1 Department of Nutrition and Dietetics, Gazi University, 06560 Ankara, Turkey
2 Department of Experimental and Clinical Medicine, University of Florence, 50134 Firenze, Italy
Abstract
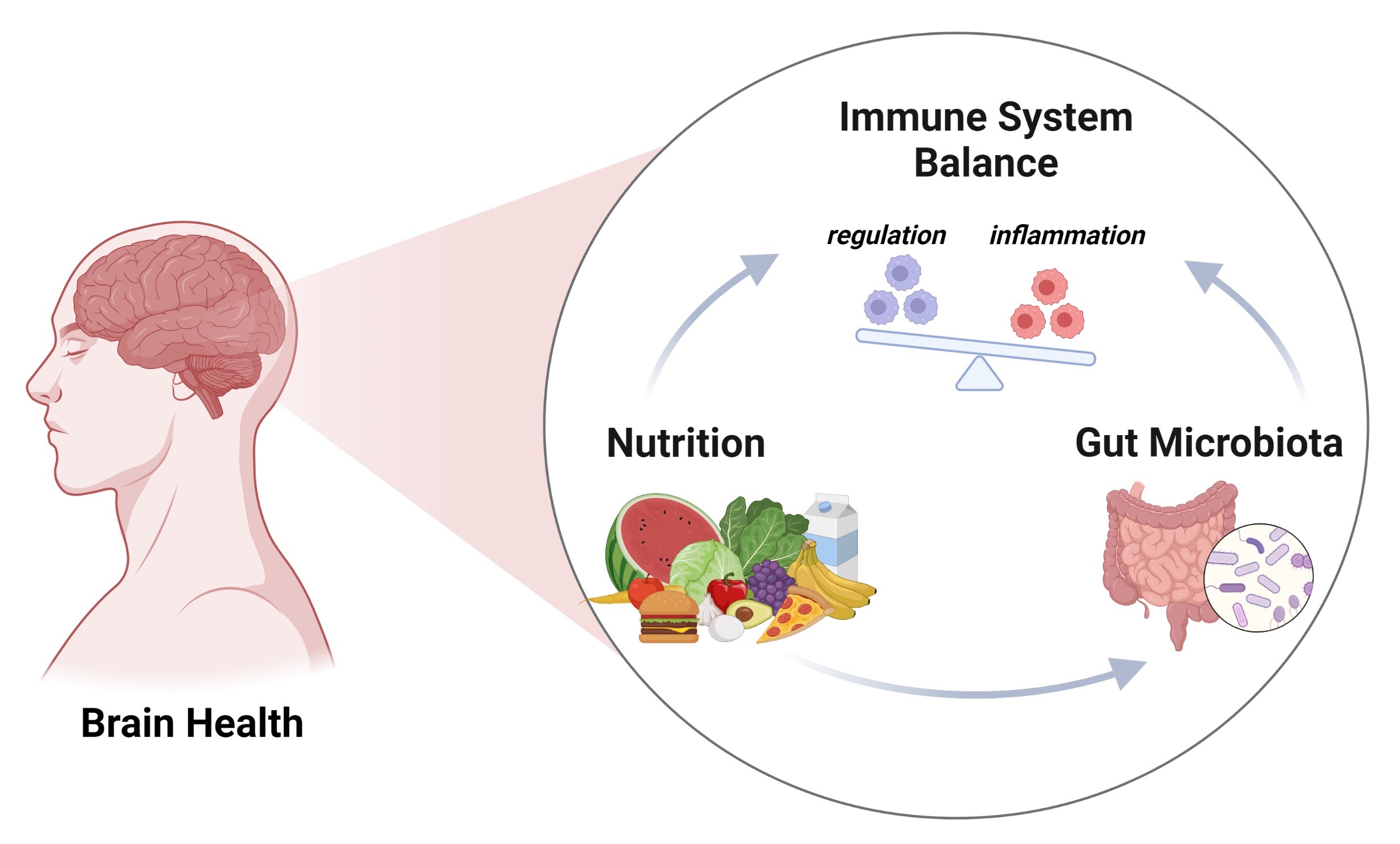
The gut-microbiome-brain axis plays a crucial role in the control of systemic metabolism and homeostasis. Recent research has shown that dietary habits and nutrients can affect immune system and inflammatory status by influencing various factors, including microbiome composition, microbial products release, gastrointestinal signaling molecules, and neurotransmitters. In addition, the gut microbiome affects the brain by altering levels of key brain transmitters, circulating cytokines, and short-chain fatty acids that can cross the blood-brain barrier. Immunonutrition, a newly born discipline, examines the relationship between diet, nutritional status, the immune system, inflammation, infection, injury, and healing. This review explores the relationship between nutrition and the immune system, focusing on immunonutrition and immunonutrients, the connections between nutrition, immunity, and the microbiome, microbiota-gut-brain communication, and potential nutritional interventions to improve neurological disorders. The manuscript provides a comprehensive overview of the complex interplay between nutrition and the immune system, highlighting the many ways in which our diets can impact our health and wellbeing, particularly in the context of neuroinflammatory and neurodegenerative conditions.
