1 Department of Pharmacology & Toxicology, College of Pharmacy, Prince Sattam Bin Abdulaziz University, Al-Kharj 11942, Saudi Arabia
2 Department of Pharmacy, Mohammed Al-Mana College for Medical Sciences, Dammam 34222, Saudi Arabia
3 Department of Pharmacognosy, College of Pharmacy, Taif University, Taif 21944, Saudi Arabia
Abstract
Background: The leaves of Origanum majorana (O.
majorana) are traditionally renowned for treating diarrhea and gut spasms. This
study was therefore planned to evaluate its methanolic extract. Methods:
Gas chromatography–mass spectrometry (GC-MS) was used to identify the
phytochemicals, and Swiss albino mice were used for an in vivo
antidiarrheal assay. Isolated rat ileum was used as an ex vivo assay
model to study the possible antispasmodic effect and its mechanism(s).
Results: The GC-MS analysis of O. majorana detected the
presence of 21 compounds, of which alpha-terpineol was a major constituent. In
the antidiarrheal experiment, O. majorana showed a substantial
inhibitory effect on diarrheal episodes in mice at an oral dosage of 200 mg/kg,
resulting in 40% protection. Furthermore, an oral dosage of 400 mg/kg provided
even greater protection, with 80% effectiveness. Similarly, loperamide showed
100% protection at oral doses of 10 mg/kg. O. majorana caused complete
inhibition of carbachol (CCh, 1 µM) and high K
Keywords
- O. majorana
- antispasmodic
- Ca++ channel blocker
- GC-MS
- verapamil
- CRCs
Most volatile oil-containing plants have been used as condiments to enhance the flavor of food since ancient times. Later on, a few species of volatile oil-containing plants were explored for managing diseases [1]. Plant families like Apiaceae, Asteraceae, Burseraceae, Lamiaceae, Lauraceae, Myrtaceae, and Zingiberaceae are known for their members containing volatile oils. In particular, members of the Lamiaceae (Labiatae) family have been explored to a massive extent during these days [2]. A few of the well-known members of the family Lamiaceae are marjoram, basil, mint, oregano, and thyme, which are used for culinary and medicinal purposes.
Marjoram (Origanum majorana L.) belongs to the family Lamiaceae, is widely recognized as sweet marjoram, and has been traditionally used for managing gastrointestinal and respiratory diseases [3, 4]. Marjoram is indigenous to the Mediterranean region and the Arabian Peninsula. The ancient Greeks and Romans believed this plant was a symbol of happiness. It is a perennial bushy plant that reaches a height of up to 12 to 24 inches. The plant bears oval leaves, which are oppositely arranged with white or red flowers. Marjoram leaves are widely used for garnishing purposes. The leaves contain essential oil, and the oil obtained from the leaves is used in mouthwashes and rubbed topically in cases of nasal congestion [5]. Marjoram has been used extensively in pharmaceuticals and food due to its flavor and medicinal properties.
Due to the dynamic uses of this plant, several studies have reported its antioxidant effect [6, 7], antibacterial effect [8], neurobiological activity [7], and skin protection effects [9]. Several studies related to its essential oil composition [10], Electrospray Ionisation Mass Spectrometry (ESI-MS) fingerprinting of essential oil [11], ratios of cis- and trans-Sabinene Hydrate [12], determination of polyphenolic compounds [8, 13, 14], and the effects of blanching on the stability of polyphenols [15] have been reported. The development of nanoemulsions as well as pectin films [16] was also done for the essential oil of marjoram.
The constituents and concentration of the compounds in the plant vary due to factors like geographical variations, soil, and climatic conditions [17]. The essential oil of marjoram majorly contributes to most of its activities. Similarly, the methanolic extract of marjoram possesses polyphenolic compounds, which majorly contribute to its antioxidant activity. Hence, for establishing a representative prototype of O. majorana L. from the Arabian Peninsula, it was worth carrying out a chemical analysis of the essential oil and the phenolic content of marjoram obtained from the Arabian Peninsula. The present study conducted a Gas chromatography–mass spectrometry (GC-MS)-assisted chemo profiling, an investigation of the antioxidants, and a detailed antidiarrheal evaluation of the methanolic extract of O. majorana leaves.
The chemicals utilized in this study were obtained from Sigma Company, located in St. Louis, MO, USA, including carbachol (CCh), acetylcholine perchlorate (ACh), and verapamil. The physiological buffer solution (Tyrode’s) was prepared using salts acquired from Merck (Darmstadt, Germany). Castor oil was acquired from a local pharmacy in Al-Kharj. All chemicals used were of analytical quality.
