and carcinogenic effects. Advances in the research of the potential pathogenic effects of LPO and 4-HNE have led to a better understanding of cardiovascular, (neuro)degenerative, metabolic, and autoimmune diseases, as well as the carcinogenesis and mechanisms of action of various medicaments and nutraceuticals [3].
Most of the above findings were obtained by qualitative immunohistochemical and/or translational in vitro and in vivo studies lacking quantification [9], due to the lack of immunosorbent enzyme-linked immunoassays (ELISA) specific for the 4-HNE-modified proteins. This was solved by the development of the first such ELISA, initially developed for in vitro research on cell cultures and specific for the 4-HNE-histidine adducts [10]. The original 4-HNE-ELISA, which uses genuine antibodies specific for the 4-HNE-histidine adducts, was further modified and validated for its applicability on plasma and serum samples and eventually compared with the 4-HNE-ELISA that uses commercial antibodies [11]. In this study, different levels of 4-HNE-protein adducts were detected depending on the type of monoclonal antibody used and the protocol applied to the same samples. However, both variants of the 4-HNE-ELISA detected higher values of 4-HNE-protein adducts in the blood of apparently healthy obese men when compared to healthy non-obese men. In the case of the 4-HNE-ELISA based on the non-commercial antibody, the average level of the 4-HNE-protein adducts was approximately 50% higher (on average, 33 vs. 20 pmol/mg protein), while in the case of the 4-HNE-ELISA with the commercial antibody, the difference between obese and non-obese subjects was 1150 vs. 950 pmol/mg protein, respectively [11]. These findings have shown that the type of monoclonal antibody used, and the protocol applied are among the most important aspects of the analysis of 4-HNE-protein adducts by 4-HNE-ELISA. Following these findings, different commercial and non-commercial antibodies were used for the development of non-commercial and commercial 4-HNE-ELISA, while for our in-house 4-HNE-ELISA, we mainly use the same non-commercial antibody specific for the 4-HNE-histidine adducts, which is also used for immunohistochemistry. It should also be mentioned that this particular in-house indirect 4-HNE-ELISA (Fig. 1) already gave similar results for the pool of human plasma samples exposed to Ultraviolet (UV) light in vitro in an earlier study without protocol modification for human sera or plasma samples and showed a very good correlation with free malondialdehyde determined by high performance liquid chromatography (HPLC) [12], which was even further improved by the modified 4-HNE-ELISA protocol [11].
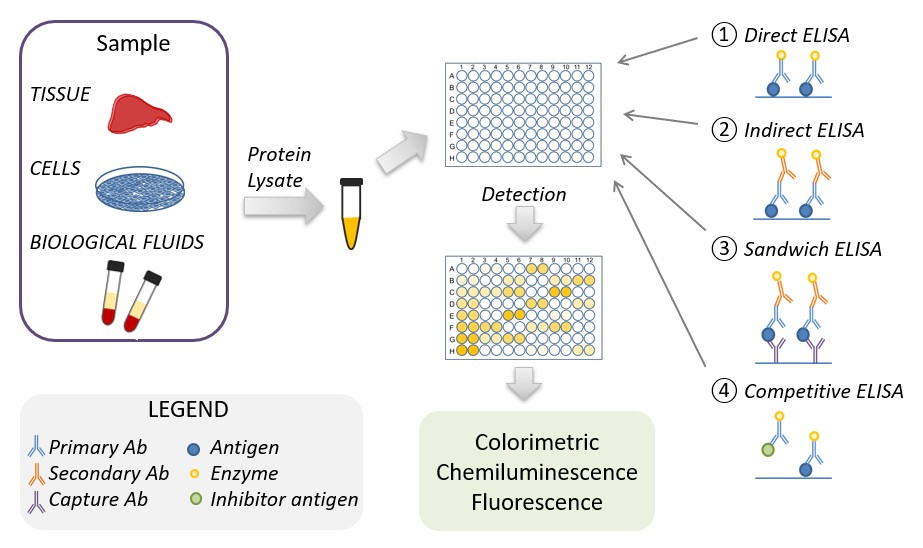 Fig. 1.
Fig. 1.Schematic representation of the ELISA procedure. ELISA, enzyme-linked immunoassays.
The antigen-containing samples or the corresponding (protein) standards are isolated from tissue, biological fluids, or cell cultures, their protein concentrations are normalized and used in the ELISA. There are four main types of ELISA: direct, indirect, sandwich, and competitive ELISA. The ELISA detection method depends on the type of enzyme linked to the antibody. The most common enzymes linked to the antibody are horse reddish peroxidase and alkaline peroxidase, which when used in combination with chromogenic substrate use a colorimetric detection method. Two other detection methods are chemiluminescence or fluorescence based, which use luminol enhancer or fluorescent substrate, respectively.
Since then, several studies have been done analyzing the levels of 4-HNE-protein adducts in human blood, especially in plasma, often comparing the results with immunohistochemical findings based on the same monoclonal antibodies. These became very attractive and convenient methods to study oxidative stress and, in particular LPO, in clinical studies [13].
The results obtained with the 4-HNE-ELISA, which are based on detection of the 4-HNE-modified proteins using specific antibodies, can additionally be partially validated by the parallel use of Western blotting. However, in the case of ELISA, the use of a standard curve allows the determination of the exact concentration of the tested molecules, while Western blotting offers only limited possibilities in this respect, since immunoblotting after electrophoretic separation mainly shows the distribution of the proteins modified by 4-HNE and allows the comparison of the intensity of the individual bands, providing only semi-quantitative results [14]. Finally, the 4-HNE-protein adducts, independently labeled with specific antibodies by Western blotting, can be extracted from the gel and analyzed by liquid chromatography coupled to mass spectrometry (LC-MS) [15, 16]. With this approach, it is possible to precisely determine the amino acid sequence of the modified protein and to identify it on the basis of proteome databases. In addition, the label-free semi-quantitative method will provide information on the amount of such modified proteins/peptides [17].
