1 Department of Pharmacy, Nanchong Central Hospital, The Second Clinical Medical College, North Sichuan Medical College, 637000 Nanchong, Sichuan, China
2 Nanchong Key Laboratory of Individualized Drug Therapy, 637000 Nanchong, Sichuan, China
Abstract
Background: Deguelin (DGL) is a natural flavonoid reported to exhibit
antitumor effects in breast cancer (BC). PEG-PCL (Polyethylene Glycol-
Polycaprolactone), as polymeric micelles, has biodegradability and
biocompatibility. The aim of this study was to investigate whether the
nanoparticular delivery system, PEG-PCL could improve the bioavailability of DGL
for suppressing proliferation of BC cells. Methods: PEG-PCL polymers
were first prepared by ring-opening polymerization, and DGL and paclitaxel (PTX)-loaded
PEG-PCL nano-micelles were formulated via the film dispersion method. The
composition and molecular weight of PEG-PCL were analyzed by nuclear magnetic
resonance and fourier Transform infrared spectroscopy (FTIR) spectra. Particle size, surface potential and hemolytic
activity of micelles were assessed by dynamic light scattering, transmission
electron microscopy and hemolysis assay, respectively. Then proliferation and
apoptosis of MDA-MB-231 and MDA-MB-468 cells were tested with Edu staining,
CCK-8, TUNEL staining, and Flow cytometer. Caspase 3 expression was also assessed
by Western blot. Results: Our results first indicated that
PEG
Keywords
- PEG-PCL
- deguelin
- paclitaxel
- breast cancer
- apoptosis
- proliferation
Breast cancer (BC) is the most frequently occurring malignancy in women with a high lethality rate [1]. The causes of BC are complex and can be related to breast density, family history, and lifestyle habits, with genetic factors accounting for 5–10% [2]. For instance, individuals with mutations in the BRCA1 or BRCA2 genes have a higher likelihood of developing BC [3]. So far, the primary treatments for BC include surgical intervention, radiotherapy, chemotherapy, and endocrine therapy [4]. Endocrine therapy depends on the tumor subtype of breast cancer (HER, PR, ER), while for triple-negative breast cancer, only radiotherapy, chemotherapy, and surgical methods can be relied upon [5]. Paclitaxel, a first-line drug for breast cancer, exerts a broad killing effect on BC cells. However, the delivery of the drug relates to its killing efficiency and directly affects the therapeutic outcome [6]. Given the above circumstances, there is an urgent need to develop a method that can treat paclitaxel (PTX)-resistant breast cancer at lower doses, with little or no damage to the patient’s normal tissues.
Systemic therapies are inefficient with a high recurrence rate due to systemic toxicity and drug resistance [7]. Additionally, Paclitaxel (PTX) - a commonly used first-line chemotherapeutic agent for BC treatment, effectively controls the disease and prolongs patient survival. However, the PTX-resistant BC will be developed after continuous chemotherapy with PTX [8]. Given this situation, there is an urgent need to develop a treatment method for PTX-resistant BC that uses lower doses and causes little to no damage to a patient’s normal tissues.
Deguelin (DGL) is a flavonoid extracted from the legume family, having the
chemical formula C
Clinically, numerous efficacious drugs are not broadly used due to limitations of their physicochemical properties, leading to low bioavailability and high toxic side effects. There are reports suggesting that the development of NDS (nanoparticular delivery systems) with biocompatible polymers can enhance the physicochemical properties of drugs [22]. NDS, as a novel field of nanotechnology application, has great potential in the field of tumor therapy [23]. The encapsulated drugs in NDS gain protection from premature degradation, thereby improving their stability [24]. Furthermore, enhanced permeability and retention (EPR) in solid tumors enable NDS to aggregate in tumor tissues, thereby enhancing the bioavailability of drugs [25]. Among them, PEG-PCL is biocompatible and can self-assemble to form polymeric nanocarriers with different structures [26]. This nanopolymer can solve the problems of poor water solubility and stability of the drug, reducing toxicity and increasing efficacy through slow release and EPR effects [27]. However, it hasn’t been reported whether NDS (DGL-PTX-PEG-PCL) can halt the malignant progression of BC cells.
In summary, we developed PEG-PCL nanoparticles laden with DGL and PTX (DGL-PTX-PEG-PCL), and evaluated their particle size, surface potential, and hemolytic properties. Additionally, we examined the nano-micelles’ impact on BC cell proliferation and apoptosis. Therefore, our current study laid the foundation for the in vivo pharmacodynamic study of DGL-PTX-PEG-PCL and provide a reference for the study of novel nano formulations of DGL and similar drugs.