Wistar albino rats (weighing 200–250 g) were used for the ex vivo
study, while Swiss albino mice (weighing 30–35 g) were used for the in
vivo study. These animals were obtained from the Animal Care Unit at the College
of Pharmacy, Prince Sattam bin Abdulaziz University (PSAU), Saudi Arabia. They
were kept under optimal conditions, with a temperature of 22
O. majorana leaves were purchased from the local market of Dammam and authenticated (PL/0445/2020-21/P-009) by the Department of Pharmacognosy, College of Clinical Pharmacy, Taif University, Saudi Arabia. The plant material was dried in the shade. All plant materials were crushed into a coarse size and later subjected to extraction.
The plant matter was crushed and macerated to prepare the extract. Ten grams of each sample were weighed into 500 mL Erlenmeyer flasks, and 100 mL of methanol was added to the plant samples. Extraction was carried out by the maceration technique with frequent agitation at room temperature for 5 days. After filtration through filter paper, the extracts of every sample were evaporated until the constant weight and respective extractives were calculated. The final residues were used in this study.
The phytochemical composition of the methanolic leaf extract of O.
majorana was analyzed by Agilent GC-MS (7890A) using HP 5MS column (30 m
Altogether, twenty mice were assigned randomly to four groups, each containing an equal number of animals. After fasting for 18 hours, the mice in the first group were given a saline solution (10 mL/kg) through oral gavage and were labeled as the negative control group. Following the pilot screening to determine the appropriate dosage, the second and third groups (referred to as test groups) were administered orally with two escalating doses of 200 and 400 mg/kg of methanolic leaves extract of O. majorana, respectively. The fourth group of mice was administered loperamide at a dosage of 10 mg/kg and designated as the positive control group. Following one hour, all