Therefore, the aim of this review is to summarize the results of the published studies to find if there are levels of 4-HNE-protein adducts in human blood that might be considered normal and to what extent these levels may change in cases of various diseases.
An altered redox balance has been implicated in the pathogenesis of diseases caused by pathogens such as viruses (e.g., SARS-CoV-2, Tick-Borne Encephalitis Virus) and bacteria (e.g., Borrelia burgdorferi and Anaplasma phagocytophilum) [18, 19]. Studies on the role of 4-HNE during infection are still limited. However, it was found that 4-HNE is generated during bacterial infection, that it impacts the growth and survival of a range of bacteria, and that many genes in bacteria are induced in response to 4-HNE exposure [20]. Although it has been known for years that LPO and 4-HNE are associated with inflammation, especially in inflammatory processes based on the oxidative burst of inflammatory cells (granulocytes and macrophages), information on the quantitative changes of 4-HNE-protein adducts in the blood of patients with inflammatory diseases, such as those obtained by 4-HNE-ELISA, is limited.
The outbreak of the COVID-19 pandemic has changed our lives and prompted many researchers to investigate the pathogenesis of this and related diseases (Table 1, Ref. [21, 22, 23, 24, 25, 26, 27, 28, 29, 30]). The occurrence of oxidative stress and its consequences in viral infections such as SARS-CoV-2 infection have been extensively studied and reviewed [19]. Higher 4-HNE levels in the blood of COVID-19 patients were independently associated with a higher risk of intubation or death after 28 days [31]. However, studies focusing on the pathophysiological mechanism of fatal cardiogenic shock in COVID-19 patients showed differences in the accumulation of 4-HNE in the myocardial and renal tissue of COVID-19 patients [21]. Cardiovascular complications, including myocarditis, are considered a potentially lethal feature in severe cases of COVID-19. This finding suggests that ferroptosis, an iron-catalysed form of regulated cell death that occurs as a result of excessive LPO, is an important factor in cardiac damage and multiple organ failure in COVID-19.
| Sample | 4-HNE level/found | Method of detection | 4-HNE-protein adducts level | Method of detection | Ref |
| 4-HNE and 4-HNE-protein adducts in COVID-19 | |||||
| Myocardial and renal tissue | Accumulation of 4-HNE | IHC | - | - | [21] |
| Lungs, brain, heart, liver, kidneys | - | - | Accumulation of 4-HNE-protein adducts | IHC | [23] |
| Lungs and plasma | Accumulation of 4-HNE in the lungs | IHC | Dynamic changes in survivors: ab. 12 pmoles/mg protein in 1st day vs. ab. 16 pmoles/mg prot. in 5th day; and in deceased: ab. 14 pmoles/mg protein in 1st day vs. ab. 16 pmoles/mg prot. in 5th day | ELISA | [22] |
| Plasma | 1.9 nmol/mL in survivors and 1.5 nmol/mL in deceased patients vs. 1.7 nmol/mL in healthy subjects | GC-MS | 5.5 pmol/mg protein in survivors and 5.9 pmol/mg protein in deceased vs. 3 pmol/mg in healthy control | ELISA | [24] |
| Plasma | - | - | 3.1 pmol/mg protein in survivors and 4.9 pmol/mg protein in deceased vs. 1.6 pmol/mg protein in healthy control | ELISA | [25] |
| 4-HNE and 4-HNE-protein adducts in TBE | |||||
| Plasma, urine, CSF | Plasma: 0.45 nmol/mL of TBE patients vs. 0.09 nmol/mL of healthy subjects | GC-MS | Plasma: 18.4 pmol/mg protein of TBE patients vs. 15.2 pmol/mg protein of healthy subjects | ELISA | [27] |
| Urine: 1161 nmol/mol creatinine of TBE patients vs. 891 nmol/mol creatinine of healthy subjects | |||||
| CSF: 14.60 nmol/mL of TBE patients vs. 12.97 nmol/mL of healthy subjects | |||||
| Plasma | - | - | Approximately 2.5-times higher level of 4-HNE-protein adducts in TBE patients in comparison to control | LC-MS | [28] |
| CSF | About 23 nmol/mL in CSF of TBE patients vs. about 13 nmol/mL of healthy subjects | GC-MS | - | - | [29] |
| Plasma | About 30 nmol/mL in plasma of TBE patients and about 17 nmol/mL in plasma of TBE patients after pharmacotherapy vs. about 11 nmol/mL of healthy subjects | GC-MS | 4.5 pmol/mg protein of TBE patients and 3.8 pmol/mg protein of TBE patients after pharmacotherapy vs. 2.7 pmol/mg protein of healthy subjects | ELISA | [30] |
| 4-HNE and 4-HNE-protein adducts in LD | |||||
| Plasma, CSF, and urine | Plasma: 0.92 nmol/mL of LD patients vs. 0.12 nmol/mL of healthy subjects | GC-MS | Plasma: 23.4 pmol/mg protein of LD patients vs. 17.8 pmol/mg protein of healthy subjects | ELISA | [26] |
| Urine: 24.88 nmol/mg creatinine LD patients; 7.51 nmol/mg creatinine of healthy subjects | |||||
| CSF: 25.72 nmol/mL of LD patients; 12.97 nmol/mL of healthy subjects | |||||
| 4-HNE and 4-HNE-protein adducts in co-infection: TBE and LD | |||||
| CSF | About 35 nmol/mL in CSF of TBE patients with coinfection vs. about 13 nmol/mL of healthy subjects | GC-MS | - | - | [29] |
| Plasma | - | - | About 2.5-times higher level of 4HNE-protein adducts in TBE vs. healthy subject and about 4-times higher level in patients with TBE and co-infection | LC-MS | [28] |
| Plasma | About 29 nmol/mL in TBE patients with coinfection and about 16 nmol/mL in TBE patients with coinfection after pharmacotherapy vs. about 11 nmol/ml healthy subjects | GC-MS | 3.5 pmol/mg protein in TBE patients with co-infection; 3.1 pmol/mg protein TBE patients with coinfection after pharmacotherapy vs. 2.7 pmol/mg protein healthy subjects | ELISA | [30] |
4-HNE, 4-hydroxynonenal; CSF, cerebrospinal fluid; GC, gas chromatography; IHC, immunohistochemistry; LC, liquid chromatography; LD, Lyme disease; MS, mass spectrometry; TBE, Tick Born Encephalitis.
