1 Institute of Antler Science and Product Technology, Changchun Sci-Tech University, 130600 Changchun, Jilin, China
2 College of Chinese Medicinal Materials, Jilin Agricultural University, 130118 Changchun, Jilin, China
3 Scientific Research center, China-Japan Union Hospital, Jilin University, 130033 Changchun, Jilin, China
†These authors contributed equally.
Abstract
Background: Type 1 diabetes mellitus (T1D) represents a severe threat
to human health. Persistent hyperglycemia and dyslipidemia can lead to damaged
liver function, while effective interventions for these complications are
currently lacking. Deer antler stem cells (AnSCs), a novel type of adult stem
cells, significantly reduced liver injury, which was speculated to be achieved
through the paracrine pathway. Methods: In this study, AnSC-conditioned
medium (AnSC-CM) was used to treat C57BL/6 mice with T1D symptoms induced by
streptozotocin (STZ). The therapeutic effects of AnSC-CM on T1D were evaluated,
and the underlying mechanism was investigated. Results: It was shown
that AnSC-CM alleviated the T1D symptom: decreased body weight, increased blood
glucose levels and islet lesions, and reduced insulin secretion. Moreover,
AnSC-CM treatment improved liver function and mitigated liver injury in T1D mice.
Impressively, the therapeutic effects of AnSC-CM on T1D were better than those of
bone marrow mesenchymal stem cell-CM (BMSC-CM). The mechanistic study revealed
that AnSC-CM significantly downregulated the NF-
Keywords
- deer antler stem cells
- conditioned medium
- type 1 diabetes mellitus
- liver injury
- NF-κB signaling pathway
Type 1 diabetes mellitus (T1D) is a severe threat to human health. The most
direct result is absolute insulin deficiency, which leads to elevated blood
glucose levels and the development of life-threatening complications from
diabetes, such as liver injury [1, 2]. The liver is a vital organ that metabolizes
glucose and lipids; persistent hyperglycemia and dyslipidemia can attenuate
metabolic pathways affecting the liver. People with diabetes have a substantially
increased risk of liver damage and liver fibrosis, which can progress to
cirrhosis and even liver cancer if left untreated [2, 3]. However, effective early
interventions for T1D and related induced liver injuries are currently lacking.
NF-
Of all the currently available treatments for T1D and its induced liver injury, mesenchymal stem cell (MSC) transplantation has repeatedly been proven as an effective approach in translational and clinical practices due to the regenerative and immunomodulatory capabilities of MSCs [5, 6]. Thus far, the accumulated evidence has demonstrated that the effects of transplanted MSCs are mainly punctuated through the paracrine pathway [5]. Therefore, conditioned medium from MSCs (MSC-CM) is reported to have similar therapeutic effects as MSCs since it would contain the paracrine factors released from cultured MSCs [7, 8, 9]. Moreover, MSC-CM provides several advantages over using MSCs, such as a lower risk of tumorigenesis, a simpler handling process, and negligible immunogenicity [1].
Deer antlers are the only mammalian organs that regenerate each year, a process known to be mediated by antler stem cells (AnSCs) [10, 11, 12]. Compared to the other types of stem cells, AnSCs have the advantages of easier acquisition and ex vivo expansion [13]. In one of our previous studies, AnSCs demonstrated significant efficacy in treating liver injuries [14]. Thus far, the role of AnSC-conditioned medium (AnSC-CM) in treating liver injury, especially in treating diabetic liver injury associated with T1D, has not been studied.
This study focused on the effect of AnSC-CM on T1D and diabetic liver injuries.
Studies have shown that AnSC-CM not only relieves T1D symptoms but also
T1D-induced liver injury and is more effective than bone marrow MSC-conditioned
medium (BMSC-CM). The mechanistic study suggests that the therapeutic effect of
AnSC-CM may be achieved by targeting the NF-
AnSC-CM was prepared using AnSCs (2–5th passage), described in our previous study [15]. Briefly, AnSCs were cultured in Dulbecco’s Modified Eagle Medium (DMEM, 01-052-1ACS, BI, Ness Ziona, Israel) supplemented with 10% fetal bovine serum (FBS, 04-001-1ACS, BI, Brisbane, Australia) until they reached 90% confluency, then, the medium was replaced with an FBS-free medium and the cells cultured for another 48 h. The supernatants were collected and concentrated via ultrafiltration using a spin column with molecular weight cut at 3 kDa (Millipore Corp, Billerica, MA, USA). The AnSC-CM concentration was measured using the BCA assay (P0012, Beo Tianmei, Shanghai, China).