mice were administered castor oil orally at a dosage of 10 mL/kg. Each animal was then placed in an individual cage with a blotting sheet on the floor. This allowed a blind observer to determine the presence or absence of diarrhea. After four hours, the blotting sheet from each cage was examined for the presence of characteristic diarrheal droppings. The protective effect of the extract was evident when no instances of diarrhea were seen, as previously documented by Jebunnessa et al. [21].
The study rats were euthanized, and the terminal portion of the small intestine
(ileum) was extracted following the protocol described by Rehman et al.
[22]. After being isolated, specific sections of ileal tissues measuring 2–3 cm
in length were thoroughly cleaned to remove nearby tissues and fecal matter.
These sections were then placed in emkaBath (France) and connected to a
transducer and IOX software (version 2.9.10.6, emka technologies, SAS, Paris,
France). The Tyrod solution (pH 7.4) was freshly prepared [22] and added to
tissue baths with a volume of 20 mL. The baths were then filled with carbogen gas
and maintained at a temperature of 37 °C. A stress of 0.7 grams was
exerted by turning the transducer knob in a clockwise direction. The tissues were
then allowed to stabilize for 30 minutes, during which they were exposed to
acetylcholine numerous times at a concentration of 0.3 µM. Following
stabilization, continuous muscle contractions were induced using CCh, and high
doses of K
Following the first relaxation of O. majorana in response to high
K
The results have been reported as the mean
The leaves of O. majorana yielded 14.7% of methanolic crude extract.
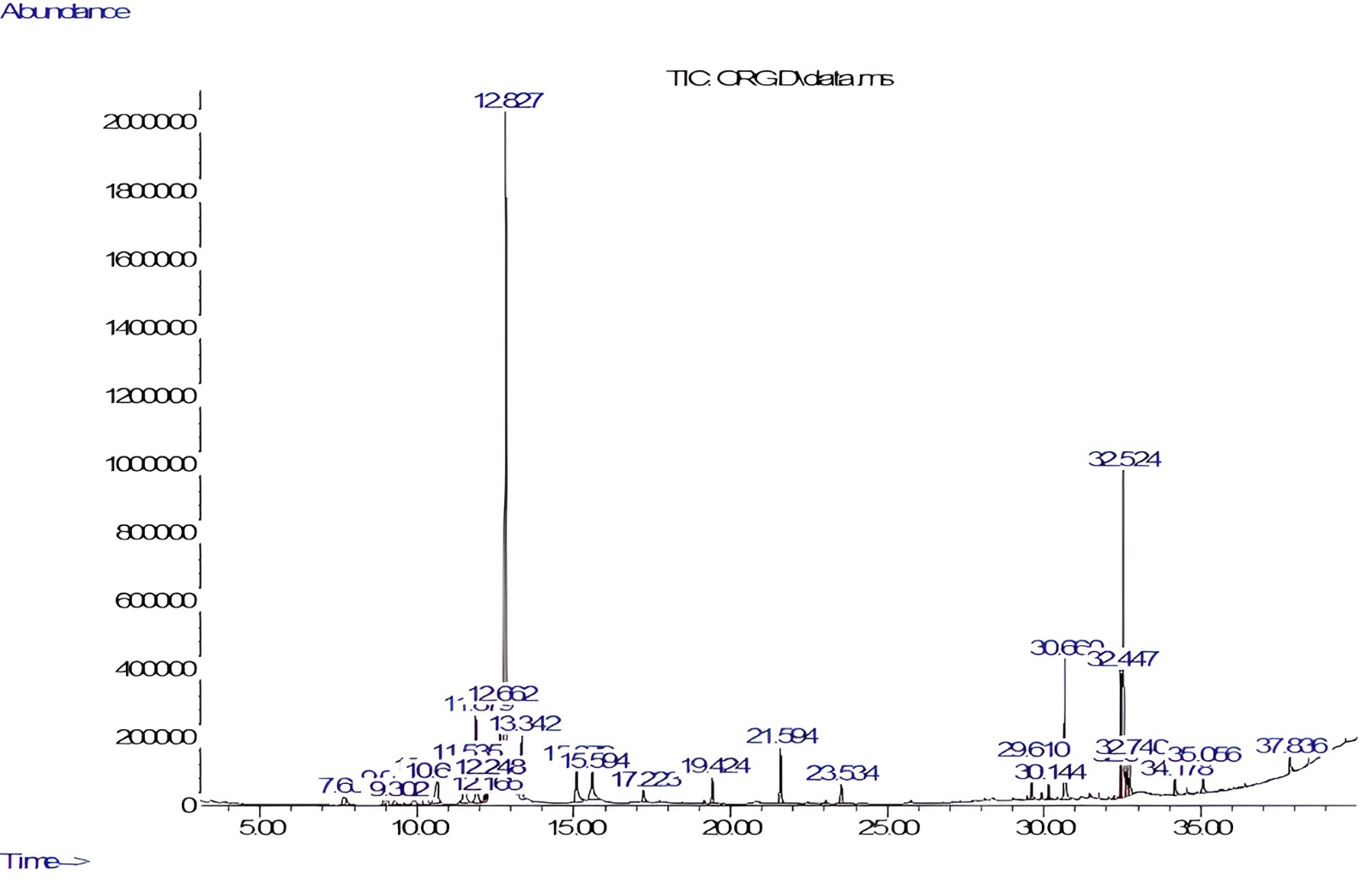
O. majorana was analyzed by GC-MS (Fig. 1). The phytoconstituents identified are listed in Table 1. GC-MS identified twenty-one constituents representing 96.27% of the extract. Alpha-terpineol (35.18%) and linoleic acid (13.3%) were characterized as major components of the methanolic extract. Sabinene, a bicyclic monoterpene found in the essential oils of various plant species, was also identified in the profiling.