Graphical Abstract
Keywords
- immunonutrition
- neuroinflammation
- microbiota
- SCFA
- brain
- immunonutrients
- immunity
The control of systemic metabolism and homeostasis is now understood to depend on the gut-microbiome-brain axis. A growing body of research has shown that dietary habits and nutrients can affect immune system and inflammatory status by influencing a wide range of factors, including microbiome’s composition, the release of microbial products, gastrointestinal signalling molecules, and neurotransmitters. These signalling molecules are then involved in the control of the immune system by either stimulating or inhibiting the synthesis of pro-inflammatory cytokines and the growth of particular leukocyte subpopulations, such as T helper (Th) 17 and regulatory T (Treg) cells, which are important in the emergence of neuroinflammatory and neurodegenerative conditions. In addition, the gut microbiome affects the brain altering levels of key brain transmitters, such as serotonin [1], circulating cytokines and short chain fatty acids, which can go above and beyond the blood brain barrier (BBB) [2, 3, 4]. The newly born discipline of immunonutrition examines the relationship between diet and nutritional status, the immune system, inflammation, infection, injury and healing [5]. Emphasizing the relationship between inflammation and nutrition will help to better understand immunonutrition. Inflammation is the vascular response of living tissue to local damage. It is a process that tries to provide repair while, on the one hand, eliminating harmful factors. There are acute and chronic types. Acute inflammation is a short-lived event, ending in a few minutes or days. Acute inflammatory responses include three basic phases: enhanced blood flow to the inflamed area, followed by vasodilatation and enhanced vascular permeability with leakage of plasma from the microcirculation, and phagocytic leukocyte migration to the surrounding tissue [6]. Chronic inflammation is characterized by persistent inflammation-induced tissue damage and repair, accompanied by progressive changes in inflammatory cells. Consequently, the absence of regulatory or tolerative mechanisms can lead to the progression of inflammation into a pathogenic state. This results in an increased number of activated inflammatory cells in the bloodstream and the primary lesion area, along with elevated plasma concentrations of various inflammatory biomarkers [7]. However, chronic inflammation presents challenges as the inflammatory response fails to effectively clear injurious stimuli, as observed in conditions such as HIV infection [8] and lipotoxicity [9, 10]. In cases of overnutrition, lipotoxicity acts as an injurious stimulus and is further exacerbated by the inflammatory response, which is an integral part of immunonutrition. The presence of inflammatory processes has led to the development of nutritional interventions that incorporate immunonutrients and anti-inflammatory dietary components.
There are many definitions in the literature on immunonutrition. earliest definitions, proposed by Calder, emphasizes the role of nutritional factors in maintaining and inducing immune homeostasis [11]. There is still debate over the relative merits of the various substrates for particular patient groups; however, immunonutrition’s primary goals are cellular defence, local or systemic inflammation, mucosal barrier function and eubiosis maintenance and restoring [12, 13]. Therefore, maintaining appropriate eating patterns should be a key element of any plan intended to stop neurological diseases brought on by systemic metabolic changes.
The current review discusses various aspects of the relationship between nutrition and the immune system. The first section focuses on immunonutrition and immunonutrients, detailing how certain nutrients can impact immune function and potentially improve health outcomes. The second section explores the connections between nutrition, immunity, and the microbiome, highlighting the importance of maintaining a healthy gut microbiota for optimal immune function. The third section delves into the fascinating topic of microbiota-gut-brain communication, exploring the ways in which the microbiome can affect the brain and vice versa. Finally, the manuscript explores the potential for nutritional interventions to improve neurological disorders, detailing the latest research on how specific nutrients and dietary patterns can impact on neuroinflammatory and neurodegenerative conditions. Overall, the manuscript provides a comprehensive overview of the complex interplay between nutrition and the immune system, highlighting the many ways in which our diets can impact our health and wellbeing.
In our search for publications related to the theme of Immunonutrition and the Microbiome in Immunity and Neuroinflammation, we employed specific criteria to ensure comprehensive and targeted results. To begin, we utilized the Medical Subject Headings (MeSH) tool available in PubMed, which enables indexing of National Library of Medicine (NLM) databases and encompasses a vast array of headings and subheadings, totaling over 23,000. This tool allowed us to refine our search and enhance its relevance. Using MeSH headings, we selected the following key headings: Diet Therapy, Neurodegenerative Diseases, Dietary Intervention, and Microbiota. To ensure a focused search, we combined these headings using boolean operators, specifically the “AND” operator. The combinations were as follows: (“Diet Therapy”[Mesh]) AND “Neurodegenerative Diseases”[Mesh], (“Neurodegenerative Diseases”[Mesh]) AND “Diet”[Mesh], (“Neurodegenerative Diseases”[Mesh]) AND “Dietary Intervention”[Mesh], and (“Microbiota”[Mesh]) AND “Neurodegenerative Diseases”[Mesh].