Preliminary findings of the study comparing the levels of 4-HNE-protein adducts in the plasma of survivors and non-survivors during a five-day hospitalization in the intensive care unit, as determined by the 4-HNE-ELISA specific for the 4-HNE-histidine adducts, revealed higher levels of the 4-HNE-modified proteins in the plasma of deceased patients at admission and two days later, although no clinical differences were observed between these two groups at that time [22]. On the fifth day, which was critical for the patients’ recovery or lethal outcome, levels of 4-HNE-protein adducts increased slightly in the plasma of survivors, whereas they decreased in the plasma of patients who later passed away. Even more interesting was finding of a dynamic change in the plasma levels of 4-HNE-protein adducts in the survivors during the five-day period, by more than tenfold in just two days, in contrast to the persistence of levels of the 4-HNE-protein adducts in the plasma of patients who did not survive. These findings suggested that the survivors had active oxidative stress response mechanisms to the aggressive COVID-19 that were not effective in the deceased patients. An autopsy of one patient revealed the abundant presence of 4-HNE-protein adducts in the lungs, according to immunohistochemical analysis performed with the same monoclonal antibody specific for the 4-HNE-histidine adducts that was used in the 4-HNE-ELISA [22].
A further study based on immunohistochemical analysis of the vital organs of other deceased COVID-19 patients (without comorbidities) confirmed the association of the 4-HNE-protein adducts with the fatal outcome of the infection, mainly due to the LPO affecting the blood vessels and the appearance of thus generated 4-HNE in the form of protein adducts in the tissues of these organs, but not due to the inflammatory processes in the lungs, brain, heart, liver, or kidneys as such [23]. Hence, further studies were based on the comparison of plasma samples collected at the time of admission of COVID-19 patients to the hospital, i.e., about a week before their death or recovery, which revealed the onset of systemic oxidative stress in all patients, altered lipid metabolism, malfunctioning of the antioxidant capacities of the entire organism, especially affecting granulocytes, and altered plasma protein composition associated with increased levels of 4-HNE-protein adducts, in particular in the patients who eventually passed away [24]. These results suggested that lipid-soluble antioxidants could regulate 4-HNE metabolism and its biological activities in COVID-19 and may be required for maintaining the physiological functions of enzymatic antioxidants, notably superoxide dismutases (SOD-1 and SOD-2).
The level of 4-HNE-protein adducts in plasma samples from COVID-19 survivors averaged 5.5 pmol/mg protein, compared to 5.9 pmol/mg protein in the COVID-19 deceased patients, while the level of 4-HNE-protein adducts in plasma from healthy individuals averaged 3 pmol/mg protein [24]. These findings were confirmed in a similar study that investigated changes in the plasma proteome induced by COVID-19. It was reported that the amount of 4-HNE-modified proteins in the plasma of COVID-19 survivors averaged 3.1 pmol/mg protein, while the amount of plasma proteins modified in COVID-19 deceased and healthy subjects averaged 4.9 pmol/mg protein and 1.6 pmol/mg protein, respectively. Accordingly, we can conclude that COVID-19 is associated with systemic LPO manifested by an increase in 4-HNE-protein adducts as determined by the 4-HNE-ELISA, which may be detected as early as one week before the fatal outcome of the disease [25].
Furthermore, serum 4-HNE levels in community-acquired pneumonia (CAP) were shown to correlate positively with the severity and poor prognosis of CAP patients, indicating 4-HNE involvement in the pathophysiology of CAP. Consequently, it was suggested that 4-HNE may be an early biomarker that enables diagnosis and a better prognosis for patients [32]. Moreover, 4-HNE was shown to be able to induce the cytomegalovirus-1 promoter, suggesting its role in viral reactivation from latency [33].
Elevated 4-HNE levels have also been found in tissues and body fluids caused by other infectious diseases [18]. For example, enhanced formation of the 4-HNE–protein adducts was observed in the blood of patients with malaria on the surface of parasitized cells, reaching several times higher levels if compared to control non-parasitized cells [34]. One of the growing global health problems are diseases caused by tick-borne pathogens, including both viruses and bacteria. The most common tick-borne diseases include Lyme disease and tick-borne encephalitis (TBE). Lyme disease, caused by Borrelia burgdorferi, is the most prevalent tick-borne infection in humans [35]. The most common and early symptom of Borrelia burgdorferi infection is a skin rash called erythema migrans [36], which, if left untreated, leads to inflammation of the joints (Lyme arthritis), the heart, or the nervous system (neuroborreliosis), which are considered the late forms of the disease [37]. The Borrelia burgdorferiinfection is associated with elevated oxidative stress and LPO. In the plasma of patients with Lyme arthritis, a significantly increased level of 4-HNE was found, which is due to increased LPO and decreased glutathione (GSH)-dependent phospholipid protection [38]. The antioxidant defense mechanisms were even more affected in the plasma of neuroborreliosis patients compared to Lyme arthritis [26]. This led to a more pronounced LPO and an elevated level of 4-HNE-modified proteins in the cerebrospinal fluid of patients with neuroborreliosis, which was accompanied by a significant reduction in plasma levels of arachidonic and docosahexaenoic acids, the precursors of 4-HNE [26]. It should be noted that the alteration of protein structures in the brain by 4-HNE-binding may increase the permeability of the blood–brain barrier promoting the spread of LPO [39, 40, 41]. Consequently, due to high 4-HNE reactivity, this may affect the plasma phospholipid profile [42] or protein structure and function [26].