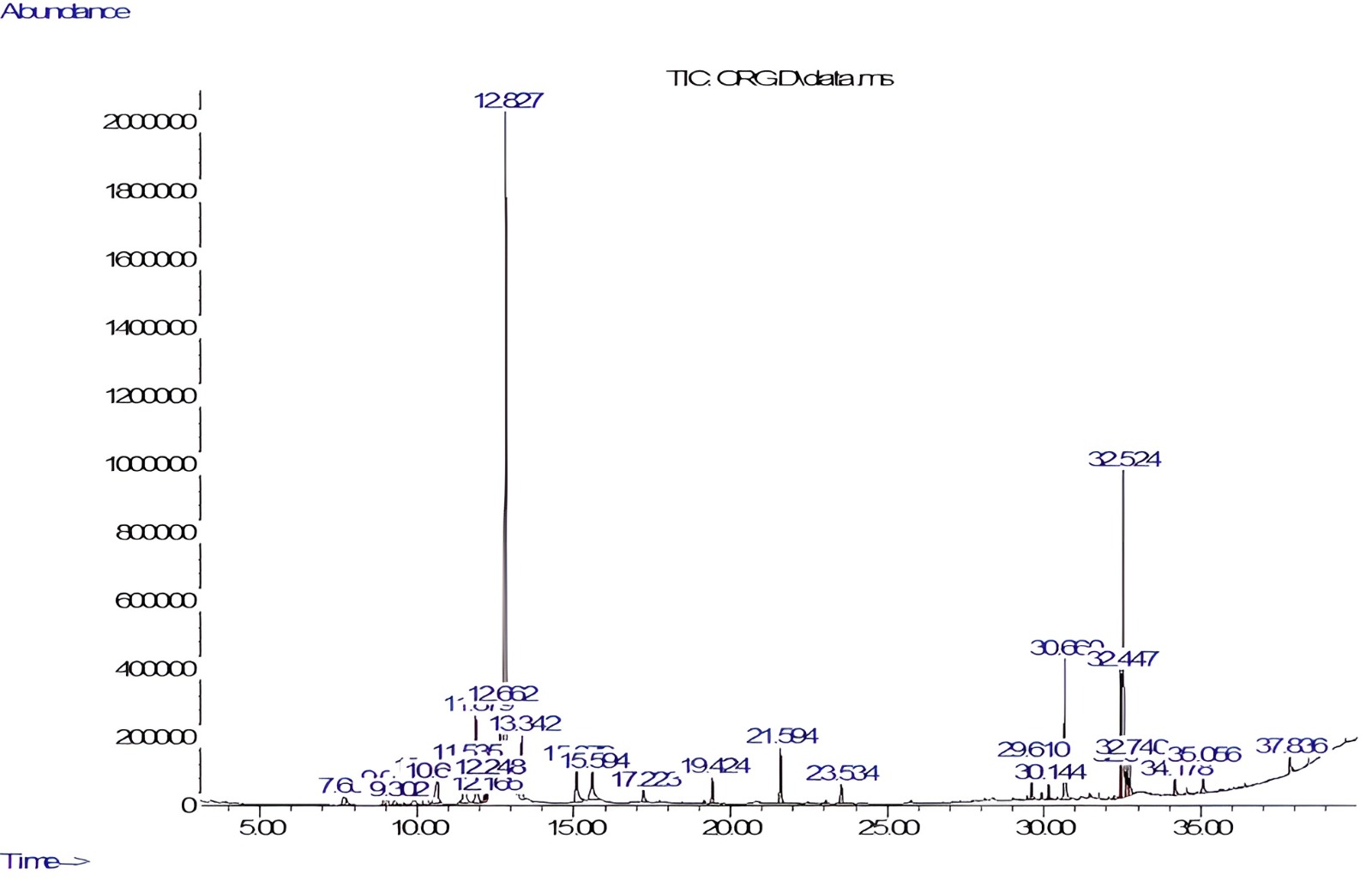 Fig. 1.
Fig. 1.Gas chromatography–mass spectrometry (GC-MS) chromatogram of Origanum majorana (O. majorana) leaves extract.
| S. No. | RT (min) | Area % | Compound name | Molecular weight | Molecular formula |
| 1 | 7.655 | 0.95 | Sabinene | 136.23 | C |
| 2 | 9.01 | 1.07 | Alpha-thujene | 136.23 | C |
| 3 | 10.30 | 2.96 | Alpha-terpinene | 136.23 | C |
| 4 | 10.65 | 2.77 | Beta-Phellandrene | 136.23 | C |
| 5 | 11.53 | 3.56 | Gamma-terpinene | 136.23 | C |
| 6 | 11.87 | 4.64 | Beta-terpineol | 154.25 | C |
| 7 | 12.66 | 4.19 | Carane | 138.25 | C |
| 8 | 12.82 | 35.18 | Alpha-terpineol | 154.25 | C |
| 9 | 13.34 | 3.04 | 3-Formyl-1-methyl-2(1H)-pyridine-thione | 153.20 | C |
| 10 | 15.07 | 3.05 | Terpinen-4-ol | 154.25 | C |
| 11 | 17.22 | 0.75 | Linalyl acetate | 196.29 | C |
| 12 | 19.42 | 1.17 | 1,3,6-Heptatriene, 2,5,5-trimethyl | 136.23 | C |
| 13 | 21.59 | 3.06 | Beta-caryophyllene | 204.36 | C |
| 14 | 23.53 | 1.30 | Gamma-Pyronene | 136.23 | C |
| 15 | 29.61 | 1.24 | Pinane | 138.25 | C |
| 16 | 30.66 | 5.68 | Methyl palmitate | 270.5 | C |
| 17 | 32.44 | 3.92 | Methyl linoleate | 294.5 | C |
| 18 | 32.52 | 13.30 | Linoleic acid | 280.4 | C |
| 19 | 32.64 | 1.53 | Phytol | 296.5 | C |
| 20 | 32.74 | 1.48 | Methyl isostearate | 298.5 | C |
| 21 | 35.05 | 1.42 | Dehydroabeityl alcohol | 290.5 | C |
S. No., Serial Number; RT, Retention Time.
Both the increasing doses of orally administered O. majorana extract in mice exhibited significant antidiarrheal effects in comparison to the control group (Table 2). When administered a dose of 200 mg/kg, two out of five mice exhibited protection, indicating a 40% protection rate. In contrast, a higher dose of 400 mg/kg resulted in 80% protection. The positive control drug, loperamide, showed 100% protection at 10 mg/kg, as detailed in Table 2.
