- Academic Editor
†These authors contributed equally.
Background: Pyroptosis is a critical form of cell death during the
development of chronic kidney disease (CKD). Tripartite motif 6 (TRIM6) is an
E3-ubiquitin ligase that participates in the progression renal fibrosis (RF). The
aim of this study was to investigate the roles of TRIM6 and Glutathione
peroxidase 3 (GPX3) in oxidative stress-induced inflammasome activation and
pyroptosis in Ang-II treated renal tubular epithelial cells. Methods: To
study its role in RF, TRIM6 expression was either reduced or increased in human
kidney-2 (HK2) cells using lentivirus, and Ang-II, NAC and BMS-986299 were served
as reactive oxygen species (ROS) inducer, ROS scavenger and NLRP3 agonist
respectively. Pyroptosis and mitochondrial ROS were measured by flow cytometry.
The levels of malondialdehyde (MDA), glutathione (GSH), and superoxide dismutase
(SOD) were determined using commercial kits, while the levels of IL-1
Chronic kidney disease (CKD) is a progressive clinical condition characterized by renal structural change and renal function loss. CKD develops under the influence of various risk factors, such as obesity, diabetes and infection [1]. The World Health Organization has listed CKD as a major public health problem worldwide, with a global prevalence of 13.4% [2]. China has a large population with CKD and an overall prevalence of 11.6% for this disease [3]. Effective therapeutic options for CKD are currently limited [4]. Therefore, elucidation of the underlying mechanism of CKD may help with disease prevention, or with the delay of disease progression.
A stable rate of production and degradation of reactive oxygen species (ROS) in the human body is required for the removal of harmful substances. However, failure to detoxify these reactive products leads to cell and tissue accumulation of ROS, thereby harming organ function [5]. Oxidative stress acts as a pivotal factor in the progression of renal fibrosis (RF). The resulting glomerular lesions and renal ischemia lead to inflammation and endothelial dysfunction [6]. Patients with advanced-stage CKD were found to have increased ROS levels. This plays a crucial role in the development of RF by initiating an inflammatory chain reaction involving the recruitment of infiltrating macrophages and the secretion of pro-fibrogenic and pro-inflammatory cytokines [7]. It has also been shown that renal dysfunction damages the antioxidant system, thereby making patients highly susceptible to oxidant stress [8]. However, the exact role of ROS in CKD requires further investigation.
Our previous published article revealed that Angiotensin (Ang) II is a powerful inducer that can not only induce production of epithelial mesenchymal transition (EMT), but also promote the production of ROS that further mediates the activation of inflammasomes, a crucial process tightly associated with different renal diseases [9]. N-acetylcysteine (NAC) is an amino acid with multiple biological activities that can exert antioxidant and anti-inflammatory effects through inhibiting the production and release of ROS as well as the release of inflammatory cytokines, which plays an important role in reducing kidney inflammation and delaying the progression of renal fibrosis.
Inflammasomes are cytoplasmic multiprotein complexes that can be triggered by
endogenous and exogenous stimuli. The mechanism of inflammasome activation
involves formation of protein complexes that activate caspase 1, which then
proteolytically activates pro-inflammatory cytokines such as IL-1
The Tripartite motif (TRIM) family of proteins has three conserved domains. From the N-terminal to the C terminal, these are a RING finger, one or two B-boxes, and a coiled-coil (CC) domain [9]. Major importance has been attached to the function of TRIM proteins due to their E3 ubiquitin ligase activity associated with the RING finger structure [16]. Pyroptosis is a type of programed cell death, also referred to as cellular inflammatory necrosis, with features of cell swelling and cell membrane rupture that eventually lead to the massive leakage of cellular contents. Studies have revealed that TRIM proteins are closely involved in a variety of biological and pathobiological processes, including pyroptosis [17, 18]. A previous study reported that enhanced expression of Tripartite motif 6 (TRIM6) was observed in RF, and that TRIM6 activates mTORC1 by ubiquitinating TSC1-TSC2, thereby promoting endoplasmic reticulum stress, EMT, and RF [9]. However, the mechanism that involves TRIM6 in CKD is not well understood. Ang II has been reported to be involved in various biological processes, including the promotion of EMT and RF [19], NLRP3 inflammasome activation [20], and the induction of ROS [9]. Ang-II is one of the major contributors to the progression of renal fibrosis, inflammation, glomerular injury, and chronic kidney disease [19]. The aim of this study was to further elucidate the functional role of TRIM6 in RF by using Ang II as a tool. The results should lead to a better understanding of the underlying molecular mechanism of RF.