Another tick-borne pathogen that affects the central nervous system is the TBE virus. The TBE virus disrupts metabolic pathways related to redox balance and reduces the protection of brain lipid structures from oxidative damage. This leads to an increased 4-HNE concentration in the plasma, but even more significantly in the cerebrospinal fluid of TBE patients [27]. Elevated plasma 4-HNE concentrations correlated with 4-HNE-modified plasma proteins and were significantly increased in TBE patients if compared to healthy subjects, suggesting that 4-HNE may be a useful biomarker for TBE diagnosis and monitoring. In addition, a study of the plasma proteome of patients with TBE and co-infections [28] revealed that 4-HNE induced by tick-borne diseases binds to proteins involved in the antioxidant response, including GSH transferase, angiopoietin-4, clathrin, protein disulfide-isomerase A3, actinin-4, and peroxiredoxin-5. The level of all 4-HNE-protein adducts was higher in TBE patients than in co-infected patients. It should also be emphasized that 4-HNE modifies structural proteins that may indirectly affect cellular signaling and stress responses [43].
It is estimated that more than 9% of the population suffers from autoimmune diseases, including psoriasis, rheumatoid arthritis, or systemic lupus erythematosus (SLE), commonly known as lupus [44]. These diseases can affect people of all ages and affect any part of their body. However, the biggest problem is the lack of knowledge about the underlying mechanisms that promote the onset and progression of these diseases. Some of them, such as lupus, run in families and may have a genetic basis, while other cases may be triggered by infections, environmental factors, or stress [45]. Regardless of the cause of the disease, the development and progression of autoimmune diseases are associated with an impaired immune response of the body to pathogenic factors [46]. It is inextricably linked to disturbances in the body’s redox status as well as to the formation of signaling molecules that are derivatives of LPO metabolites [47], such as isoprostanes or reactive aldehydes, including 4-HNE [48, 49, 50].
Psoriasis is an example of one of the most common autoimmune diseases. It is caused by an increased interaction between the active cells of the immune system and the keratinocytes, which continuously stimulates them to proliferate, keratinize, and exfoliate. As a result, psoriatic lesions appear on the skin [51]. There are different types of psoriasis in which the active immune system not only affects the function and appearance of the skin (psoriasis vulgaris; PsV), but also, for example, inflammation in the joints (psoriatic arthritis; PsA) [52]. Inflammatory reactions associated with the development of psoriasis are always accompanied by oxidative stress and increased LPO throughout the body, both in the affected skin/joint areas and in the peripheral blood [53, 54].
Today, it is well documented that alterations in lipid metabolism, including ROS-dependent changes that promote the peroxidation of lipids, play an important role in the development of psoriasis [3, 55, 56, 57] (Table 2, Ref. [58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72]). Analysis of 4-HNE protein adducts in skin biopsies revealed a significantly higher incidence of proteins modified with 4-HNE in psoriatic lesions compared to healthy skin [58, 59]. Interestingly, the same was not observed in animal models [60]. In another study, the presence of 4-HNE protein adducts was found to be twice as high in epidermal keratinocytes, the main cells involved in the formation of psoriatic scales [61]. A similar pattern of changes may also be observed in blood cells isolated from psoriatic patients. In erythrocytes, lymphocytes, and granulocytes, the level of 4-HNE measured by gas chromatography–mass spectrometry (GC-MS) appeared to be up to 30% higher in cells isolated from psoriatic patients than in cells obtained from healthy individuals [62]. In addition, although the lymphocytes from PsA patients contained more free 4-HNE when compared to the lymphocytes of PsV patients, the presence of 4-HNE-protein conjugates was greater in PsV [63]. Similarly to immune system cells, plasma samples from both PsV and PsA patients can have elevated amounts of the free 4-HNE and 4-HNE-protein conjugates if compared with healthy volunteers [64].
| Sample | 4-HNE level | Method of detection | 4-HNE-protein adducts level | Method of detection | Ref |
| 4-HNE and 4-HNE-protein adducts in psoriasis | |||||
| Human skin biopsy | Increase in Ps vs. healthy subjects | IHC | - | - | [58] |
| Human skin biopsy | Increase in Ps vs. healthy subjects | WB | - | - | [59] |
| Skin biopsy (mouse model) | No changes in this model | IHC | - | - | [60] |
| Keratinocytes from patients with PsV and heathy subjects | 6.5 pmol/mg protein PsV patients; 12 pmol/mg protein healthy subjects | GC-MS | No differences | ELISA | [61] |
| Keratinocytes and lymphocytes from patients with PsV and heathy subjects | - | Keratinocytes: 3-times higher in PsV than in control cells; Lymphocytes - 2-times higher in PsV than in control cells | LC-MS/MS | [65] | |
| Erythrocytes, lymphocytes, granulocytes from patients with PsV and healthy subjects | Erythrocytes: 29.5 nmol/mL PsV patients; 17.5 nmol/mL healthy subjects | GC-MS | Erythrocytes: 1.15 pmol/mg protein PsV patients; 0.65 pmol/mg protein healthy subjects | ELISA | [62] |
| Lymphocytes: 1.4 nmol/mL PsV patients; 1.1 nmol/mL healthy subjects | Lymphocytes: 1.70 pmol/mg protein PsV patients; 0.5 pmol/mg protein healthy subjects | ||||
| Granulocytes: 9.5 nmol/mL PsV patients; 15.5 nmol/mL healthy subjects | Granulocytes: 2.35 pmol/mg protein PsV patients; 1.80 pmol/mg protein healthy subjects | ||||
| Lymphocytes from patients with PsV and PsA healthy subjects | 10% and 90% higher, in PsV and in PsA respectively than in healthy subjects | GC-MS | 100% and 30% higher in PsV and in PsA than in healthy subjects | ELISA | [63] |
| Plasma from patients with PsV, PsA and healthy subjects | 15.36 nmol/mL PsV patients; 11.01 nmol/mL PsA patients; 8.90 nmol/mL healthy subjects | GC-MS | 21.59 pmol/mg protein PsV patients; 17.45 pmol/mg protein PsA patients; 15.24 pmol/mg protein healthy subjects | ELISA | [64] |