In order to narrow down the research to specific neurodegenerative disorders, we incorporated additional keywords such as Multiple Sclerosis (MS) and Amyotrophic Lateral Sclerosis (ALS), which we combined with the MeSH headings. This allowed us to focus on publications specifically related to these disorders within the context of our study. We restricted the research to studies published in the last 5 years, with a few exceptions due to the necessity to include more studies focusing on ALS and MS.
Throughout the selection process, our priority was to favour papers that presented Meta-Analysis and Systematic Reviews, as these provide comprehensive and consolidated data. We also included Randomized Controlled Trials to gain insights from past and ongoing clinical studies. Finally, we considered reviews that offered new perspectives and future implications in order to stay abreast of emerging research trends.
By employing these criteria, we aimed to ensure a thorough and well-rounded selection of publications that encompassed various study designs and provided a comprehensive overview of the topic at hand.
The relationship between nutrition and immunology is quite intricate. The immune system is impacted by an individual’s nutritional status and dietary intake. This impact is seen at the levels of physical barriers, the innate and adaptive immune systems, and gut microbiota. In contrast, the immune system affects nutrient metabolism and requirements as well as the body’s physiological responses to food [14]. Food components alone can regulate autoimmune and chronic inflammatory illnesses and mediate pro- and anti-inflammatory responses [15]. In addition, supporting the immune system through dietary intake is just a small aspect of immunonutrition. Another significant aspect is the nutrition produced by the human immune system itself. Initially, apoptosis was considered to be the primary form of programmed cell death. However, subsequent research revealed its subtypes, including pyroptosis and necroptosis [16]. These mechanisms actively eradicate damaged cells, effectively clearing invasive stimuli such as viruses or bacteria and converting them into immunonutrition for the regeneration of cells and tissues. Consequently, the immune system eliminates around 330 billion cells per day, primarily blood and gut epithelial cells, to maintain tissue homeostasis [17]. When faced with harmful triggers like invading viruses or bacteria, mechanical injuries, chemical insults, or lipotoxicity caused by misplaced lipids, our immune system employs a “self-destruct and rebuild” strategy to target the affected cells. By utilizing programmed cell death mechanisms such as pyroptosis [18] and necroptosis [19] to actively remove damaged cells, these invasive threats are effectively eliminated and transformed into immunonutrition, facilitating the regeneration of cells and tissues. Malnutrition is the most widespread global cause of immunodeficiency, and it influences immune system responses. A considerable reduction in cell-mediated immunity, phagocytic function, the complement system, immunoglobulin A secretion, and cytokine production have all been linked to protein-energy malnutrition [5]. It is known that the immune system decreases with age. However, changes in organ functions, physiological changes, and lifestyle changes in elderly individuals may cause hyponutrition, exacerbating already-impaired immune function [20]. Wherever malnutrition is mentioned, it is possible to talk about the other side of the coin, obesity. Excessive adiposity is characterized by high circulating inflammatory biomarkers and low-grade chronic inflammation. Changes in white adipose tissue in obesity stimulate the release of tumor necrosis factor-alpha (TNF-
 Fig. 1.
Fig. 1.Anti-inflammatory mechanisms of calorie restriction strategy. The multiple mechanisms through which calorie restriction exerts an anti-inflammatory effect, including an increase in ghrelin production, reduction in adiposity and circulating adipokines, decrease in glycemia and glycation end products, sympatholytic activity and reduction in parasympathetic tone, and an increase in glucocorticoid production. AGE: advanced glycation end products. The figure was crafted using the professional design tools https://www.biorender.com/.
A crucial part of protein synthesis, arginine is a semi-essential amino acid for catabolism. It encourages T cells differentiation, increases their activity, and prompts neutrophil phagocytosis. In addition, arginine enhances wound regeneration and healing, and it controls the immune response and inflammation [32]. It has been demonstrated that dietary arginine, which is included in immunonutrition formulas, increases protein and collagen deposition in experimental wounds and upregulates T cell activity [13, 33]. Nitric oxide (NO), a free radical that maintains the microcirculation and destroys microorganisms, uses arginine as a substrate. It should be emphasized that despite the various advantages of arginine supplementation, excessive nitric oxide generation by inducible nitric oxide synthase has been linked to tissue injury and refractory hypotension. Additionally, arginine supplementation in individuals with severe inflammation may worsen their inflammatory condition. For this reason, the rational use of arginine supplementation in clinical applications and the evaluation of optimum dose, timing, and patient conditions are extremely important. Clinicians should be aware that overdosing on arginine supplementation should be avoided, especially in patients with an inflammatory status [32].