Dodecanol (1.86 g, 10 mmol) was dried under vacuum at 50 °C for 5 h,
followed by the addition of Sn (Oct)2 and continued drying for 0.5 h. Then the
dried
PCL-OH (6.0 g, 3 mmol) was dried under vacuum at 50 °C for 8 h, dissolved in 30mL of trichloromethane, added with succinic anhydride (1.5 g, 15 mmol) at 70 °C for 48 h. After cooling, the precipitated succinic anhydride was removed by filtration, precipitated in cold ethanol, and dried by filtration to obtain PCL-COOH.
PCL-COOH (4.0 g, 2 mmol) was dried under vacuum for 5 h, dissolved in 20 mL dichloromethane, and supplemented with NHS (0.46 g, 4 mmol). The mixture was placed in an ice bath, after which, DCC (0.82 g, 4 mmol) was added, and the reaction continued with stirring for 24 h. Then the insoluble DCU was removed by filtration, and the filtrate was precipitated in cold ether. After filtration, the filtrate was washed once with ether and isopropanol, and dried to obtain PCL-NHS.
PEG (2.0 g, 1 mmol) was dried under vacuum at 90 °C for 8 h. After dissolving in 10 mL dimethyl sulfoxide (DMSO), PEG was added with 10 mL THF solution containing PCL-NHS (2.0 g, 1 mmol) and stirred for 24 h. The resulting solution was dialyzed in deionized water for 3 d to obtain the PEG-PCL. In this study, the PEG-PCL micelle materials were provided by Tanshui Technology Co., Ltd. (Guangzhou, China) as a technical service.
For
40 mg of PEG-PCL was dissolved in 2 mL of chloroform, to which 20 mL of ultrapure water was slowly added under ultrasonication, using a sonicator (Scientz, Ningbo, China), to form an emulsion. The aqueous phase solution of blank micelles was obtained by rotary evaporator to remove chloroform at 30 ℃. The blank micelles were concentrated by ultrafiltration three times, aggregates were removed by filtration using a 0.22 µm filter, and the product was stored at 4 °C. 3 mg PTX and 3 mg DGL (Selleck Chemicals, USA) were dissolved in 1 mL DMSO, which were added to 1.5 mL chloroform containing 40 mg polymer. Then 20 mL ultrapure water was added slowly under ultrasonication to form an emulsion, and the chloroform was removed by rotary evaporation. The product was added to a dialysis bag with a molecular retention capacity of 14000 Da for more than 24 h. After ultrafiltration concentration and filtration, the product was stored at 4 °C.
The micelle particle size and surface potential were measured using a Brookhaven
Instruments BI-200 SM DLS system at 25 °C. The scattered light was
detected at 90
10 µL of DGL and PTX-loaded PEG-PCL micelles (0.5 mg/mL) were deposited onto TEM copper grids and then placed in a desiccator for 8 h. The samples were stained with a 2% solution of acetyldioxymethylcellulose for 1 min, and the excess solution was then blotted with filter paper. After drying overnight at 60 °C, the samples were examined under a Transmission Electron Microscope (TEM) with an operating voltage of 120 kV.
Blood samples were centrifuged at 1500
Both MDA-MB-231 and MDA-MB-468 cells were all purchased from the ATCC and then
induced then into PTX-resistant cells. Both types of cells were grown in L-15
medium (GIBCO, Thermo Fisher Scientific, MA, USA) including 10% fetal bovine
serum (Gibco, USA) at 37 °C with 5% CO
EdU solution (Solarbio, Beijing, China) was diluted with culture medium to
prepare 50 µM EdU medium. MDA-MB-231 and MDA-MB-468 cells (1
Each group of cells were digested using trypsin (0.25%) and suspended in culture medium. After that, cells in each well were addressed with 10 µL of CCK-8 (Dojindo, Kumamoto, Japan) and incubated for another 3 h. The OD value was tested using a microplate reader at 450 nm.
The cells of each group were fixed using 4% paraformaldehyde for 15 min after removing the culture medium, then incubated with 0.1% TritonX-100 in ice bath for 2 min. After washing, the cells were exposed to 0.3% hydrogen peroxide in methanol for 20 min. Subsequently, appropriate TUNEL solution (Beyotime, Shanghai, China) was added to each well of cells and incubated for 1 h at 37 °C protected from light. After washing, the cells were treated with TUNEL working solution for 10 min, ethanol (dehydration) for 2 min, and xylene (transparency) for 2 times. After sealing, TUNEL positive cells were observed under a fluorescence microscope.