| Plasma from patients with PsV and healthy subjects | - | 2-times higher in PsV than in healthy subject; various functions of proteins creating adducts with 4-HNE | LC-MS/MS | [66] | |
| 4-HNE and 4-HNE-protein adducts in RA | |||||
| Synovial fluid and plasma from patients with RA and with osteoarthritis | Synovial fluid: 0.54 µmol/L RA patients; 0.24 µmol/L osteoarthritis patients | GC-MS | - | - | [67] |
| Plasma: 0.34 µmol/L RA patients; 0.09 µmol/L osteoarthritis patients | |||||
| Plasma from patients with RA and healthy subjects | 375 pmol/mL in RA patients vs. 169 pmol/mL in healthy subjects | GC-MS | 22.4 pmol/mg protein in RA patients; 17.5 pmol/ mg protein in healthy subjects | ELISA | [68] |
| About 8.5 µg/mL [RA patients without treatment] and about.13 µg/mL [RA patients with treatment] vs. about 3.5 µg/mL in healthy subjects | ELISA | - | - | [69] | |
| About 9.5 µmol/mL in RA patients vs. about 2 µmol/m in healthy subjects | HPLC | - | - | [70] | |
| - | - | About 2-times higher in RA than in healthy subject | ELISA | [71] | |
| Serum from patients with RA and healthy subjects | - | - | One-third higher in RA than in healthy subject | WB-MS | [72] |
| Urine from patients with RA and healthy subjects | 76.1 nmol/mg creatinine in RA patients; 10.7 nmol/mg creatinine in healthy subjects | GC-MS | - | - | [68] |
GC, gas chromatography; IHC, immunohistochemistry; LC, liquid chromatography; MS, mass spectrometry; Ps, psoriasis; PsA, psoriatic arthritis; PsV, psoriasis vulgaris; WB, Western Blotting.
The changes in 4-HNE levels observed in the skin, blood cells, and plasma of psoriatic patients lead to enhanced levels of 4-HNE-protein adducts, which could be measured by 4-HNE-ELISA. In addition to the ELISA, changes of the 4-HNE-protein adducts can be assessed by proteomic analysis, which, in addition to providing information on the amount of 4-HNE-protein modifications, also completes the data with the identity and type of modified proteins [6, 73, 74, 75]. However, one should bear in mind that, due to the differences in the nature of the techniques used, they may give different results. For example, the 4-HNE-ELISA showed no statistically significant differences between psoriatic and control keratinocyte samples [61], whereas LC-MS analysis detected approximately three times more 4-HNE-modified proteins in psoriatic keratinocytes than in controls [65]. Proteomic studies also showed that the proteins adducted with 4-HNE differ between control samples and the samples of psoriatic patients. In controls, the 4-HNE-protein adducts were mainly found on structural proteins, whereas in patients, the 4-HNE adducts were identified on catalytic, antioxidant, apoptotic, and binding proteins [65, 66]. In addition, 4-HNE was found to modify receptors, signal transducers, and enzymes in the cells of psoriatic patients, whereas in the cells of healthy volunteers 4-HNE modifications were found mainly on binding proteins [65].
Rheumatoid arthritis (RA), an autoimmune disease that affects the lining of the joints, causing painful swelling and leading to bone erosion and joint deformation, is accompanied by oxidative stress and inflammation [76]. Therefore, the markers of LPO and their interactions with proteins are being investigated as potential biomarkers or pharmacological therapeutic targets [3, 48, 77, 78, 79](Table 2). Increased levels of 4-HNE were found in the synovial fluid of patients with RA as early as the 1990s [67]. This is also reflected in the plasma of these patients, where the levels of 4-HNE, measured by HPLC, GC-MS, or ELISA, were up to four times higher in RA if compared to healthy individuals [68, 69, 70]. In addition, an up to 7-fold higher level of 4-HNE was found in the u rine of RA patients [68]. Since 4-HNE is an integral component of numerous intracellular pathways, its increased level in synovial cells from RA patients or synoviocytes cultured in vitro is a factor that directly induces pro-inflammatory signaling in these cells [80, 81, 82, 83]. Elevated amounts of 4-HNE promote the formation of 4-HNE-protein adducts, as evidenced by 4-HNE-ELISA in the plasma/serum of RA patients [68, 71, 72].
Moreover, mass spectrometry analysis has shown that 4-HNE forms adducts with various amino acids in RA patients compared to healthy individuals [72]. These results are also reflected in the case of another LPO product, malondialdehyde (MDA), which was also elevated in the blood of RA patients [84, 85], along with an increase in the amount of MDA-protein adducts [86].
Different autoimmune diseases have been found to be closely associated with amyotrophic lateral sclerosis (ALS), enabling the development of strategies targeting the associated autoimmune inflammation [87]. Oxidative stress markers, especially 4-HNE, have been found elevated in ALS and may represent a reliable indicator of the disorder [88].
Autoimmunity is also closely associated with insulin resistance [89, 90]. Adipocytes from insulin-resistant individuals accumulate more endogenous 4-HNE if compared with insulin-sensitive individuals, yielding significantly more proteins modified with 4-HNE as determined by the 4-HNE-ELISA [91]. Moreover, 4-HNE affects insulin signaling, thus triggering the insulin-resistance phenotype [91]. In addition, plasma levels of 4-HNE, determined by the 4-HNE-ELISA, were markedly increased in non-diabetic obese individuals and were negatively correlated with insulin sensitivity [92]. The interrelation between inflammation and adipocytes, which is at least in part attributed to 4-HNE, results in a vicious cycle that maintains persistent inflammation and consequently insulin resistance [93]. Non-alcoholic fatty liver disease (NAFLD) is closely associated with resistance to insulin and high oxidative stress. Treatment of patients with antioxidant vitamin E significantly reduced the presence of 4-HNE protein adducts in liver biopsies of NAFLD patients [94], while similarly elevated 4-HNE-protein adducts were found in patients with alcoholic liver disease [95].