Glutamine is a plentiful free amino acid; skeletal muscle glutamine accounts for more than 60% of the total free amino acid pool [13]. Glutamine controls several cellular pathways and associated processes and serves as a precursor for the production of proteins, nucleotides, and nucleic acids [32]. The primary function of glutamine in controlling immunity depends on its conversion through the mechanism of glutaminolysis into glutamate, aspartate, and alanine [12]. Rapidly proliferating cells such as neutrophils, lymphocytes, and enterocytes often use glutamine as fuel [32]. It is also the most significant substrate for renal ammoniagenesis. There is sufficient glutamine reserve to maintain the integrity of the intestinal mucosa. However, in hypercatabolic and hypermetabolic clinical conditions such as surgical interventions, burns, and infections, there is a serious decrease in intracellular glutamine levels. This glutamine-depleting environment has shown adverse effects on the immune system, and this has been shown to be improved by enteral and parenteral glutamine supplementation [13]. Additionally, glutamine enhances intestine, lymphocyte, and neutrophil performance. This amino acid supports healthy gut-associated lymphoid tissue (GALT) activity and respiratory immunity. As a result, glutamine is crucial for intestine health and function, a healthy immune system, and antioxidative balance [32].
Omega-3 (
The effects of some vitamins and minerals on adaptive and innate immunity were examined, and their immunological outcomes were revealed by studies [15]. Vitamin A is essential for maintaining the function of innate immune cells and preserving the integrity of mucosal membranes in barriers like the skin and respiratory tract. Moreover, it affects the development and differentiation of T helper (Th1) and Th2 cells as well as the appropriate functioning of T and B lymphocytes. Vitamin C is a powerful antioxidant and anti-inflammatory nutrient, which provides protection against reactive oxygen species (ROS) and reactive nitrogen species (RNS), activates important antioxidants, and provides integrity and continuity of the epithelial barrier by supporting collagen synthesis. Moreover, it stimulates leukocyte production and function and it is involved in various anti-inflammatory mechanisms. Vitamin D has an essential inhibitory effect on adaptive immunity. By promoting immune cell proliferation and cytokine production, it influences innate immunity by enhancing monocyte differentiation. In addition, the active form of vitamin D, 1,25-dihydroxyvitamin D3, controls the antimicrobial proteins cathelicidin and defensin, and innate immune cells express the vitamin D receptor [35]. Vitamin E works well to control cellular immunity, modify membrane integrity, transmit signals, and reduce oxidative stress in immune cells. Zinc reduces Toll-like Receptor (TLR)4 signals as well as IL-1
Significant connections exist between the gut and brain on a physical, biochemical, and metabolic level. In this connection a relevant role is mediated by the microbiota. The central nervous system (CNS) interacts with various intestinal targets, including the enteric nervous system (ENS), muscular layers, and gut mucosa, along both afferent and efferent autonomic pathways, regulating motility, immunity, permeability, and mucus secretion. Conversely, the intestine sends many signals to the CNS. According to research, the gut microbiota interacts not only locally with intestinal cells and the ENS but also directly with the CNS in a bidirectional communication, known as microbiota-gut-brain axis [36]. This network involves neuroendocrine-immune pathways and is influenced by diet quality and mealtimes [20, 21, 22]. Microbial-derived metabolites such as short-chain fatty acids (SCFAs), secondary bile acids, and lipopolysaccharide (LPS) play a role in this communication [36] and their altering can impact mental health. SCFAs regulate feeding behavior and energy balance and have immune functions, promoting host intestinal barrier integrity [37]. They stimulate GLP-1 secretion in enterocromaffin L cells, controlling feeding behaviour and energy balance [38, 39]. The gut microbiota plays a vital role in producing important neuroactive substances that have a significant impact on the autonomic nervous system and neuronal activity in the brain. These substances, including GABA, catecholamines, serotonin, tryptophan metabolites, and precursors, contribute to regulating feeding behavior associated with maintaining balance in the body and experiencing reward [36]. For instance, certain bacteria, like Escherichia spp. and Lactobacillus spp. [40, 41] produce active molecules that interact with the autonomic nervous system and can either directly engage with it or stimulate vagal sensory neurons present in the gut. This stimulation leads to the activation of neurons located in the nucleus tractus solitarius (NTS) [38, 39]. The activation of the NTS enables the transmission of information to various other brain structures, including the hypothalamus, nucleus accumbens, and ventral tegmental area. These brain regions collectively control feeding behavior related to maintaining homeostasis and experiencing reward [38, 39, 42]. In essence, the gut microbiota’s synthesis of neuroactive molecules and their interaction with the autonomic nervous system and specific brain areas contribute to the regulation of feeding behavior associated with balance and reward.