Annexin-FITC/PI apoptosis kit (BD Biosciences, NJ, USA) was utilized for
apoptosis analysis. After processing, each group of cells (5
BC cells from each group were added with 100 µL protein lysate. After ice
bath and centrifugation, the supernatant was harvested. Protein concentration was
confirmed by applying BCA kit (Beyotime, Shanghai, China). 20 µg of protein
was mixed with 5
All experiments were independently repeated 3 times and the data were denoted as
mean
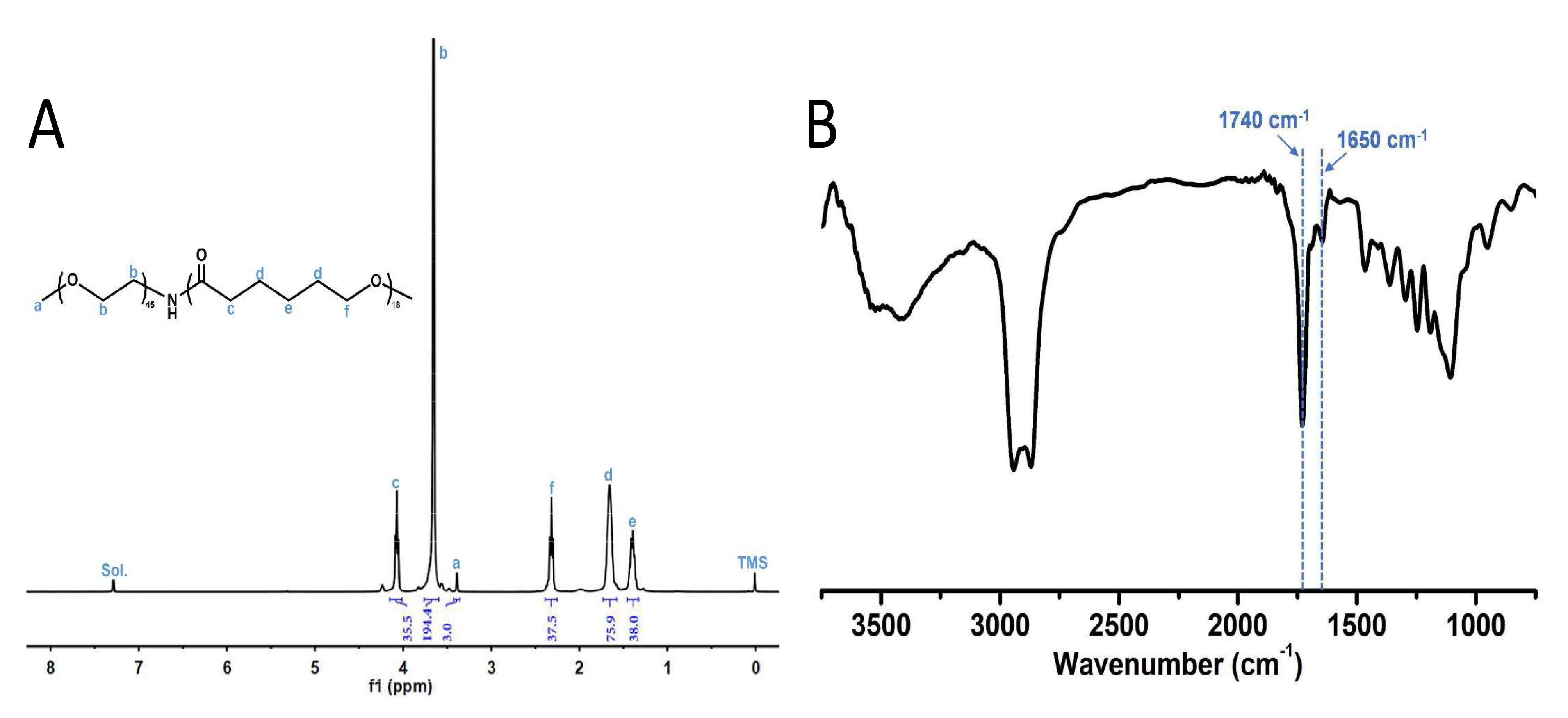
The structure of PEG-PCL was confirmed by 1H NMR (nuclear magnetic resonance)
(Fig. 1A). The molecular weight of PCL was calculated as 2054 Da in the same way,
which is consistent with our designed molecular weight of PEG2000-PC2000.