| Group | Dose | No. of mice with diarrhea | % Protection |
| Castor oil | 10 mL/kg | 5/5 | 0 |
| Castor oil + O. majorana | 200 mg/kg | 3*/5 | 40 |
| Castor oil + O. majorana | 400 mg/kg | 1*/5 | 80 |
| Castor oil + loperamide | 10 mg/kg | 0**/5 | 100 |
*p
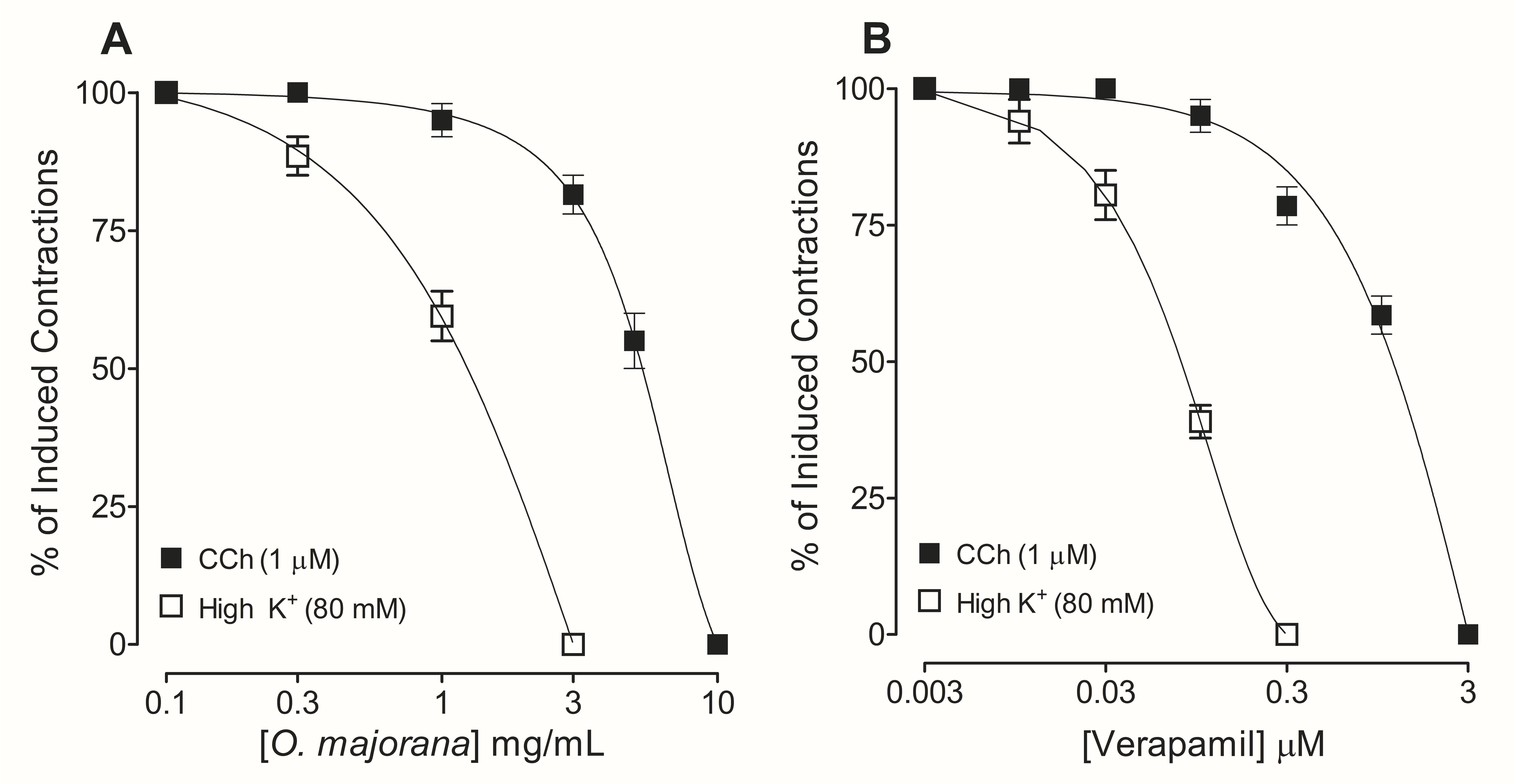
When tested against CCh and high K
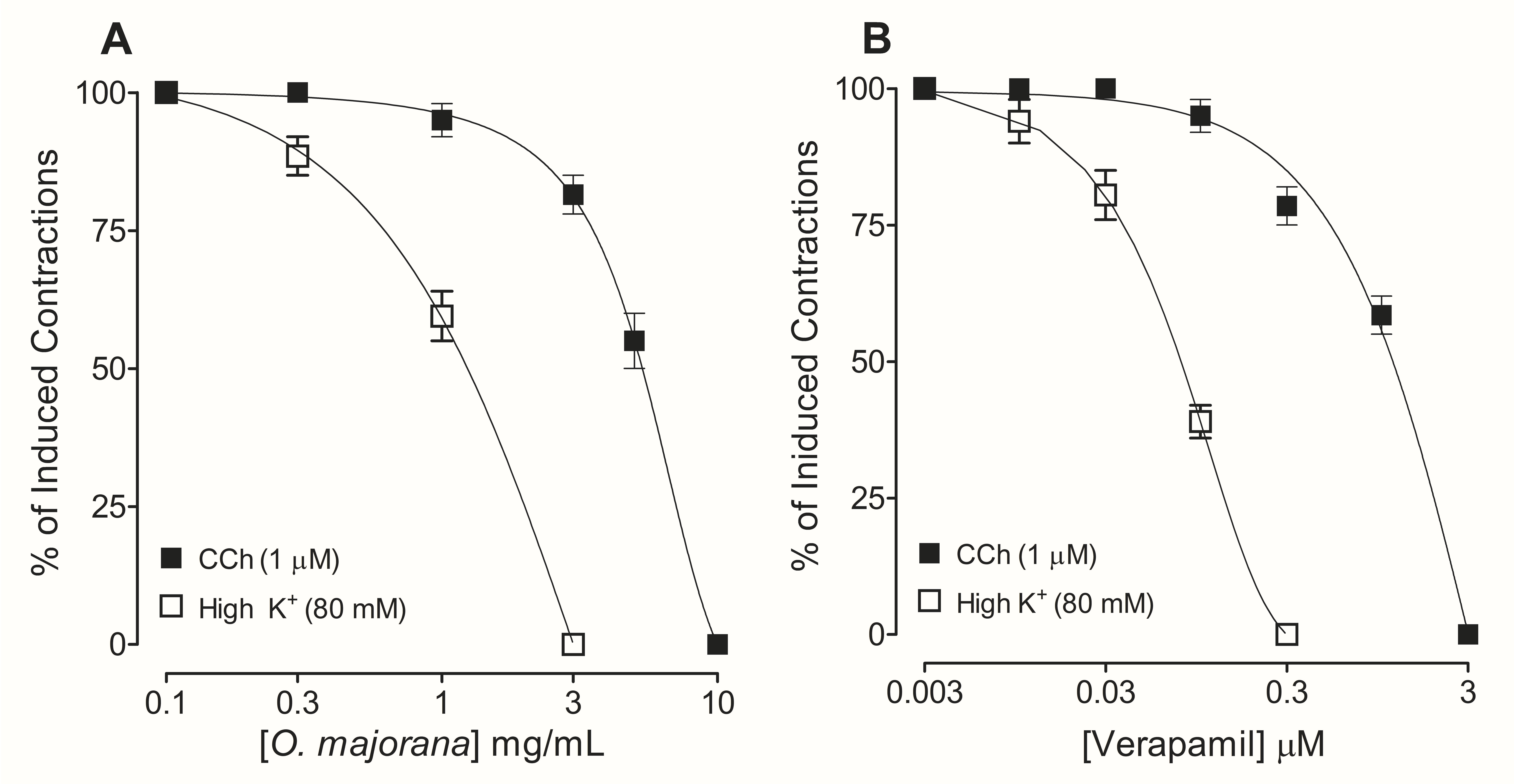 Fig. 2.
Fig. 2.The concentration-response curves compare the inhibitory effect
of the methanolic Origanum majorana (O.