Mouse BMSC-CM was prepared following the same procedure as for AnSC-CM.
All cell lines were validated by STR profiling and tested negative for
mycoplasma. Cells were all cultured in a humidified incubator at 37 °C
and 5% CO
Female C57BL/6 mice, 6–8 weeks old, were chosen for this study, and the procedure for handling animals was approved by the Animal Ethics Committee of Changchun Sci-Tech University (No. CKARI202110). In total, 24 mice were allocated to the treatment groups and injected intraperitoneally with streptozotocin (STZ, A610130, Shangon Biotech, Shanghai, China) (35 mg/kg) for five days (1/day) to induce T1D [16]; 8 mice were in the control group and injected intraperitoneally with PBS for five days (1/day). The STZ-treated mice were divided into three groups: STZ (negative control), BMSC-CM (positive control), and AnSC-CM conducted preventive treatment with PBS, 100 µg/50 µL of BMSC-CM, and 100 µg/50 µL of AnSC-CM, respectively, in the second week. The control (CTRL) group mice were injected with PBS. All injections were performed through the mouse tail vein for 4 weeks (2/week), and the mice were euthanized on day 5 after the last CM injection. Body weight and blood glucose changes in each mouse were measured once a week. Four weeks after treatment, an oral glucose tolerance test (OGTT) was performed [17].
The blood samples were collected and centrifuged to separate the serum (1200 g, 10 min). Then, the concentrations of alanine aminotransferase (ALT), aspartate aminotransferase (AST), malondialdehyde (MDA), and superoxide dismutase (SOD) were measured using the respective kits (COO9-1-1, CO10-1-1, A001, and A003-1, Nanjing Jiancheng, Nanjing, China).
A total of 0.5 mL of physiological saline was added to 50 mg of mouse liver
tissue for homogenization, then centrifuged at 3000 rpm and 4 ℃ for 10 min to
collect the supernatant. Next, the levels of interleukin 1
Pancreas and liver tissues were fixed for 24 hours (10% formalin was chosen as the fixing solution) and dehydrated by a gradient of ethanol solutions. Then, the tissues were embedded in paraffin, cut into 5 µm sections, and stained with hematoxylin and eosin (H&E) before immunohistochemical (IHC) analysis was performed.
IHC staining was performed using the kit (KIT-9710, Maixin, Fuzhou, China), as described in the instructions. The primary antibodies, anti-PCNA (ab15497, Abcam, Cambridge, UK) and anti-INS (tech66198-1-Ig, Proteintech, Wuhan, China), were used in this experiment. Images were captured under a microscope (M8 PreciPoint, Freising, Germany) and evaluated using Image-Pro Plus 6.0 software (MEDIA CYBERNETICS, Rockville, MD, USA).
Western blot analysis was performed using the methods reported in a previous
study [18]. Briefly, the protein was extracted from the pancreas and liver
tissues of mice using radioimmunoprecipitation assay (RIPA) lysis buffer.
Proteins were separated on SDS-polyacrylamide gels and then electrically
transferred onto a nitrocellulose membrane. Next, membranes were incubated with
the indicated primary antibody: p65 (CST8242), p-p65 (CST3033),
IKB
Data are presented as mean
The changes in body weight and fasting blood glucose level of mice treated with
AnC-CM were measured. The results showed that STZ treatment significantly
decreased body weight and increased fasting blood glucose levels in mice.