The development of cancer is associated with a disturbed redox balance and a shift towards oxidants. The alteration of major antioxidant defense systems in tumorigenesis in a number of different cancers was recently reviewed [96]. In addition, inflammation, a hallmark of cancer, is closely linked with oxidative stress and can determine the fate of tumor cells. For example, an inflammation-induced moderate increase in oxidative stress may decrease the rate of tumor growth or even promote tumor regression, while the formation of ROS in excessive amounts promotes tumor progression [97, 98, 99]. Consistent with the observed level of oxidative stress is the extent of reactive aldehyde formation, as well as their dual role in tumorigenesis [3, 41, 99]. The amount of 4-HNE protein adducts was found to correlate with the severity of the disease, as evidenced, for example, for brain tumors [41, 100] and squamous cell carcinoma of the oropharynx [101]. It has also been suggested that the formation of 4-HNE-protein adducts in benign and in neoplastic cells may be an important mechanism for controlling tumor growth [102]. For example, a number of early studies have shown that the formation of protein-HNE adducts in renal and colon cancer cells and tissues is related to the growth and progression of kidney and colon cancer [103, 104]. On the other hand, silencing the catalase of cancer cells contributes to triggering both apoptosis and necrosis of cancer cells [105] (Table 3, Ref. [106, 107, 108, 109, 110, 111, 112, 113, 114]).
| Sample | 4-HNE level | Method of detection | 4-HNE-protein adducts level | Method of detection | Ref |
| Plasma of benign and cancer patients undergoing midline laparotomy | Not determined | - | Concentration of 4-HNE-adducts in plasma samples was decreased by 50% in both groups postoperatively | ELISA | [109] |
| Lung tissue of SCC patients and normal tissue | 9.63 nmol/mL in SCC; 3.46 nmol/mL in no-changed tissue | GC-MS | 73.02 pmol/mg protein in SCC; 50.07 pmol/mg in no-changed tissue | ELISA | [106] |
| Lung tissue of AC patients and non-changed tissue | 8.08 nmol/mL in AC vs. 3.46 nmol/mL no-changed tissue | GC-MS | 103.49 pmol/mg protein in AC vs. 59.87 pmol/mg in no-changed tissue | ELISA | [106] |
| Primary NSCLC and secondary lung tumors (metastasis) | Primary and secondary malignant cells showed similar 4-HNE intensity; negative correlation was observed between 4-HNE intensity of the surrounding tissue | IHC | Intensity of 4-HNE-protein adducts in the surrounding lung tissue was more pronounced in the metastatic than in the primary tumors | IHC | [110] |
| Serum from patients with lung cancer | Women: 31.87 ng/mL lung cancer; 14.82 ng/mL control | ELISA | - | ELISA | [111] |
| Men: 19.97 ng/mL lung cancer; 13.76 ng/mL control | |||||
| Breath samples from patients with NSCLC, lung SCC and control subject | ab. 0.002 nmol/L in NSCLC and ab. 0.012 nmol/L in SCC patients with control ab. 0 nmol/L | FT-ICR-MS | Not determined | - | [107] |
| Human osteosarcoma cells | Not determined | - | Amount of HNE-protein adducts is higher in malignant cells compared to normal cells | ELISA | [112] |
| Human osteosarcoma cells | Nondifferentiated cells are more efficient in metabolizing 4-HNE compared to differentiated cells | LC-UV | Amount of HNE-protein adducts in differentiated HOS is double compared to nondifferentiated cells, one hour post exposure to 4-HNE | ELISA | [113] |
| Prostate carcinoma tissue and plasma | Absence of 4-HNE in prostate tissues, both cancer and non-malignant cells | IHC | Highly increased 4-HNE-protein level in plasma of patients with prostate cancer (about 14.5 vs. about 4 pmol/mg protein) | ELISA | [108] |
| Plasma samples of breast cancer patients | Not determined | - | In cancer patients 4-HNE-PDGF adducts were significantly lower compared to controls | ELISA | [114] |
AC, adenocarcinoma; GC, gas chromatography; IHC, immunohistochemistry; LC, liquid chromatography; MS, mass spectrometry; NSCLC, non-small cell lung cancer; SCC, squamous cell carcinoma; FT-ICR-MS; nanoelectrospray Fourier transform-ion cyclotron resonance mass spectrometry.
Because of their structure and function, the lungs are extremely susceptible to oxidative stress, while the production of ROS is further exacerbated by air pollutants [115, 116]. In addition, data from the literature show decreased antioxidant activity in cancerous lung tissue and increased antioxidant activity in the blood cells of patients with lung cancer [106]. This promotes the oxidation of cellular components, including phospholipids, yielding reactive aldehydes such as 4-HNE, MDA, and 4-oxonon-2-enal, which accumulate in non-small cell lung cancer (NSCLC), which accounts for more than 80% of all lung cancer cases [39, 106]. The changes in lipid metabolism during carcinogenesis also depend on the type of tumor, even if it originates from the same organ. This has also been observed in lung cancer, reflecting great variability between the same tumor cell types. The amount of 4-HNE in plasma significantly differs between patients with small cell lung cancer and patients with NSCLC, and could therefore be used in combination with computed tomography for additional non-invasive differentiation of these types of lung cancer [107]. Histochemical studies suggest that 4-HNE may also act as a specific anti-cancer compound produced by inflammatory cells, and may act as a kind of natural cytostatic agent [99]. Immunohistochemical studies have also shown that a low level of 4-HNE in lung squamous cell carcinoma (SCC) cells is a predictor of poor prognosis and resistance to chemotherapy [117]. Regardless of the level of 4-HNE in cancerous tissue, the 4-HNE content in the exhaled air of patients also distinguishes between patients with lung cancer and those with benign lung nodules [118], which could support such a non-invasive diagnostic.