Numerous neurological disorders display specific gut microbial profiles, and it has been shown that alteration of microbiota has a significant role in brain health [43, 44, 45]. In the meta-analysis by Nikolova et al. [44], specific psychiatric disorders such as depression, bipolar disorder, schizophrenia, and anxiety shared gut microbiota perturbations, such as the enrichment of pro-inflammatory bacteria and the depletion of anti-inflammatory butyrate-producing bacteria. In particular, various psychiatric and neurologic disorders, including amyotrophic lateral sclerosis (ALS) [46], have been linked to lower levels of SCFA-producing bacteria and higher levels of lactic acid-producing bacteria [30, 31, 44, 45]. Patients with severe mental illness and chronic fatigue have also been found to have higher levels of zonulin, LPS, and gut-related systemic inflammatory markers in their bloodstream than healthy controls [43]. Studies have suggested that alterations in the gut microbiome may contribute to the development of Alzheimer’s disease (AD). For example, one study found that AD patients had decreased levels of beneficial gut bacteria, such as Bifidobacterium and Faecalibacterium, compared to healthy controls [47, 48]. Another study found that fecal microbiota transplantation from AD patients into germ-free mice led to cognitive impairment and increased brain deposition of amyloid beta, a hallmark feature of AD [49]. However, more research is needed to establish a causal link between the gut microbiome and AD. In multiple sclerosis (MS), an autoimmune disorder that affects the central nervous system, was found that MS patients had decreased levels of butyrate-producing bacteria in their gut compared to healthy controls, and that supplementation with a butyrate-producing probiotic reduced disease severity in a mouse model of MS [50]. Another study found that MS patients had altered gut microbiota composition and decreased microbial diversity compared to healthy controls [51]. Furthermore, Parkinson’s disease (PD) patients had decreased levels of Prevotella bacteria in their gut compared to healthy controls [52, 53]. Another study found that transplantation of fecal microbiota from PD patients into germ-free mice led to motor deficits and
Recent randomized controlled trials (RCTs) have also suggested that dietary interventions may be effective in improving brain health outcomes, including depression symptoms [41, 42, 43, 55, 56, 57], autoimmune [58] and neurodegenerative disorders [59, 60].
The following sections will explore the complex mechanisms through which diet and nutrition can influence brain health, including microbiota gut-brain communication and the latest researches on how specific dietary patterns, micronutrients, and gut microbiota modulation can impact neuroinflammatory and neurodegenerative conditions.
Recently, it has been demonstrated that the gut microbiota functions as an endocrine organ, are stimulating immunity, regulating and contributing to the development of inflammatory, metabolic and infectious diseases [20]. It is suggested that the possible effects of nutrition on the immune system may be mediated by microbiota. Nevertheless, fibers, which are non-digestible parts of fruits, vegetables and grains, are well recognized to be a substantial source of energy for the SCFAs-producing bacteria. Fibers have been linked to the maintenance of intestinal homeostasis in multiple investigations employing various fiber treatments by improving epithelial barrier function, reducing pathogen-induced cytotoxicity, and preventing colonization with pathogenic bacteria [14]. In addition, by binding to the arylhydrocarbon receptor and the G-protein-coupled receptors, anti-inflammatory nutrients, including dietary fiber, omega-3 fatty acids, some vitamins, tryptophan and tryptophan-derived products, and SCFAs, can activate the production of anti-inflammatory cytokines (IL-10 and IL-22) [61]. Plant-based diets containing these components, like the Mediterranean diet (MD), Japanese diet, and vegetarian diet have been associated with non-inflammatory status, healthy intestinal mucosa and eubiosis [20]. On the other hand, diets high in energy, saturated fat, and processed foods, such as the Western diet and hypercaloric diets, have been associated with inflammatory status, disruption of the intestinal mucosa, and dysbiosis [20]. Emulsifiers, which are abundant in Western diets, have been shown to impair intestinal barrier function and contribute to the development of obesity and metabolic syndrome by stimulating low-grade inflammation in the wild-type host in animal models [62]. Researches have demonstrated a connection between high-fat diets, microbiota and inflammation. High-fat diets are directly linked to obesity-related dysbiosis, which shows up as altered bacterial diversity and abundance as well as an increase in intestinal permeability [63, 64]. Moreover, a study reported that the Nfkb1 inflammatory pathway was triggered by intestinal microbiota transplantation from mice on a high-fat diet to germ-free mice [65]. This result again raises the possibility that inflammation may be caused directly by diet-induced dysbiosis [66]. Dietary components in these diets have an impact on the inflammatory or anti-inflammatory process. As previously mentioned, calorie restriction is believed to have an impact on the immune system through the microbiota. In the fasting state, bacteria in the gut use endogenous substrates releasing beneficial metabolites for the host. Different studies have shown that, along with the increase of these metabolites, butyrate-producing bacteria also increased [67, 68]. In a research including individuals with type 2 diabetes who were on a special diet with calorie restriction, an increase in A. muciniphila and Faecalibacterium was observed [69, 70]. A. muciniphila protects the lumen of the gastrointestinal tract and stimulates mucin production, that is able to reduce the translocation of pro-inflammatory LPS and peptidoglycan. In conclusion, fasting diets increase microbial diversity, improve intestinal barrier function, and have positive effects on the immune and inflammatory response. However, there is still a need for more well-planned studies on the microbiota-mediated immune system effects of fasting diets [68]. In addition, early colonization is also very important in the development of immune regulation. The most important nutritional factor affecting early colonization is maternal and infant nutrition. In this sense, it is expected that early-life nutrition will affect the microbiota-mediated immune response. Increasing evidence on the effects of the gut microbiota on inflammation and the immune system has suggested that microbiota interventions may affect these processes, bringing prebiotics and probiotics to the forefront. Although not all, some specific prebiotics and probiotic strains show immunomodulatory properties by affecting dendritic cells, epithelial cells, T lymphocytes, natural killer T cells and B cells [71]. In the light of all this information, taking into account the complex and bidirectional relationship between nutrition and the immune system, it is extremely important to create personalized diet programs that are suitable for the nutritional status of individuals.