Additionally, these changes were significantly attenuated four weeks after the
treatment by administering AnSC-CM (p
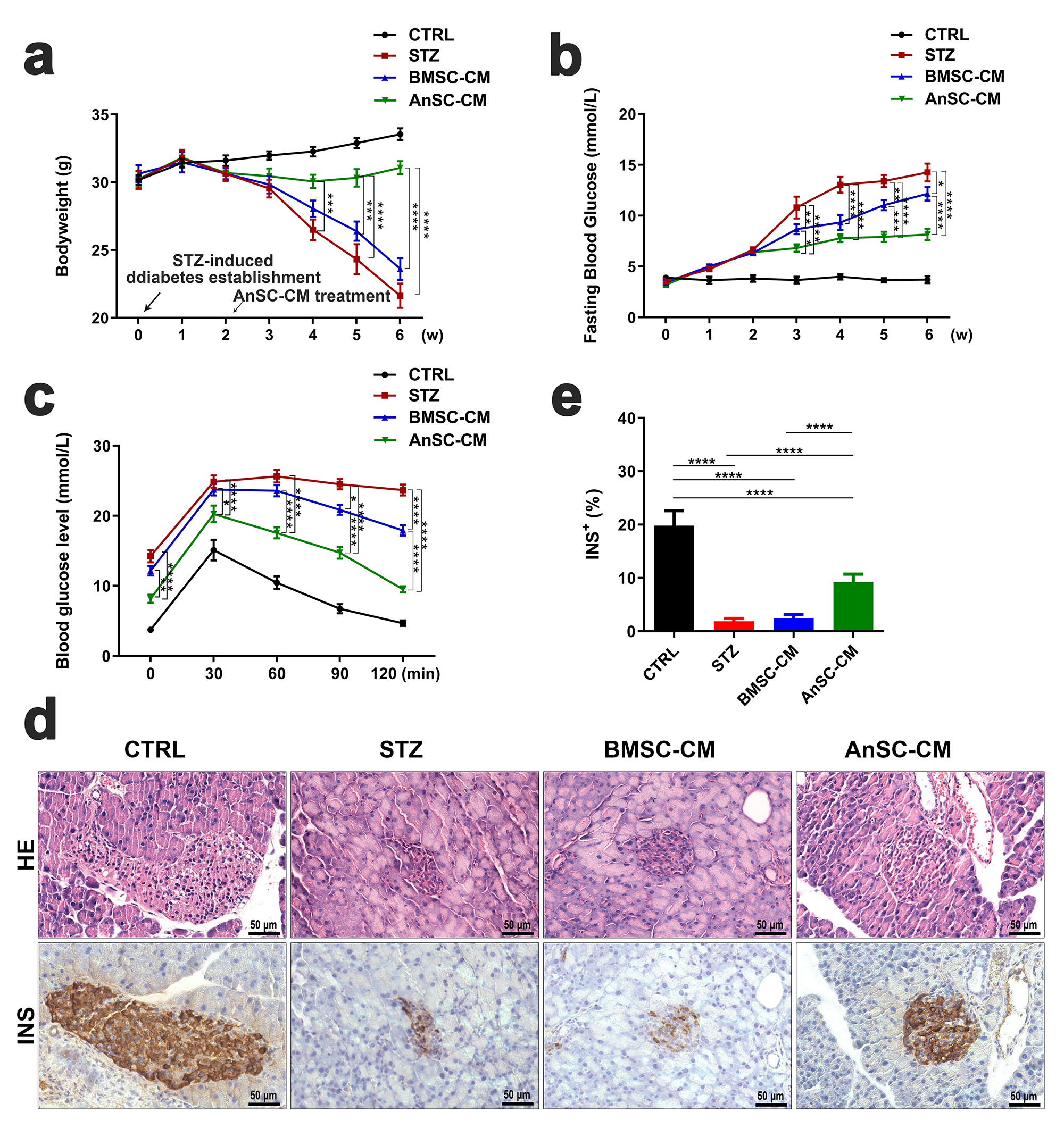 Fig. 1.