Previous studies indicated that reactive aldehydes formed in the process of LPO and interacting with nucleophilic compounds, including proteins, are involved in intracellular signaling pathways and the activation of transcription factors in cancer cells. Proteins rich in nucleophilic amino acids, such as cysteine, histidine, and lysine, are extremely susceptible to interactions with electrophilic aldehydes, including 4-HNE [39, 119]. Data from the literature suggest that up to 8% of 4-HNE formed in cells can interact with proteins to form 4-HNE-protein adducts, particularly via the thiol group of cysteine [39, 120]. Since thiol groups often function as redox switches that control cell signaling and metabolism, the binding of 4-HNE to thiol groups may have pathophysiological consequences [39].
As a signaling molecule, 4-HNE stimulates gene expression by regulating
transcription factors such as nuclear factor erythroid 2-related factor 2 (Nrf2)
and nuclear factor kappa B (NF
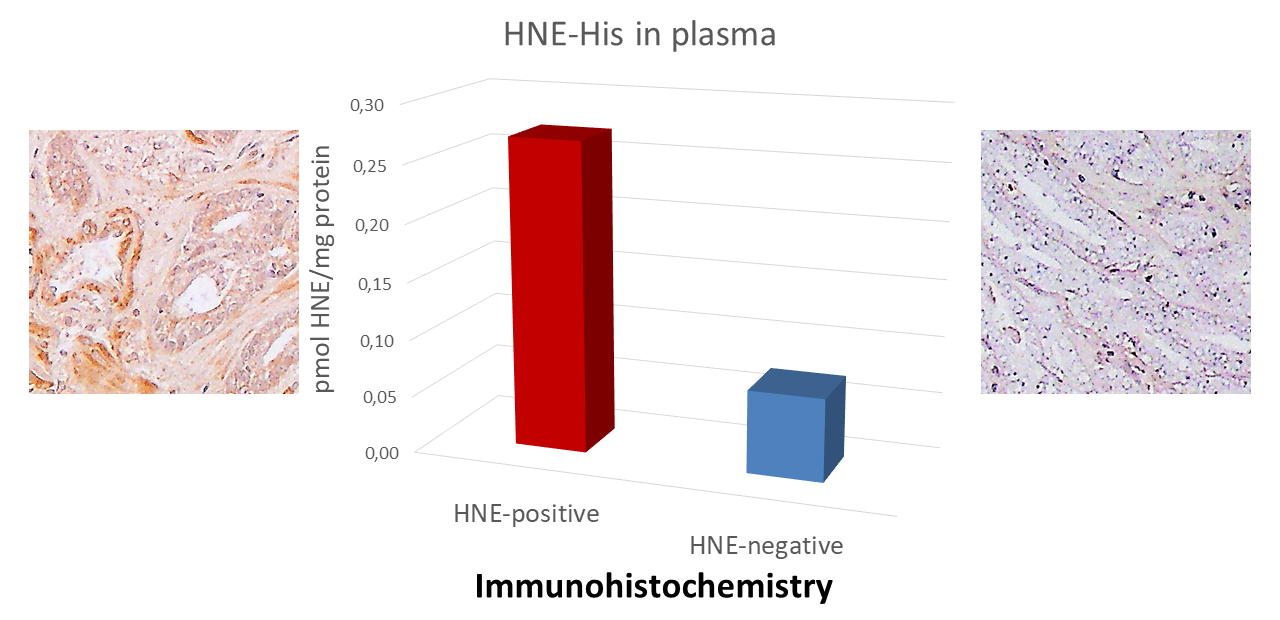 Fig. 2.
Fig. 2.Comparison of the ethylenediaminetetraacetic acid tetrasodium salt dihydrate (EDTA)-plasma levels of the 4-HNE-protein adducts determined by the 4-HNE-ELISA with the immunohistochemistry of the prostate carcinoma tissue. 4-HNE, 4-hydroxynonenal.
Plasma samples were collected before surgery from the two patients with similar
status and laboratory parameters. The left example patient age = 63, PSA = 5.82
(ng/mL), cancer stage GS 3+4=7 (pT2c+N0MX), the right example patient age = 64,
PSA = 5.22 (ng/mL), cancer stage GS 3+4 = 7 (pT2c+N0MX). Immunohistochemistry of
the prostate carcinoma tissue specimens obtained by surgery was done using the
same monoclonal antibody as for the 4-HNE-ELISA specific for the 4-HNE-histidine
adducts. The presence of the 4-HNE-protein adducts was visualized by the
3,3
Approximately 30% of proteins modified by 4-HNE are localized in the mitochondria [128]. Therefore, it is hypothesized that strategies focusing on the manipulation of mitochondrial ROS production and 4-HNE formation may have therapeutic significance for the treatment or prevention of cancer [129, 130]. Today various effective anticancer treatments, including pharmacotherapy [130], surgery, and radiotherapy, rely on oxidative stress and LPO generating 4-HNE, which is more cytotoxic for malignant cells than for normal cells [131].
As mentioned already, acrolein was found to be useful for prediction of prostate cancer progression (relapse) with 90% accuracy if tumor-positive surgical margins, stage of disease, and the intensity of acrolein presence in tumor stroma were taken together [127]. However, the appearance of acrolein in prostate cancer might not be due to the LPO, but also the spermine/spermidine metabolism [132]. On the contrary, the recent study found in more than 90% of prostate carcinoma specimens no presence of 4-HNE-protein adducts at all, neither in the tissue of prostate cancer [108], nor even in the non-malignant prostate tissue adjacent to the cancer, which is different from the other types of human malignancies studied so far by immunohistochemistry specific for the 4-HNE-histidine adducts. While it is not unusual that increased levels of reactive aldehydes, such as 4-HNE, can initially be increased in carcinogenesis to be reduced afterwards in developed cancer tissue [133], the increase of 4-HNE in the normal cells in the vicinity of cancer might be considered a defense mechanism against cancer [108]. Namely, 4-HNE may act as a systemic not only local tissue factor of carcinogenesis [133, 134] on one side, while on the other, 4-HNE may also be considered a natural anti-cancer substance. Accordingly, 4-HNE may also be considered an important factor in the spontaneous regression of cancer [101].