Human kidney-2 (HK2) cells free of mycoplasma contamination were obtained from
the Cell Bank of the Chinese Academy of Sciences, Shanghai Biology Institute
(#SCSP-511, Shanghai, China). HK2 cells were cultured in RPMI 1640 medium
(#11875093, Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum
(#16140071, Gibco), penicillin (100 IU/mL), and streptomycin (100 µg/mL)
in an incubator (37 °C, 95% humidity, 5% CO
Full length human GPX3 was cloned into pLVX-puro (Clontech, Palo Alto, CA, USA).
The lentivirus expressing short hairpin RNA (shRNA) oligos targeting TRIM6
(shTRIM6#1, #2), TRIM6 (oeTRIM6) or GPX3 (oeGPX3) was produced in 293T cells,
along with the packaging plasmids, psPAX2 and pMD2.G as previously described (2
µg/mL) [9]. The viral supernatant was harvested after 48 h
(1
2
At the end of cells intervention, samples were collected and washed with ice-cold PBS. Then, cells were incubated with a caspase-1 detection probe (FAM-FLICAR Caspase 1 Assay Kit, #ICT-97, ImmunoChemistry Technologies, Davis, CA, USA) for 1 h at 25 °C in the dark. To remove non-combined caspase-1, the cells were washed with PBS for three times and incubated with propidium iodide solution (Propidium (PI), #P3566; Invitrogen, Carlsbad, CA, USA) at 3 µM for 15 min in the dark. Thereafter, the cells were analyzed using flow cytometry (BD FACSCalibur, Franklin Lake, NJ, USA). The pyroptosis cells were determined by forward scatter/side scatter (FSC/SSC). The cells in Upper Right quadrant (Q1 UR) and Upper left quadrant (Q1-UL) were PI positive cells, and the cells Q1-UR and Q1-LR are caspase-1 positive cells. Activate caspase-1 and PI double positive cells were defined as pyroptotic cells. Each assay was repeated in triplicate.
The levels of malondialdehyde (MDA) and glutathione (GSH) were measured using
the thiobarbituric acid method and the DTNB (5,5
The supernatant containing IL-1
The procedure for quantitative reverse transcription polymerase chain reaction
(RT-PCR) analysis was described in our previous report [9]. Total RNA was
extracted from HK-2 cells with Trizol reagent (Invitrogen, Waltham, MA, USA). Reverse transcription
was performed using a first-strand cDNA reverse transcription kit (Takara, Dalian, Liaoning, China ).
Quantitative RT-PCR was employed to measure the mRNA levels of specific genes
with the SYBR®Green kit (Thermo Fisher Scientific, Waltham, MA,
USA). The mRNA level of genes was measured with the
2
The co-IP assay was used to examine the interaction between TRIM6 and GPX3. The experimental workflow was described in a previous report [21]. Cell lysates were incubated for 1 h at 4 °C with anti-TRIM6 (#Orb623826, Biorbyt, Cambridge, MA, USA), anti-GPX3 (Abcam, Ab275965, Cambridge, MA, USA), or control IgG (#sc-2748, Santa Cruz Biotech, Santa Cruz, CA, USA), followed by protein A/G-agarose for 3 h at 4 °C. Precipitates were washed three times in lysis buffer and then evaluated by Western blot analysis.