Fig. 1.Effects of AnSC-CM on mice with T1D. (a,b) Body weight and
fasting blood glucose levels of mice were measured weekly (w). (c) Glucose
tolerance levels in each group. (d) H&E staining and immunohistochemical
evaluation of INS in the pancreatic tissue; scale bar = 50 µm. (e)
INS-positive staining in the pancreatic tissue. H&E, hematoxylin and eosin; T1D,
type 1 diabetes mellitus; AnSC-CM, antler stem cell conditioned medium; BMSC-CM,
bone marrow mesenchymal stem cell conditioned medium; CTRL, control; INS,
insulin; STZ, streptozotocin. *p
Serum parameters (AST, ALT, and MDA), which are associated with liver injury,
were measured. The results showed that AST, ALT, and MDA levels in the AnSC-CM
group were significantly lower than in the STZ group (Fig. 2a–c; p
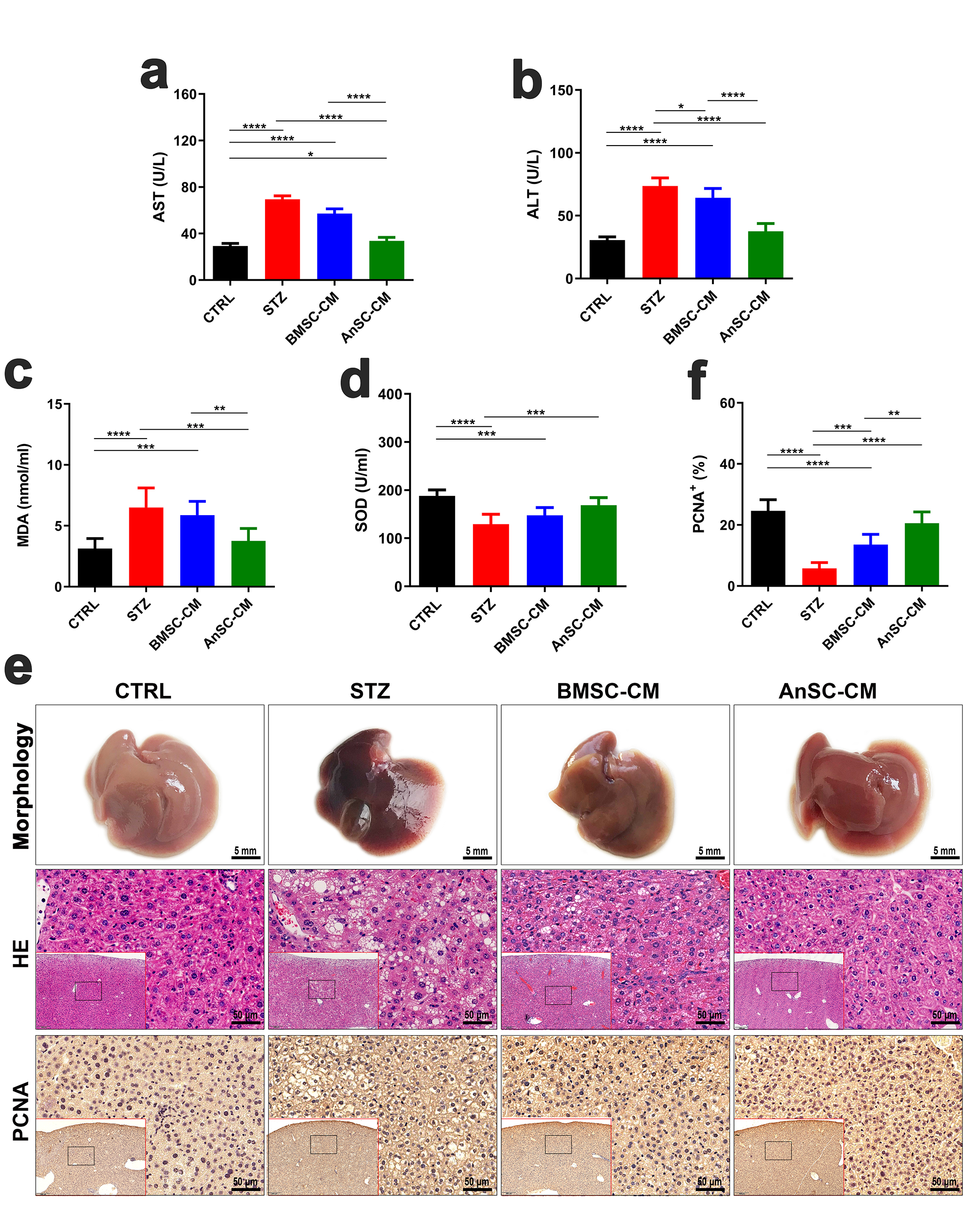 Fig. 2.
Fig. 2.Effects of AnSC-CM on injured livers induced by STZ in T1D mice. (a,b,c,d) Serum levels of aspartate aminotransferase (AST), alanine
aminotransferase (ALT), malondialdehyde (MDA), and SOD (superoxide dismutase).