Therefore, results showed that PEG-PCL has been successfully synthesized. The
characterization of FTIR (fourier transform infra-red) spectra showed that 1740
cm
 Fig. 1.
Fig. 1.nuclear magnetic resonance (NMR) spectrum and fourier transform infra-red (FTIR) spectra of PEG-PCL. (A)
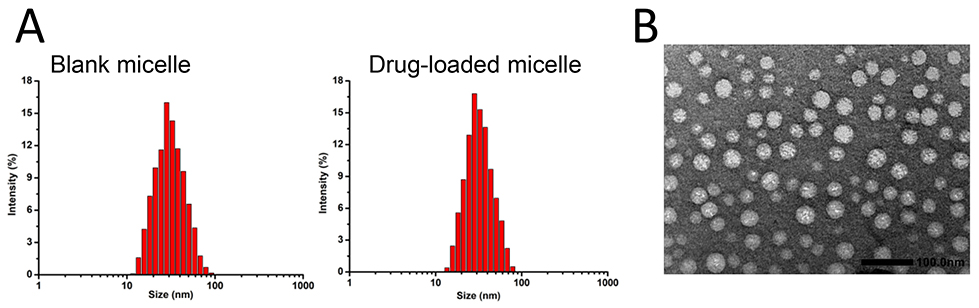
Next, the data of DLS signified that the average particle size and surface
potential of micelles formed by blank carriers were 30.86
 Fig. 2.
Fig. 2.Particle size and morphology characterization of deguelin (DGL) and paclitaxel (PTX)-loaded polyethylene glycol-polycaprolactone (PEG-PCL) nano-micelle. (A) Particle sizes of blank micelle (left) and drug-loaded micelle (right) through dynamic light scattering assay. (B) Drug-loaded micelles were observed using TEM with uranyl acetate. DGL, deguelin.
Specifically, the acetonitrile solution of PTX showed the maximum absorption at
227 nm, while the DMSO solution of DGL showed the maximum absorption at 272 nm.
The standard curve concerning concentration for PTX was: A = 0.02951c + 0.00173,
R2 = 0.99627; whereas, for DGL with respect to concentration, it was: A =
0.05549c - 0.02276, R2 = 0.99829. Having obtained the absorbance of PTX and DGL
in the micelles, their loading capacities were calculated to be 5.3
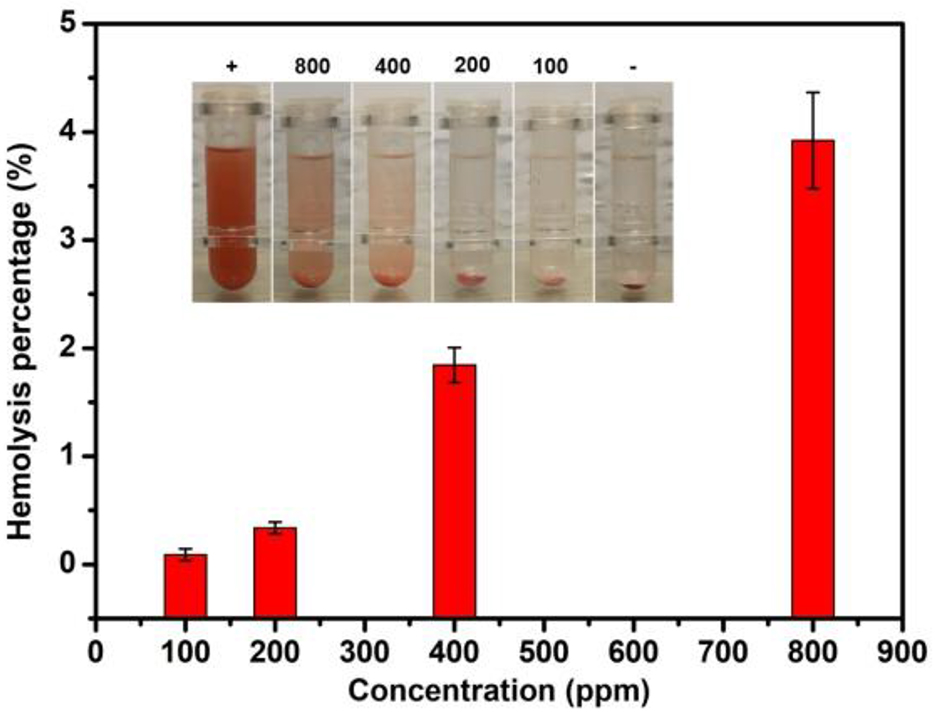
Crucially, we further validated the blood biocompatibility of the micelles. Once hemolysis occurs, the hemoglobin in the red blood cells will be released into the solution. The redness of this solution, indicating hemolytic activity, can be estimated by measuring the UV absorbance of the supernatant at 541 nm. As represented in Fig. 3, there was no significant hemolysis in micelles at the experimental concentrations (100–800 ppm). Even at a concentration of 800 ppm, only about 4% hemolysis occurred in micelles at 3 h exposure time. Therefore, the hemolytic activity of micelles was negligible.