Several studies have examined the role of various nutrients and dietary patterns in reducing neuroinflammation and the risk of neurodegenerative diseases. For example, a MD diet, which is rich in fruits, vegetables, whole grains, legumes, fish, and healthy fats, has been shown to have anti-inflammatory effects and to reduce the risk of cognitive decline and Alzheimer’s disease [72]. Similarly, the Dietary Approaches to Stop Hypertension (DASH) diet, which emphasizes fruits, vegetables, low-fat dairy, and whole grains, has been associated with lower levels of inflammation and a lower risk of cognitive impairment [73, 74].
Studies have also examined the potential of specific nutrients to combat neuroinflammation. For example, omega-3 fatty acids, which are found in fatty fish and certain nuts and seeds, have been shown to have anti-inflammatory effects and to potentially reduce the risk of cognitive decline in Alzheimer’s disease [75]. Curcumin, a compound found in turmeric, has also been studied for its potential anti-inflammatory effects and its ability to improve cognitive function in people with mild cognitive impairment [76].
Additionally, there is growing interest in the potential role of the gut microbiome in neuroinflammation and neurodegenerative diseases. Studies have shown that certain gut bacteria, such as Akkermansia muciniphila and Faecalibacterium, may have anti-inflammatory effects and may be protective against neurodegenerative diseases [77, 78]. Therefore, dietary interventions that aim to promote the growth of these beneficial bacteria may be a promising approach to reducing neuroinflammation and the risk of neurodegenerative diseases.
Here we provide a description of the most interesting clinical and preclinical studies examining the role of dietary patterns, nutrients or microbiota in reducing neuroinflammation and the risk of neurodegenerative diseases [79].
A number of studies have evaluated the impact of Mediterranean Diet on neurodegeneration. One of the first studies on MD was conducted in Spain between 2003 and 2010: a multicenter, randomized control trial PREDIMED, assessed the efficacy of the MD in lowering the risk of coronary heart diseases [80]. Participants, male and female, had a prior condition of either type II diabetes mellitus (T2DM), hypertension, obesity, high low density lipoprotein (LDL) or low high density lipoprotein (HDL), but none of them had a previous heart condition. They were randomly assigned to 3 different groups: (1) Traditional MD (TMD) with extra-virgin olive oil (VOO) enrichment, (2) TMD + Nuts enrichment, (3) a controlled low-fat diet. In 3 months, the transcriptome of individuals showed a modulation of neuroinflammation in TMD + Nuts group and a downregulation of triggering receptors expressed on myeloid cells-1 (TREM1) signalling pathway in TMD + VOO group. Neurodegenerative diseases are regulated through these pathways so this study was crucial to understand how nutritional therapy can prevent neuroinflammation [80].