(e) Morphology (scale bar = 5 mm), H&E staining (scale bar = 50 µm), and
immunohistochemical localization of proliferating cell nuclear antigen (PCNA)
(scale bar = 50 µm) in the liver tissues. (f) Quantification of
PCNA-positive staining. *p
We further measured the expression levels of genes related to the NF-
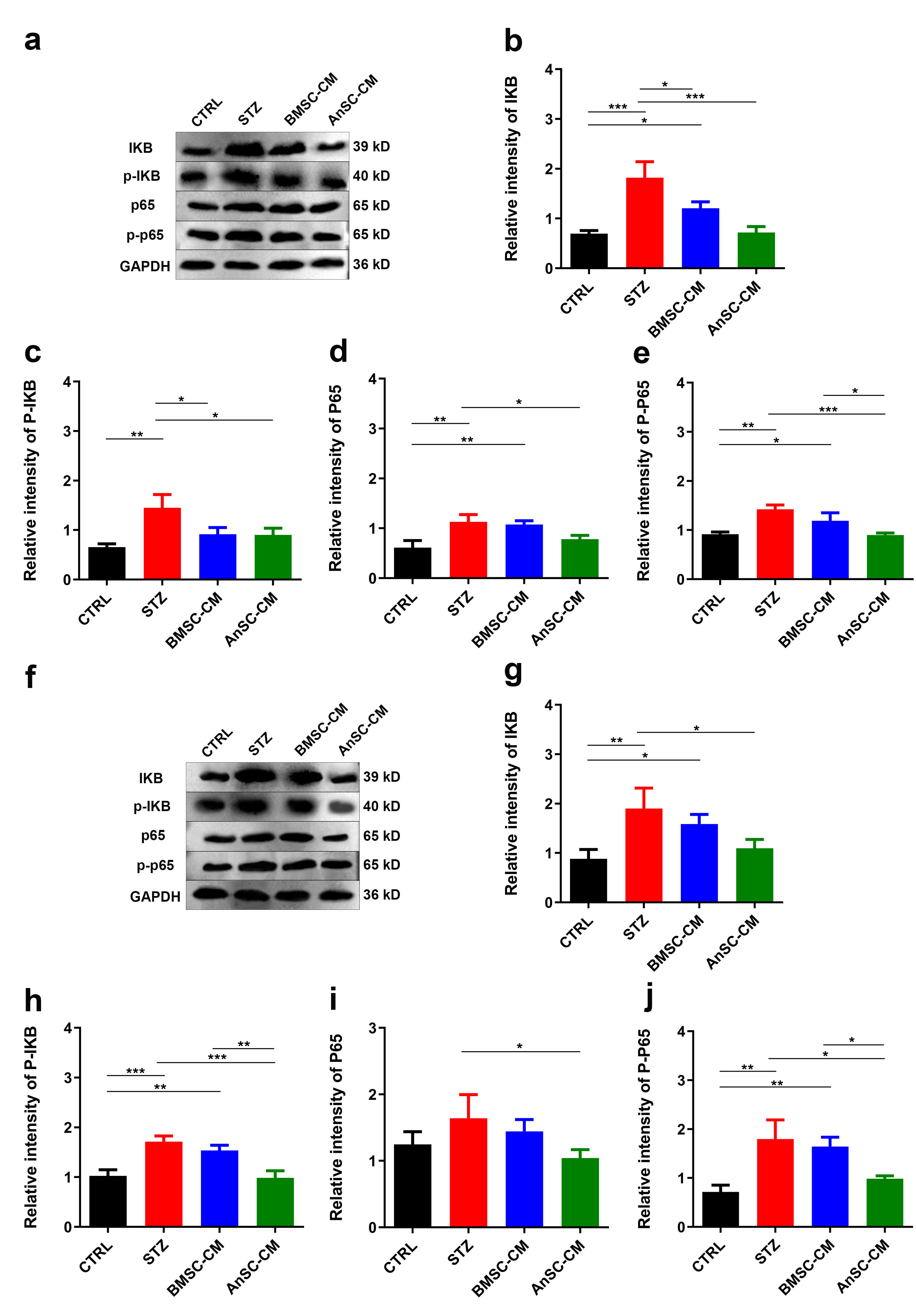 Fig. 3.