 Fig. 3.
Fig. 3.Hemolysis caused by micelles at different concentrations. Hemolysis assay was conducted after processing with different concentrations of micelles. Deionized water was served as a positive control (+) and phosphate buffered saline (PBS) as a negative control (-). PBS, phosphate buffered saline.
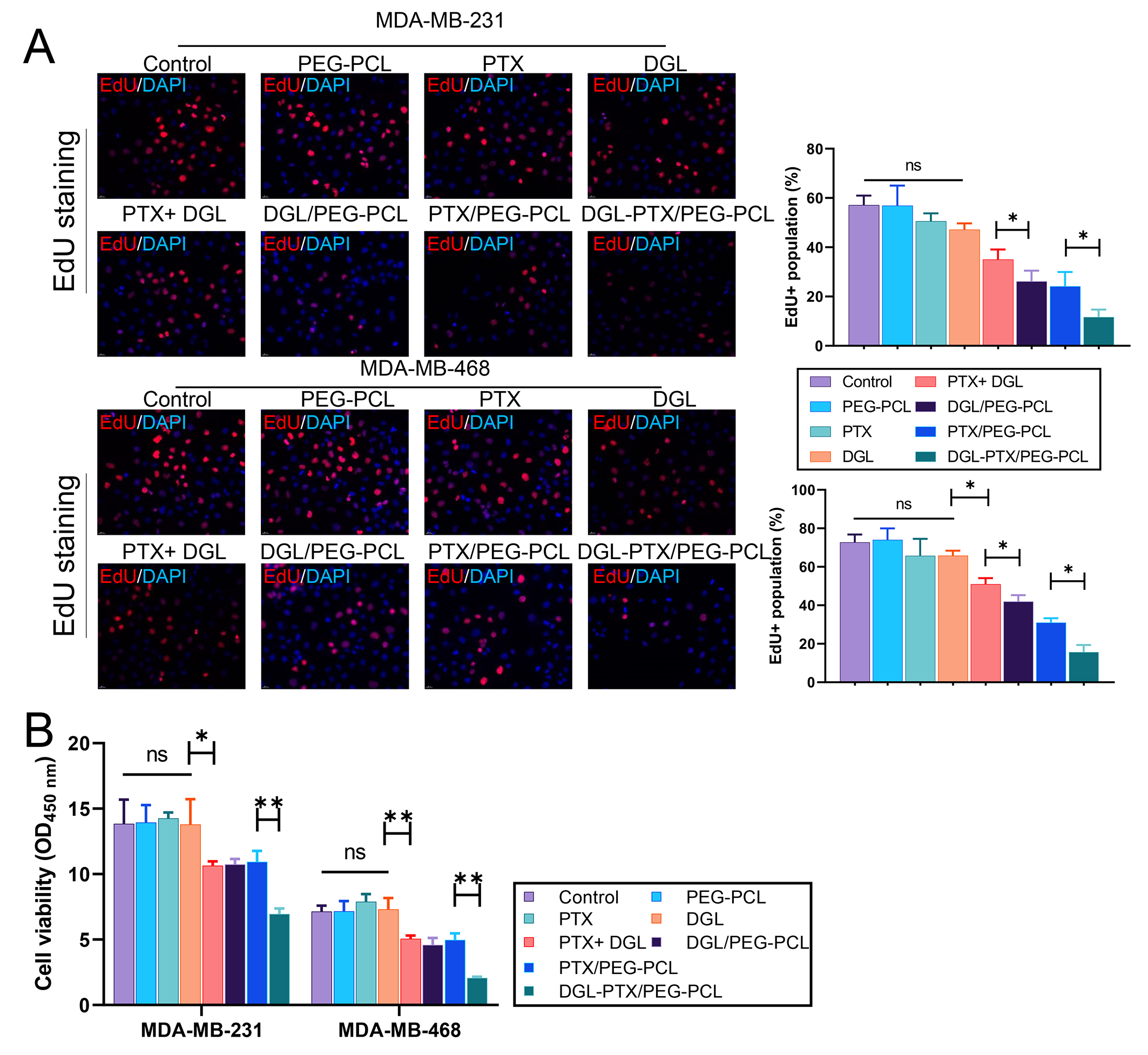
Subsequently, we validated the impact of DGL and PTX-loaded PEG-PCL nano-micelles on the proliferation of BC cells. MDA-MB-231 and MDA-MB-468 cells were treated with PEG-PCL, PTX, DGL, PTX+DGL, DGL/PEG-PCL, PTX/PEG-PCL, and DGL-PTX/PEG-PCL. EdU staining results displayed that PEG-PCL, PTX, and DGL treatment alone did not significantly affect the proliferative activity of the BC cells; Four combined treatments including PTX+DGL, DGL/PEG-PCL, PTX/PEG-PCL, and DGL-PTX/PEG-PCL could observably reduce BC cell proliferation activity, especially DGL-PTX/PEG-PCL (Fig. 4A). Similarly, CCK-8 data showed that treatment of PEG-PCL, PTX, and DGL alone also failed to induce changes in BC cell viability; Combined treatments (PTX+DGL, DGL/PEG-PCL, PTX/PEG-PCL, and DGL-PTX/PEG-PCL) also could markedly restrain BC cell proliferation viability, and this suppression effect was strongest in the DGL-PTX/PEG-PCL group (Fig. 4B). Overall, these data suggest that DGL and PTX-loaded PEG-PCL nano-micelles can cause a remarkable reduction in BC cell proliferation.