Several other studies elucidated protective effects of MD bioactive compounds such as monounsaturated and polyunsaturated fatty acids (MUFA and PUFA). Besides olive oil, walnuts are also rich in polyunsaturated fatty acids, flavonoids, phenolic acid, folates, all of them being antioxidant and anti-inflammatory components. In a randomized controlled trial, cognitive functions and memory capabilities were tested in 447 participants randomly assigned to a MD plus extra virgin olive oil (1 L/week) and a MD plus nuts (30 g/day) or to a control diet with low intake of fats [81]. Subjects who received an implementation of olive oil or nuts showed an improvement of cognitive capabilities [81]. Anti-inflammatory effects of a walnut diet, are observed also in murine models (with LPS induced oxidative stress), where the expression of IL-6, TNF-
About other protective food items, a multi-centered case control study was conducted on ALS patients and healthy controls from 4 different Italian regions (Lombardy, Piedmont and Aosta Valley, and Apulia), between 2011 and 2015 [83]. The participants were asked to compile a validated and reproducible food-frequency questionnaire (FFQ) where they have to indicate the consumption of food items together with its serving size per day/week. It has been reported that among ALS patients there was a significantly low intake of whole grain products, vegetables, folate, Vit. E, vegetable fats, and conversely a high intake of red and processed meat, zinc, glutamic acid and sodium. Whole grains, with a high concentration of fibers, reduce inflammatory cytokines and the risk of developing ALS. Folate and Vit. E have shown effectiveness against oxidative-stress and in the prevention of neurodegenerative disorders like ALS, Alzheimer and Parkinson; raw vegetables and fruits with their richness in polyphenols, minerals and vitamins are highly antioxidant; also tea and coffee intake (that already tested positive on Parkinson and Alzheimer patients) seems to play a neuropharmacological role in ALS prevention. The study limitation resides in the sample made by patients who follow an Italian diet already rich in olive oil, with moderate intake of vegetables and coffee. To validate food protective and risk factors, a larger sample from other countries with different dietary patterns should be considered [83].
In Mild Cognitive Impairment (MCI) and Alzheimer Disease (AD) patients, obesity, hypertension, dyslipidemia, atherosclerosis, T2DM and insulin resistance were often conditions present prior to the rise of the diseases. Which means that a diet rich in saturated fatty acid, processed foods, low fibers carbohydrates can enhance inflammatory processes of the CNS. Furthermore, from systematic reviews it is evident that an adherence to a MD and a consumption of whole grains, legumes and vegetables is linked to a reduced risk to develop MCI and AD, also to a lower progression of diseases [84]. A similar study approach has been tested with an anti-inflammatory diet on Multiple Sclerosis (MS) patients [85]. In MS, inflammatory cytokines TNF-
Another dietary approach has been attempted to assess anti-inflammatory and neuroprotective benefits of weight loss in MS patients. A normal calorie diet was compared to Calorie Restricted (CR) and CR Intermittent diet [86]. The highest benefits were seen in the CR Intermittent Diet where T effector memory cells and the Th1 were reduced, while naïve T cells were instead increased, showing anti-inflammatory and neuroprotective advantages of this dietary approach [86].
The Keto Diet (KD), a dietary regime extremely poor in carbohydrates intake and rich in protein components, has been proven to lower the overactivity of innate immune cells, the free radicals’ production (reducing oxidative stress), and to increase the mitochondrial level of ATP, modulating neuroinflammation in Alzheimer and Parkinson diseases [87]. To test KD on ALS, a prospective, open-label pilot study is ongoing on patients diagnosed with ALS. The study monitors the safety of the dietary regimen of KD for 8 weeks, in addition to other clinical outcomes such as inflammatory status, force capacity, gut dysbiosis. ALS determines a loss of body mass, so an adequate caloric diet is fundamental to contrast this loss. This pilot study could establish if KD may be tolerable and effective in ameliorating a disability condition and the chances of survival. Results have not yet been published [88].
Research has found that, in addition to smoking and low levels of vitamin D, high intake of saturated fats and sodium, and low consumption of
In a double-blind, placebo-controlled clinical trial, researchers investigated the effects of micronutrients on Alzheimer’s Disease (AD) by administering an integration of 500 mg DHA and 150 mg EPA, 10 g lutein, 10 g meso-zeaxanthin, 2 mg zeaxanthin (carotenoids), and 15 mg vitamin E to one intervention group (n = 50), while the control group (n = 27) received placebo. The intervention group showed improvement in skin and blood carotenoids, omega-3, Vit. E concentrations, as well as in memory and mood, indicating that fish oil, Vit E, and carotenoids can be valuable supplements in ameliorating the quality of life of AD patients [91]. Additionally, research has shown that AD patients have lower serum concentrations of Vit. D than healthy controls, and a randomized, double-blind, placebo-controlled trial found that Vit. D supplementation can potentially delay the progression of the disease, improving cognitive function as measured by Full Scale Intelligence Quotients (FSIQ) tests [92].
Similarly, patients with ALS also have low concentrations of Vit. D3, and although a randomized study found that administering 3 different concentrations of Vit. D3 (50,000, 75,000, and 100,000 international units (IU)/month) for 6 months did not significantly improve motor dysfunction, Vit. D3 intake was still recommended due to its neuroprotective properties [93].