Fig. 3.Effects of AnSC-CM on protein expressions of p65, p-p65,
IKB, and p-IKB in liver and pancreatic tissues of T1D mice.
(a) Western blot protein bands of p65, p-p65, IKB, and p-IKB in
liver tissues. (b–e) Quantification of relative intensities of protein bands in
(a). (f) Western blot protein bands of p65, p-p65, IKB, and
p-IKB in pancreatic tissues. (g–j) Quantification of relative
intensities of protein bands in (f). *p
Mesenchymal stem cell therapy has been recognized as a promising strategy over
the past several decades for treating a wide range of conditions, especially T1D
(mainly caused by autoimmune destruction), owing to the regenerative and
immunomodulatory potential of MSCs [5]. Studies have shown that the leading cause
of tissue repair in vivo is the paracrine effect by MSCs [5]. Thus, it
is evident that CM that contains cultured-MSC paracrine factors can induce tissue
repair as effectively as MSCs [15]. In one of our previous studies, we found that
AnSCs effectively attenuated liver injury, improved liver function, and reduced
inflammation in rats [14]. Thus, we speculate that AnSC or AnSC-CM treatment
would effectively alleviate the symptoms of diabetes mellitus and induced liver
injury. This study showed for the first time that AnSC-CM effectively improved
symptoms related to STZ-induced T1D, which was found to be, at least partially,
by inhibiting the NF-
In addition to the elevated blood glucose in diabetes mellitus mice, absolute or relative insulin deficiency also leads to imbalances in lipid metabolism and hepatic lipid accumulation [1, 2]. The early manifestation of T1D is the fat accumulation in the liver parenchyma. Subsequently, a large quantity of the accumulated lipids progresses to the formation of lipotoxicity. The latter causes dysfunctions in the endoplasmic reticulum, mitochondria, and other cellular organelles and promotes inflammatory reactions, which lead to hepatocyte damage and even cell death [8, 14]. It has been reported that paracrine secretions of BMSC and human urine-derived stem cells can improve STZ-induced diabetes and its complications [19, 20]. Studies have shown that AnSC-CM reduced fasting blood glucose levels and promoted gains in body weight in T1D mice. Additionally, AnSC-CM was more effective than BMSC-CM in treating these symptoms. AnSC-CM enhanced insulin synthesis and secretion in T1D mice alongside alleviating liver dysfunction and improving lipid metabolism, which could be well