 Fig. 4.
Fig. 4.DGL and PTX-loaded PEG-PCL nano-micelles suppresses the
proliferation of breast cancer (BC) cells. MDA-MB-231 and MDA-MB-468 cells were
dealt with DMSO, PEG-PCL, PTX, DGL, PTX+DGL, DGL/PEG-PCL, PTX/PEG-PCL, and
DGL-PTX/PEG-PCL, respectively. (A) Cell proliferative activity was confirmed with
Edu staining. Magnification, 200
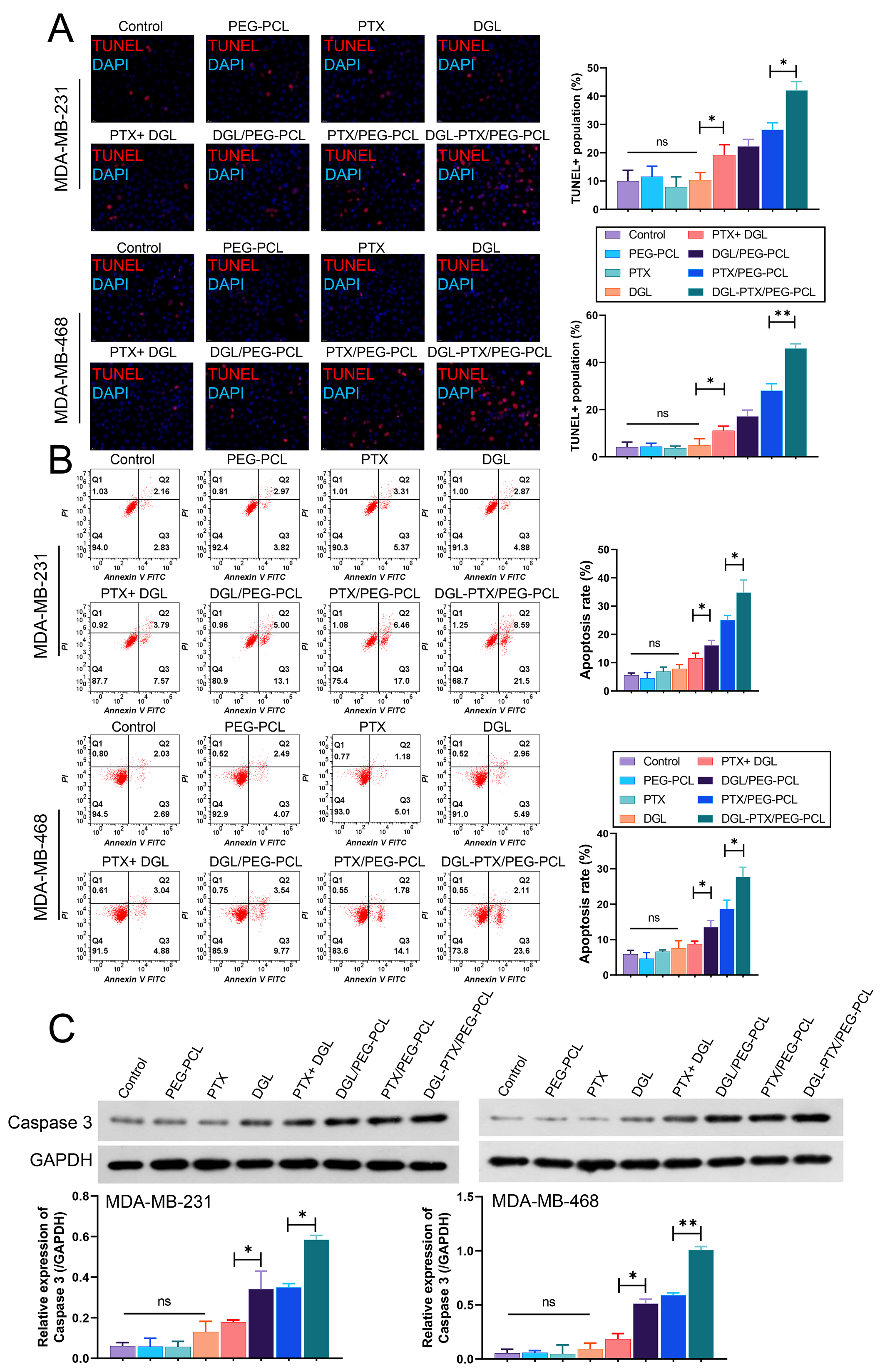
We also further confirmed the changes in apoptosis of BC cells after treatment with DGL and PTX-loaded PEG-PCL nano-micelles. Data from TUNEL indicated that PEG-PCL, PTX, DGL alone did not lead to prominent changes in the apoptotic capacity of BC cells; relative to the control (DMSO) group, the apoptotic capacity of BC cells was significantly increased in PTX+DGL, DGL/PEG-PCL, PTX/PEG-PCL, and DGL-PTX/PEG-PCL groups, especially in DGL-PTX/PEG-PCL group (Fig. 5A). Flow cytometer data also indicated that PTX+DGL, DGL/PEG-PCL, PTX/PEG-PCL, and DGL-PTX/PEG-PCL could dramatically induce BC cell apoptosis, and relative to other groups, DGL and PTX-loaded PEG-PCL nano-micelles (DGL-PTX/PEG-PCL) had the strongest induction of apoptosis in BC cells (Fig. 5B). Additionally, western blot data showed that Caspase 3 expression was notably upregulated in PTX+DGL, DGL/PEG-PCL, PTX/PEG-PCL, and DGL-PTX/PEG-PCL groups versus that in DMSO group; and the degree of