The procedure for Western blot analysis was described in our previous report
[9]. After blocking with 5% skim milk, the membranes were incubated overnight at
4 °C with primary antibodies against active IL-1
Statistical analyses were conducted using GraphPad Prism 7.0 (Graphpad, La Jolla, CA, USA).
The normality of data was assessed and found to obey normal distribution
characteristics. Data was presented as the mean
To study the role of TRIM6 in ROS production, TRIM6 expression in HK2 cells was silenced with lentivirus, or induced by Ang II (1 µM) treatment for 24 hours (Fig. 1A,B). Ang II significantly increased the levels of ROS (Fig. 1C,D) and MDA (Fig. 1E), but decreased the levels of GSH (Fig. 1F) and SOD (Fig. 1G). All of these changes were ameliorated by the silencing of TRIM6 expression. These results indicate that Angiotensin II-induced ROS production was mediated by TRIM6.
 Fig. 1.
Fig. 1.Angiotensin II-induced ROS production is mediated by TRIM6.
Transfection of HK2 cells was performed with TRIM6-interfering lentivirus or with
control virus for 24 h. The cells were then treated with 1 µM Ang II for 24
h. (A) TRIM6 protein expression. (B) TRIM6 mRNA expression. (C,D) Flow cytometry
detection of ROS. (E–G) Biochemical detection of MDA, GSH, and SOD. ***p
To study the role of TRIM6 in pyroptosis, HK2 cells in which TRIM6 was silenced
were challenged with Ang II (1 µM) for 24 hours. Flow cytometry
results showed that Ang II significantly promoted the pyroptosis of HK2 cells
(Fig. 2A,B) and increased the levels of the inflammatory factors IL-1
 Fig. 2.
Fig. 2.Ang II-induced inflammation and pyroptosis are mediated by
TRIM6. HK2 cells were transfected with TRIM6-interfering lentivirus or control
virus for 24 h, then treated with 1 µM of Ang II for 24 h. (A,B) Flow
cytometric assay of pyroptosis. (C–F) ELISA assay of IL-1
Next, TRIM6 was successfully over-expressed in HK2 cells, which were then
treated with the ROS scavenger NAC (100 µM) for 24 h. Overexpression
of TRIM6 markedly up-regulated the levels of ROS (Fig. 3A,B) and MDA (Fig. 3C),
but decreased the levels of GSH and SOD (Fig. 3D,E). Overexpression of TRIM6 also
sharply increased the level of pyroptosis (Fig. 3F,G) and the levels of
IL-1
 Fig. 3.
Fig. 3.NAC suppresses TRIM6-induced ROS production and pyroptosis.
TRIM6- overexpressing HK2 cells were treated with the ROS inhibitor NAC (100
µM) or control solvent for 24 h. (A,B) Flow cytometry evaluation of ROS.
(C–E) Biochemical evaluation of MDA, GSH, and SOD. (F,G) Flow cytometry
evaluation of pyroptosis. (H,I) ELISA evaluation of IL-1
We further investigated the roles of Ang II-mediated ROS production and the NLRP3 signal in inflammasome activation and pyroptosis. The ROS scavenger NAC and the NLRP3 agonist BMS-986299 (1 µM) were added to Ang II-treated HK2 cells. Ang II was found to induce ROS production, inflammation, and pyroptosis, which were all reduced by NAC treatment (Fig. 4A–D). However, co-treatment with BMS-986299 abolished the effects of NAC, except for the ROS level. Immunoblotting results revealed that Ang II notably up-regulated expression of the pyroptosis marker proteins active Caspase-1, active IL-1, and GSDMD-N (Fig. 4E,F). These Ang II-mediated effects were all significantly reduced by NAC treatment, while co-treatment with BMS-986299 abolished the effects of NAC on protein expression (Fig. 4E,F).
 Fig. 4.
Fig. 4.ROS induces HK2 cell pyroptosis by activating NLRP3. Ang
II-treated HK2 cells were incubated with the ROS inhibitor NAC (100 µM) or/and BMS-986299 (1 µM) for 24 h. (A,B) Flow cytometry evaluation of ROS.