explained by a significant decrease in the serum levels of alanine aminotransferase (ALT), alanine aminotransferase (AST), and MDA, and an increase in the serum levels of SOD. Compared to other MSC-CM-based therapies, AnSC-CM-based treatment is likely more effective in preventing and treating T1D. We speculated that AnSC-CM performs better than BMSC-CM, which may be due to the dual properties of both embryonic stem cells and mesenchymal stem cells from AnSCs [21], meaning there may be more active components that are anti-inflammatory in their CM components.
Inflammation is strongly associated with the development of diabetes [22, 23].
NF-
In summary, the results of the present study highlight that AnSC-CM has
advantages over BMSC-CM in ameliorating STZ-induced T1D and diabetic liver injury
in our mouse model, which is possibly achieved through the downregulation of the
NF-
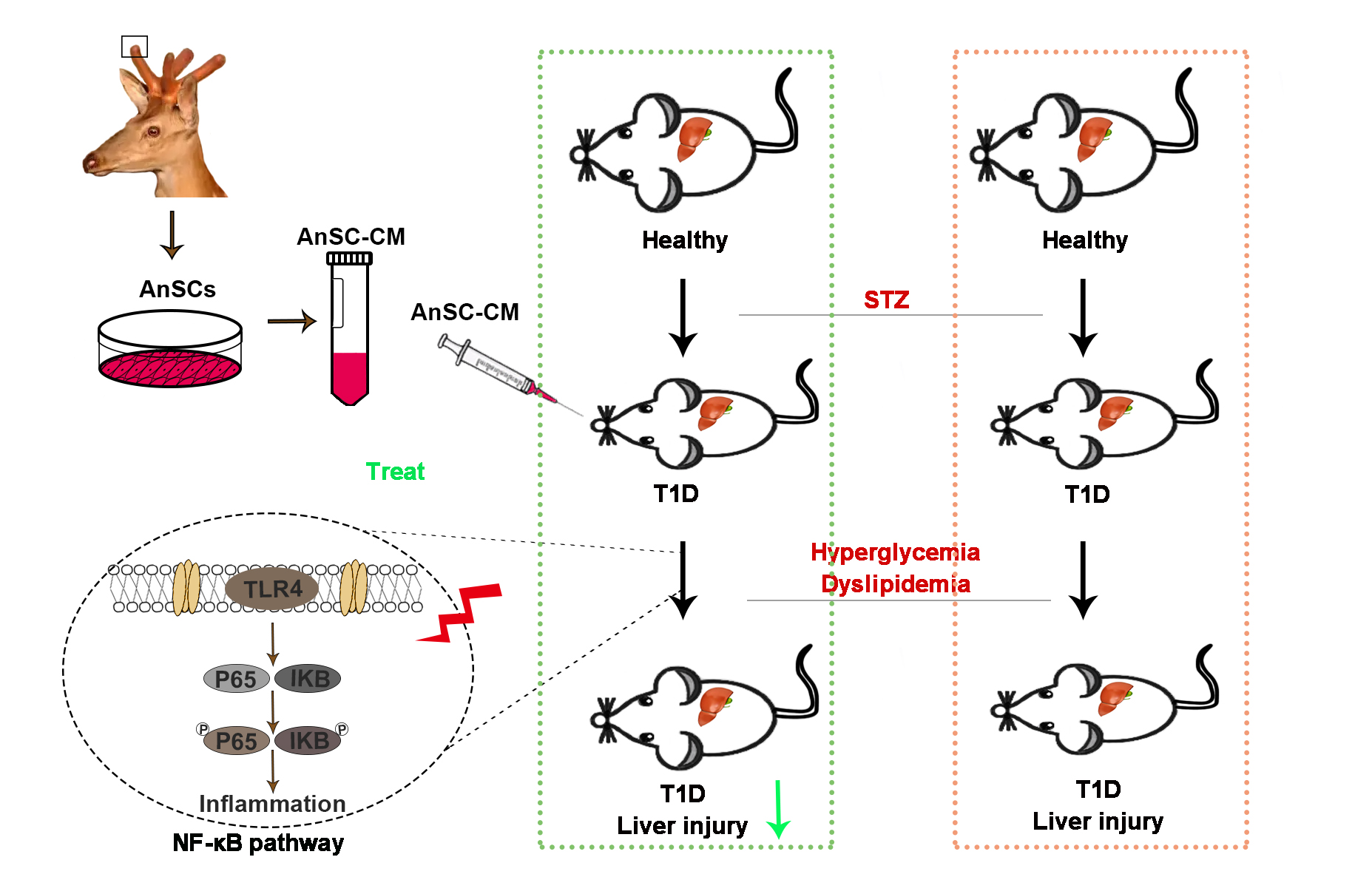 Fig. 4.
Fig. 4.Schematic diagram of the mechanism through which AnSC-CM
alleviates STZ-induced T1D liver injury symptoms. AnSC-CM
alleviates STZ-induced T1D liver injury symptoms by potentially downregulating
the NF-
ALT, alanine aminotransferase; AST, aspartate aminotransferase; AnSCs, deer
antler stem cells; DMEM, Dulbecco’s Modified Eagle’s Medium; FBS, etal bovine
serum; HE, hematoxylin and eosin; IHC, immunohistochemistry; IL-1
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
GZ and CL designed the study. DW, XL, JL and JR performed the research. ZP, JY, TJ, QG and ZW analyzed the data. DW, GZ, and CL wrote the paper. All authors contributed to editorial changes in the manuscript. All authors give final approval of the version to be published. All authors have participated sufficiently in the work to take public responsibility for appropriate portions of the content and agreed to be accountable for all aspects of the work in ensuring that questions related to its accuracy or integrity.
The animal experiments were approved by the Animal Ethics Committee of Changchun Sci-Tech University (Approval No.: CKARI202110).
We thank Peter Fennessy for his critique of the manuscript and valuable suggestions.
This work was supported by the National Natural Science Foundation of China (No. U20A20403, 82000765), the Doctoral Research Start-Up Fund of Changchun Sci-Tech University (202303), the Natural Science Foundation of Jilin Province (No. YDZJ202301ZYTS508, YDZJ202201ZYTS435).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.