Finally, in traditional medicine, saffron has been used for sedation, to cure depression, and as a coadjuvant in cardiovascular and cancer treatments. In a double-blind, randomized, placebo-controlled trial on 40 MS patients treated daily with 2 crocin capsules or placebo for 28 days, the antioxidant effect of saffron was tested. The results showed high antioxidant serum capacity and a decreased presence of inflammatory oxidative biomarkers such as lipid peroxidation, DNA damage, TNF-
While a conclusive causal link between gut microbiota and ALS remains to be established, alterations in microbiota composition have been observed in ALS [46, 95]. This provides a foundation for the intestinal microbiota manipulation as a potential treatment for this debilitating disease [96].
In a comprehensive study by Blacher et al. [96], it was found that supplementing the gut microbiota of transgenic SOD1G93A mice with Akkermansia muciniphila alleviated the symptoms of ALS. The study further demonstrated that the beneficial effect of gut-supplemented Akkermansia muciniphila relied on increased levels of nicotinamide in the CNS of SOD1G93A mice. It was also noted that systemic nicotinamide levels were downregulated in ALS patients. However, it is worth noting that mucin degradation produces SCFA, and some SCFA-producing bacteria are adversely affected in ALS models [97, 98] and patients [99].
In a trial involving 50 ALS patients and 50 healthy individuals, the participants were randomly assigned to receive either probiotic treatment with Lactobacillus strains (Lactobacillus fermentum, Lactobacillus delbrueckii, Lactobacillus plantarum, Lactobacillus salivarius) or a placebo for a duration of 6 months [100]. The purpose was to assess if there were any changes in the microbiota composition and progression of the disease. The results indicated that probiotic supplementation did not have any effect on the progression of the disease. However, changes in microbiota composition among the study participants suggested a potential role in the etiopathogenesis of ALS.
At present, a multicenter randomized controlled trial is underway in Italy, which involves transplanting fecal microbiota (FMT) into 42 ALS patients. The plan is to monitor the immunological and microbiota profile in 12 months follow-up and evaluate the FMT effects on disease progression and symptoms [101].
Akbari et al. [59] conducted a randomized controlled trial to investigate the effect of probiotic supplementation on cognitive functions in AD. The study found that probiotic consumption for 12 weeks positively affects cognitive function, improving the metabolic status and decreasing inflammation [59]. Similarly, Tamtaji et al. [60] conducted a randomized controlled trial to investigate the effect of a probiotics on individuals with Parkinson’s disease. The study found that treatment improved the Movement Disorders Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), and the metabolic profile of patients.
Previous studies have indicated that neurodegenerative disorders are associated with increased homocysteine concentrations [102]. Since folates, Vitamin B12, and Vitamin B6 are involved in the metabolism of homocysteine, they could be useful in treating the symptoms of ALS. In a murine study, Song et al. [103] found that a prebiotic Galacto-oligosaccharides (GOS) and GOS-rich prebiotic yogurt administered to SOD1G93A mice improved Vitamin B synthesis and absorption/concentration of folates, leading to prevention and a longer life in SOD1G93A mice. The results also showed that GOS and GOS-rich prebiotic yogurt treatments were able to lessen motor neuron loss, muscle atrophy, and factors related to inflammation and apoptosis. GOS and GOS-rich prebiotic yogurt are valid candidates for future ALS nutritional therapy [103].
Finally, several studies have demonstrated that postbiotic compounds, such as SCFAs, inhibit histone-deacetylase and could be beneficial for neurodegenerative disorders. SCFAs may influence the composition of the gut microbiota, and together with prebiotics, probiotics, and postbiotics, they could shape a complete nutritional therapy to be tested on neurological patients in clinical trials [104].
In conclusion, the gut-microbiome-brain axis plays a crucial role in regulating systemic metabolism and homeostasis. Research has demonstrated that dietary habits and nutrients can impact the immune system and inflammatory status by influencing various factors, such as the composition of the microbiome, the release of microbial products, gastrointestinal signaling molecules, and neurotransmitters. These signalling molecules then affect brain homeostasis and can sustain neuroinflammation, being involved in neuroinflammatory and neurodegenerative disease development or progression. Maintaining proper dietary habits should be a fundamental aspect of any plan aimed at preventing neurological diseases caused by systemic metabolic changes. However, the relationship between nutrition and the immune system is complex, and the discipline of immunonutrition has emerged to explore ways to modify immune system activity through specific nutrient interventions. Given the strong evidence linking intestinal dysbiosis to neurodegenerative and neuroinflammatory diseases, future research should focus on investigating the impact of immunonutrition interventions on disease onset and progression, taking into account the potential effects mediated by the gut microbial communities and their derived components.
ED, IL, EN designed the research study. ED, IL, EN, GN wrote the manuscript. EN, GN revised the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Not applicable.
We thank the Prof. Amedeo Amedei for the mentoring and supervising of our work.
This research received no external funding.
The authors declare no conflict of interest. Given the role as Guest Editor, Elena Niccolai had no involvement in the peer-review of this article and has no access to information regarding its peer-review. Full responsibility for the editorial process for this article was delegated to Graham Pawelec.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.