upregulation of Caspase 3 expression was most significant in DGL-PTX/PEG-PCL group (Fig. 5C). Thus, we testified that DGL and PTX-loaded PEG-PCL nano-micelles can result in a noteworthy elevation in apoptotic capacity of BC cells.
 Fig. 5.
Fig. 5.DGL and PTX-loaded PEG-PCL nano-micelles induces the
apoptosis of BC cells. MDA-MB-231 and MDA-MB-468 cells were also processed with
dimethyl sulfoxide (DMSO), PEG-PCL, PTX, DGL, PTX+DGL, DGL/PEG-PCL, PTX/PEG-PCL, and DGL-PTX/PEG-PCL,
respectively. (A) Cell apoptosis was determined with TUNEL staining.
Magnification, 200
Breast cancer (BC) is characterized by its aggressive progression and high mortality rate. The killing effect of drugs on tumor cells is fundamental to anti-tumor therapy. PTX is a taxane compound with strong anti-malignant effects [30]. PTX, as a chemotherapeutic agent, has been widely utilized in malignant tumors, especially BC [8, 31]. Moreover, drug resistance is one of the key reasons for therapeutic failure in BC patients [31]. The main factors causing PTX resistance mainly cover gene mutations, ABC transporters, and side effects, etc [8]. Currently, the carrier of PTX is mostly polyoxyethylated castor oil, which can lead to allergic reactions [32]. Literature suggests, we discovered that PTX nanoparticles for BC-targeted therapy offer a stable and effective therapeutic intervention [33].