(C,D) Flow cytometry evaluation of pyroptosis. (E,F) Western blot evaluation of
pyroptosis marker protein expression. ***p
The expression of GPX3 was evaluated to gain a better understanding of TRIM6 function in this model. TRIM6 overexpression was found to significantly decrease the level of GPX3 protein. Silencing of TRIM6 significantly increased the level of GPX3 protein, but neither TRIM6 overexpression or silencing affected GPX3 at the mRNA level (Fig. 5A). The decrease in GPX3 level with TRIM6 overexpression was suppressed by the proteasome inhibitor MG132 (Fig. 5B). Co-IP results showed that TRIM6 interacted with GPX3 (Fig. 5C). Importantly, the overexpression of TRIM6 in HK2 cells significantly promoted the ubiquitination of GPX3 (Fig. 5D). Together, these results demonstrate that TRIM6 interacts with GPX3 and promotes its ubiquitination.
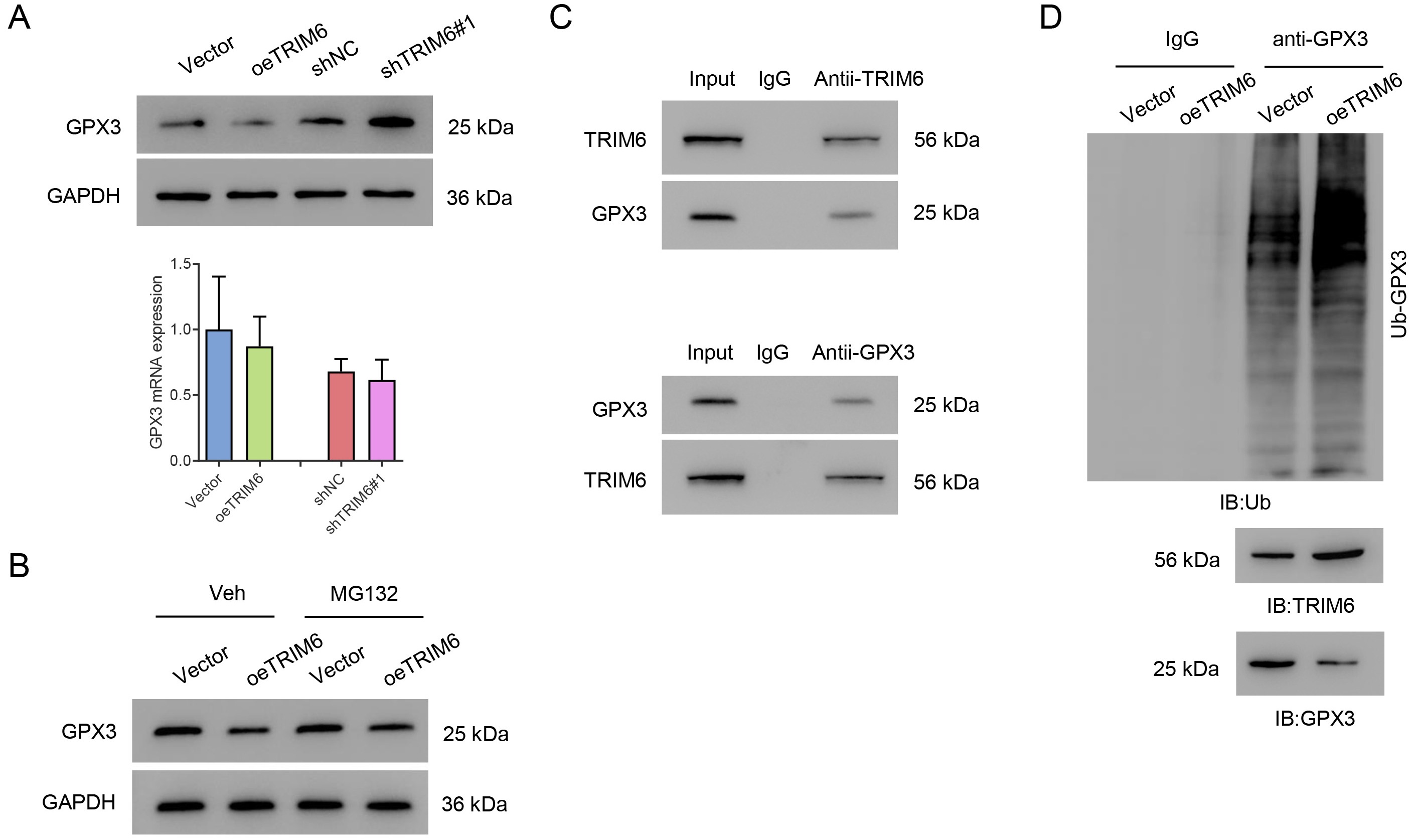 Fig. 5.