majorana) leaf extract (A) and verapamil (B) against carbachol (CCh, 1
µM) and high K
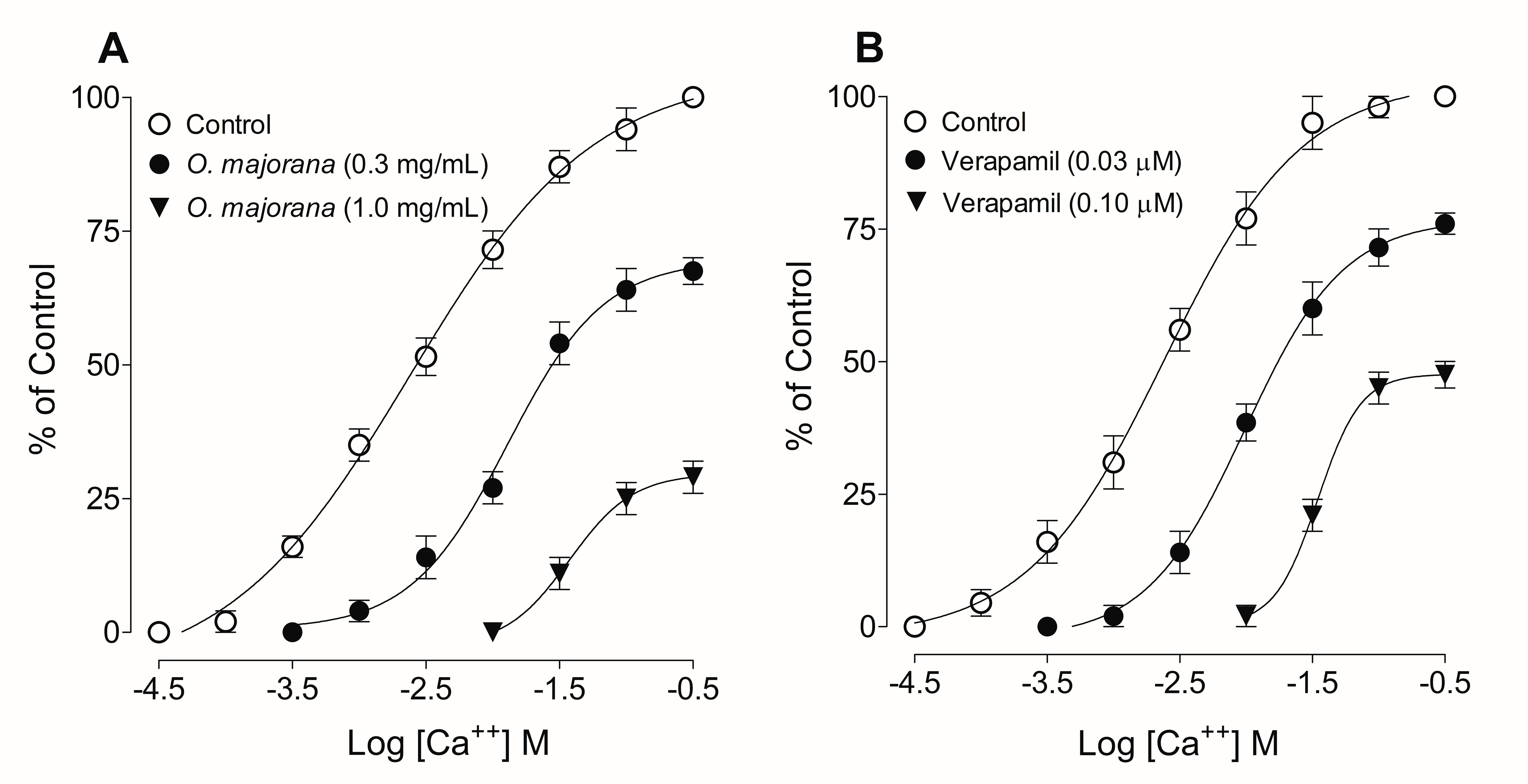
To further confirm the Ca
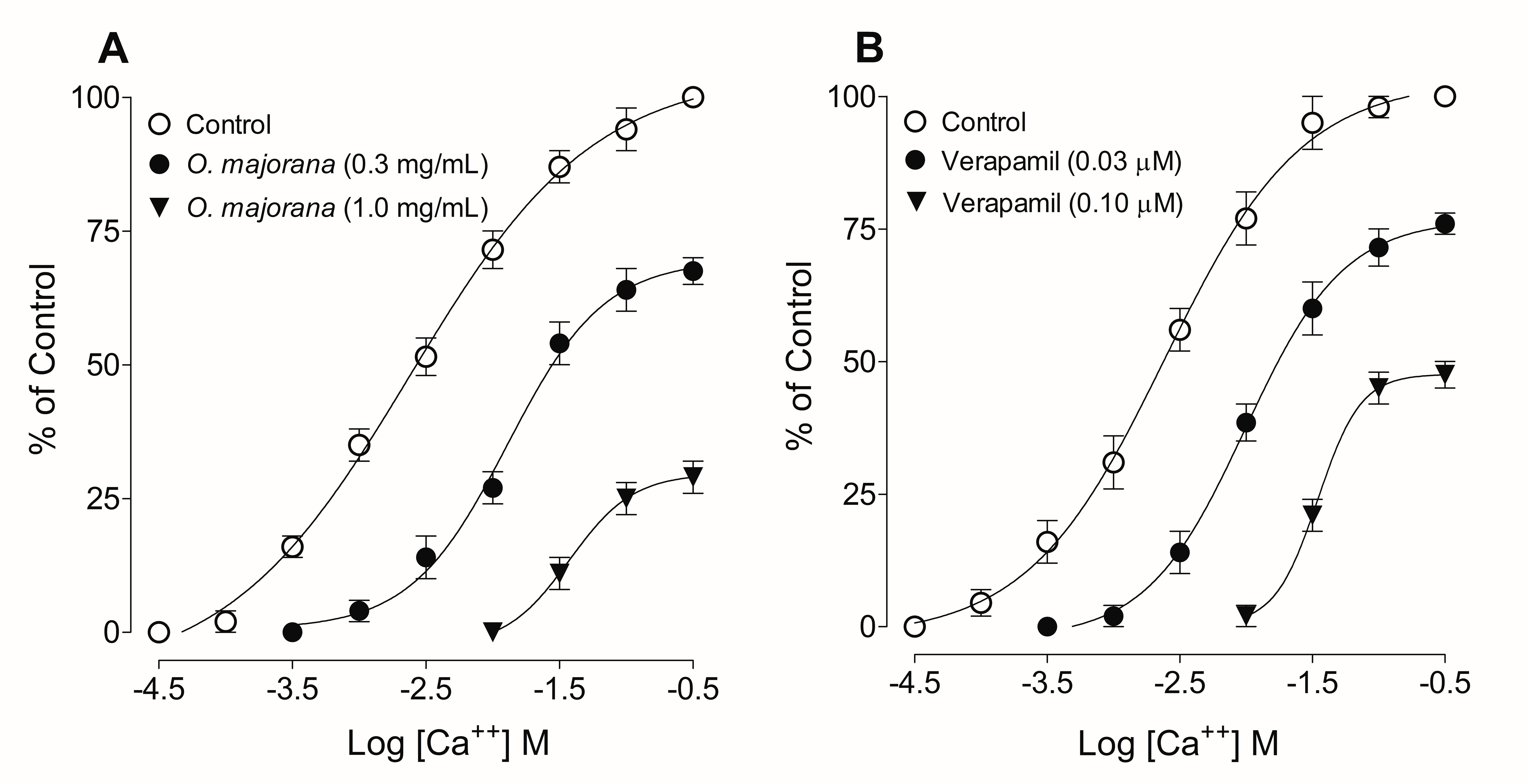 Fig. 3.
Fig. 3.The concentration-response curves of Ca
The therapeutic effect of O. majorana in digestive disorders [24, 25] raised our interest in evaluating this plant scientifically using rodents and employing GC-MS to determine its phytochemical analysis. In the castor oil-induced diarrhea model, the preincubated mice with plant extract resulted in a dose-dependent antidiarrheal effect by protecting the mice from typical diarrheal drops at 200 and 400 mg/kg when compared to a saline pretreated group where no protection was observed. The dose selection of the plant extract was based on a previously reported acute toxicity study that revealed the safety of O. majorana ethanolic extract up to the maximum tested dose of 5 g/kg [26, 27]. The induction of diarrhea in normal mice was achieved by administering castor oil, which, upon hydrolysis into ricinoleic acid, alters the transportation of electrolytes and water. This disruption leads to spasms in the gastrointestinal tract [28]. Thus, a potential antidiarrheal agent may exhibit its antidiarrheal effect by inhibiting bowel contraction. The antidiarrheal activity of the plant extract of O. majorana can be attributed to multiple inhibitory components present in the plant.
To identify the potential pharmacodynamics responsible for the observed
antidiarrheal activity, the methanolic O. majorana extract was tested in
increasing doses on isolated rat ileum, as previously documented [29]. Based on
previous findings that antispasmodic medicines typically have inhibitory effects
on the gut by blocking calcium channels [30], we conducted further experiments to
assess the impact of O. majorana extract on induced contractions in rat
ileum using CCh and high K
Godfraind et al. [33] reported that high concentrations of K
The study’s findings show that the methanolic leaf extract of O.
majorana inhibits with higher potency the contractions against high K
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
NUR and WA designed the research study. MNA, NUR, AA, and WA performed the research. NUR, MNA, and WA analyzed the data. MNA, NUR, AA, and WA wrote the manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work. All authors read and approved the final manuscript.
The study has obtained approval from the Bio-Ethical Research Committee (BERC) at PSAU, with the reference number BERC-004-12-19.
The authors are thankful to the Department of Pharmacology and Toxicology, College of Pharmacy, PSAU, for providing the facility to perform the study.
The authors extend their appreciation to Prince Sattam Bin Abdulaziz University for funding this research work through the project number (PSAU/2023/03/25570).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.