Changes in the presence of 4-HNE conjugates, as observed during tumorigenesis, are not only specific for cancer, but very similar findings were also observed for patients with post-traumatic stress disorder (PTSD). Even thirty years after the combat stress that caused PTSD, the difference in the levels of 4-HNE-protein adducts in their plasma increased “only” by about 20% on average (26 vs. 22.5 pmol/mg protein) when compared to the levels found in the other warriors who did not suffer from PTSD [135]. The high values observed in the healthy men again confirmed that the type of monoclonal antibody and the protocol used are crucial aspects in the analysis of 4-HNE-protein adducts by 4-HNE-ELISA, as well as the kind of samples analyzed. Indeed, in most of the studies presented in this review, the 4-HNE-ELISA was used on human sera or ethylenediaminetetraacetic acid tetrasodium salt dihydrate (EDTA) plasma samples, whereas in this study, the acid citrate dextrose was used for the plasma collection.
It has also been suggested that the accumulation of 4-HNE-protein adducts in some patients is the main cause of age-associated neurodegenerative diseases. Elevated levels of 4-HNE and 4-HNE-protein adducts have previously been found in the neurons of Parkinson’s patients [136, 137, 138]. In addition, increased levels of 4-HNE-protein adducts have been reported in various brain regions and in the body fluids of patients with Alzheimer’s disease, Huntington’s disease, and amyotrophic lateral sclerosis, as well as in animal models of these diseases [139, 140].
This review covered currently available information on the applicability of different commercial and non-commercial 4-HNE-ELISAs in clinical studies involving patients with different diseases known to be associated with oxidative stress, LPO, and 4-HNE (Fig. 3). Despite the differences in the protocols and antibodies used, all studies confirmed the usefulness of 4-HNE-ELISA for research purposes. In addition, although mass spectrometry analysis is a valuable tool for the identification and quantification of proteins modified with 4-HNE, due to its timely and costly analysis, the 4-HNE-ELISA is frequently a preferred choice to determine the amount of proteins modified with 4-HNE.
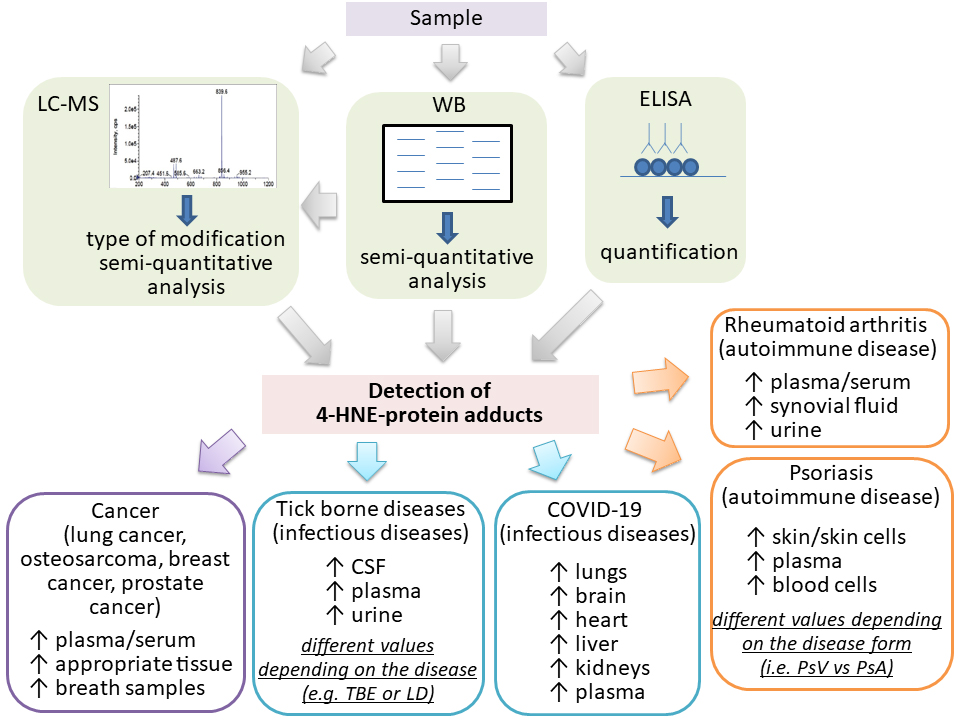 Fig. 3.
Fig. 3.Increased levels of the 4-HNE-protein adducts in human plasma and/or samples measured by LC-MS, WB or ELISA in the human diseases studied, which also included healthy controls. LC–MS, liquid chromatography–mass spectrometry; WB, western blotting; CSF, cerebrospinal fluid; Arrows indicate increase.
Because different protocols and the antibodies used could give very different values of the 4-HNE-protein adducts, the 4-HNE-ELISA should be combined with the other complementary analytical methods and should be used to allow comparisons between the values obtained for patients and healthy people. In spite of protocol and antibody differences and the use of the different non-SI units, all the studies analyzed have shown mostly significantly increased levels of 4-HNE-protein adducts for about 50 to 300% in the samples obtained from patients when compared with the healthy people of the respective control group.
As in any other biomarker studied in patients. it is preferred to have not only single time analysis, but multiple time point measurements done to allow monitoring of the dynamics of the onset of oxidative stress and the systemic response to the disease causing it. That is valid in particular for acute diseases because individual values of 4-HNE-protein adducts could range in the blood in dependence of the health condition even more than 3-fold in a period of days, as was shown for the COVID-19 patients.
NŽ and ES designed the study. NŽ, AG, WŁ, MJ, SBŠ, KŽ and ES acquired, analyzed and interpreted the data. NŽ, AG, WŁ, MJ, SBŠ, KŽ and ES wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
The authors extend their appreciation to the COST Action B35 and project CRO_A-00033.
This research received no external funding.
The authors declare no conflict of interest. Given the role as Editorial Board Member, Neven Žarković had no involvement in the peer-review of this article and has no access to information regarding its peer-review. Full responsibility for the editorial process for this article was delegated to Giuseppe Murdaca.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.