Fig. 5.TRIM6 promotes GPX3 ubiquitination in HK2 cells. (A,B) Western blot (upper panel) and qPCR (lower panel) were used to evaluate GPX3 expression after the silencing or overexpression of TRIM6 in HK2 cells. (C) TRIM6-overexpressing HK2 cells were treated with 10 µM MG132 for 4 h and Western blot was then used to evaluate GPX3. (D) Co-IP assay detects the ubiquitination of GPX3. GPX3, Glutathione peroxidase 3; Co-IP, Co-Immunoprecipitation.
GPX3 was successfully overexpressed in HK2 cells that overexpress TRIM6 (Fig. 6A). The overexpression of GPX3 significantly abolished TRIM6-induced ROS
production (Fig. 6B,C) and MDA content (Fig. 6D). Overexpression of GPX3 also
attenuated the decreased GSH level (Fig. 6E) and SOD level (Fig. 6F) associated
with TRIM6 overexpression. Moreover, overexpression of GPX3 reversed the
following changes induced by TRIM6 overexpression: increased pyroptosis (Fig. 6G,H), IL-1
 Fig. 6.
Fig. 6.GPX3 mediates ROS production and pyroptosis induced by TRIM6.
(A) Cells were infected with oeGPX3 virus or control vector, and GPX3 expression
was evaluated by Western blot assay. (B,C) Flow cytometry evaluation of ROS.
(D–F) Biochemical evaluation of MDA, GSH, and SOD. (G,H) Flow cytometry
evaluation of pyroptosis. (I,J) ELISA evaluation of IL-1
The inappropriately activated renin-angiotensin system (RAS) plays a critical
role in renal tissue injury and remodeling in CKD progression. Ang II mediates
the majority of the classical biological functions of the RAS system and
regulates renal cellular and physiological responses in the renal system [22].
Ang II is also related to renal injury and fibrosis via activating renal tubular
cells as well as local immune cells to increase inflammation release, such as
IL-6, and TNF-
ROS-mediated redox signaling plays a very important role in various biological
processes. Redox balance is critical, as elevated ROS levels may damage cells
[27]. The accumulation of ROS also has been shown to trigger an inflammatory
response [7]. Furthermore, ROS-induced activation of the NLRP3 inflammasome
pathway has been reported in triple-negative breast cancer [28]. Activation of
the NLRP3 inflammasome can regulate IL-1
Pyroptosis is one of the main forms of programmed cell death and serves as a
defensive barrier against endogenous and exogenous pathogens [33]. As mentioned
above, ROS can activate the NLRP3 inflammasome, resulting in the production and
release of pro-inflammatory cytokines. Activation of the NLRP3 inflammasome also
leads to the cleavage of gasdermin D (GSDMD). This causes release of its
N-terminus and ultimately the formation of pores on the cell membrane, resulting
in cell rupture and the release of cytokines [29]. Pyroptosis has been
demonstrated to regulate numerous biological activities. For instance, increased
expression of pyroptosis-related proteins has been associated with a lower risk
of breast cancer [34]. Inhibition of GSDMD-dependent pyroptosis reduces the
progression of silica-induced pulmonary inflammation and fibrosis [35].