Currently, Nanodelivery systems have garnered significant attention in recent years for their potential in drug delivery, with nanoparticles, liposomes, and micelles being among the most prevalent. Nanoparticles, typically composed of natural or synthetic polymers, encapsulate drugs for sustained release [34]. Liposomes, vesicles formed from phospholipid bilayers, can encapsulate both hydrophilic and lipophilic drugs [35]. Micelles, self-assembled from amphiphilic molecules, stand out for their hydrophilic shell and lipophilic core, making them particularly adept at delivering lipophilic drugs [36]. This unique structure of micelles enhances drug solubility and stability, offering a promising avenue for targeted and efficient drug delivery. PEG-PCL copolymer exhibits biocompatibility and biodegradability, implying that they do not induce adverse side effects in vivo and can naturally degrade upon fulfilling their therapeutic purpose [37]. PEG-PCL micelles significantly enhance the solubility of pharmaceutical agents, particularly those inherently insoluble [38]. Furthermore, PEG-PCL micelles can be engineered to target specific cells or tissues, ensuring precise drug delivery [37]. In fact, multiple studies have utilized PEG-PCL as a carrier to construct drug delivery systems [26, 39]. PEG-PCL polymer is synthesized by ring-opening polyaddition of caprolactone monomer at high temperature with stannous octanoate as catalyst and methoxy-polyethylene glycol as macroinitiator [40]. PEG is a hydrophilic segment and PCL is a hydrophobic segment [41]. The length and molar ratio of hydrophilic and hydrophobic segments can be controlled during the synthesis of PEG-PCL to produce polymers with different molecular weights and mass ratios of hydrophilic and hydrophobic segments [27]. In vivo, after the hydrolysis of PCL ester bonds, the long chains break and form small fragments, which are eventually metabolized and absorbed by the body [42]. It was also discovered that PCL had a mild inflammatory response for a short time after entering the body, which then disappeared, and that PCL had little influence on immune cell activity [43]. Overall, PCL and PEG are biocompatible materials. PEG is water-soluble, non-antigenic and immunogenic, and can evade recognition by the human macrophage system [44]. Studies have shown that drug-loaded PEG-PCL micelles can be applied in cancer therapies through drug delivery. For instance, ditelluride-loaded PEG-PCL can enhance cancer therapy [45]; Gambogic acid-loaded PEG-PCL can improve anti-tumor efficiency against gastric cancer [46]; docetaxel-loaded PEG-PCL can serve an effective antitumor agent against prostate cancer [47]; and doxorubicin-bridged PEG-PCL-PEG has an inhibitory effect on the BC progression [48]. Therefore, we suggested that the PEG-PCL micelles have a time-controlled release property in vitro and are expected to be a stable, effective, and safe vehicle in cancer chemotherapy. In our study, we also successfully synthesized PEG-PCL through ring-opening polymerization based on previous research [39].
Deguelin (DGL), an isoflavone, has been reported to have regulatory roles on cancer cell proliferation, growth, cell cycle distribution, and apoptosis [9]. DGL has a high safety profile and a promising application as a potential natural antitumor agent. Multiple studies also have reported that DGL can prevent the growth of BC cells [16, 49], suggesting that DGL is effective in BC therapy. Additionally, DGL is a novel Akt inhibitor with strong anti-tumor effects [50, 51]. Multiple studies revealed that Akt pathway is in connection with the PTX resistance of BC [52, 53]. Furthermore, deguelin analogues can hinder the progression of PTX-resistant non-small cell lung cancer [54]. Therefore, we speculated that the combined application of DGL and PTX might have an obvious effect on BC therapy. To achieve a lower dosage, reverse drug resistance, and ensure high safety in BC therapy, we constructed an efficient NDS using PEG-PCL as a carrier to co-deliver DGL and PTX. Our results indicated that DGL and PTX-loaded PEG-PCL nano-micelles had stronger effects on cell proliferation inhibition and apoptosis induction in BC cells than DGL alone, PTX alone or DGL and PTX combination. Additionally, the BC inhibitory effects of DGL-loaded PEG-PCL or PTX-loaded PEG-PCL were not as potent as DGL and PTX-loaded PEG-PCL nano-micelles.
In summary, we enhanced the anti-BC properties of DGL and PTX and increased the drug’s in vitro effectiveness through the construction of PEG-PCL nanoscale micelles (NSM). Future studies will explore the distribution and anti-tumor activity of DGL and PTX-loaded PEG-PCL nano-micelles in vivo.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
QY and YW designed the research study. YW and YL performed the research. LW, SZ and QS analyzed the data. YW and YL wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
Not applicable.
This research was funded by Sichuan Scientific Research Subject on Traditional Chinese Medicine (2021MS196), North Sichuan Medical College Doctoral Research Project (CBY19-QD06).
The authors declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/j.fbl2902090.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.