Pyroptosis was also found to be involved in renal interstitial fibrosis in the
unilateral ureteral occlusion model [36]. Another study found that renal tubule
injury induced by ureteral obstruction is highly susceptible to the initiation of
fibrosis, with pyroptosis playing a major part in this process [37]. The present
results showed that Angiotensin II significantly promoted pyroptosis, as shown by
increased levels of IL-1
GPX3 has important antioxidant activity. It uses glutathione to catalyze the reduction of intracellular ROS, thereby protecting cells, DNA, and proteins against ROS damage [38]. Studies have shown that GPX3 overexpression exerts anti-oxidative stress protection against acute kidney injury, and improves the viability of tubular cells [39]. On the other hand, decreased GPX3 expression diminished its anti-oxidative stress effects and activated pro-tumorigenic redox signaling, thereby promoting tumor growth, proliferation, and metastasis [40]. GPX3 also participates in fibrosis, and oxidative stress is considered a regulator in the development of idiopathic pulmonary fibrosis, which can be attributed to the upregulation of GPX3 [41]. The current study found that overexpression of TRIM6 significantly decreased the level of GPX3 protein, whereas the silencing of TRIM6 expression significantly increased the level of GPX3 protein. Furthermore, our results suggest that TRIM6 decreased the GPX3 protein level by promoting its ubiquitination. These results suggest a new role for GPX3 in RF and broaden our understanding of the pathogenesis of RF.
Protein ubiquitination is a post-translational modification that regulates all
aspects of cell activity, including the cell cycle, proliferation, apoptosis and
differentiation [42]. Recently, TRIM proteins have aroused the interest of
researchers due to their E3 ubiquitin ligase activity. For example, TRIM23 has
been shown to regulate adipocyte differentiation via the ubiquitination of
PPAR
It is worth noting that further research on TRIM6/GPX3-mediated RF will require the development of an animal model. In vitro studies with other cell types in addition to the HK-2 cell line should also provide more evidence. Although further experiments are necessary, the current findings highlight the importance of TRIM6/GPX3 in RF.
The main limitation of this experiment is the lack of in vivo experiments. Besides, Ang II could trigger various signaling, the present reveled the role of TRIM6/GPX3 in CKD in vitro. The protective role of TRIM6 might also associate with other signaling pathways, and we will explore this in future experiments.
These research findings provide novel insights into the role of TRIM6/GPX3 signaling in RF. They suggest that Ang II-induced ROS levels and pyroptosis in HK2 cells are mediated by TRIM6 via the promotion of GPX3 ubiquitination. Our findings shed light on the molecular mechanism of TRIM6/GPX3 in relation to RF, and may lead to the development of novel therapeutic options for RF and CKD.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conceptualization, JL, LL, PT and QX. Methodology, LL, PT, WC and JH. Software, WC, WWL and WRL. Validation, QX, JC and WWL. Formal analysis, LL, JL and JH. Investigation, LL, JH and QX. Resources, JL. Data curation, JL, JC, WWL and WRL. Writing—original draft preparation, JL, LL, PT and QX. Writing—review and editing, all authors. Visualization, LL, WRL and JH. Supervision, JL. Project administration, JL. Funding acquisition, JL. All authors have read and agreed to the published version of the manuscript. All authors have participated sufficiently in the work to take public responsibility for appropriate portions of the content and agreed to be accountable for all aspects of the work in ensuring that questions related to its accuracy or integrity. All authors contributed to editorial changes in the manuscript.
The cellular study was reviewed and approved by Ethics committee of Seventh People’s Hospital of Shanghai University of Traditional Chinese Medicine (2019-AR-005).
Not applicable.
This work was supported by the National Natural Science Foundation of China (NSFC-82074261).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
