1 Infinitus (China) Co., Ltd., 510000 Guangzhou, Guangdong, China
2 College of Chemistry and Materials Engineering, Beijing Technology & Business University, 100048 Beijing, China
3 Beijing Key Lab of Plant Resource Research and Development, 100048 Beijing, China
4 Beijing Lan Divine Technology Co.Ltd., 100048 Beijing, China
†These authors contributed equally.
Abstract
Background: Propionibacterium acnes causes upregulation of inflammatory
factors, such as cycloxygenase-2, prostaglandin E2, interleukin-1
Keywords
- Schisandra chinensis fruit oil
- propionibacterium acnes
- HaCaT cells
- anti-inflammatory
- NF-κB signalling pathway
- COX-2/PGE2 signalling pathway
- LL-37
Schisandra chinensis (Turcz.) Bail (SC) is a dried ripe fruit of the
family Magnoliaceae [1]. It is a traditional Chinese herbal medicine with a long
history of use. In traditional Chinese medicine, it is used to treat
gastroenteritis, respiratory failure, cardiovascular disease, physical weakness,
excessive sweating, insomnia, depression, anxiety, and many other disorders [1].
SC’s main distribution sites are in the northeast and Inner Mongolia, Hebei, and
Shanxi, China. Through literature research, it was found that SC has
anti-inflammatory and antibacterial pharmacological effects [2, 3, 4, 5, 6]. Among them,
lignans are the main potent constituents in SC, including schisanol A, schisanol
B, schisanin A, schisanin B, and schisanin C [7]. The anti-inflammatory mechanism
works by regulating the activity of inflammatory signalling pathways and reducing
the production and release of inflammatory factors such as TNF-
Acne is a chronic inflammatory disease of the sebaceous glands of hair follicles [15]. The massive proliferation of Propionibacterium acnes in the pilosebaceous unit and chemical and cellular mediators that cause inflammation destroy the follicular sebaceous glands. As a result of their interaction, the skin microenvironment is altered, leading to an inflammatory response in the host and fostering acne lesion progression [16, 17]. In addition to its direct antimicrobial action, LL-37 has a powerful immunomodulatory function and can act as an anti-inflammatory mediator with various immunomodulatory effects [18, 19].
Cycloxygenase-2 (COX-2) is undetectable in most tissues under physiological conditions but can be abundantly expressed in response to the induction of inflammatory factors [20, 21]. COX-2 is the rate-limiting enzyme in prostaglandin E2 (PGE2) synthesis and is essential for regulating PGE2 activity [22, 23]. PGE2 is an important inflammatory mediator that directly causes an increase in vascular tissue permeability and promotes the secretion of inflammatory factors such as interleukin (IL)-6 from relevant tissues to induce an inflammatory response [24, 25]. Activation of the COX-2/PGE2 pathway triggers skin pain, erythema, and pruritus.
NF-
Many endogenous antimicrobial peptides (AMPs) have been shown to play essential
roles in innate immunity [33, 34]. The antibacterial protein LL-37 is the only
antimicrobial peptide of the cathelicidin family expressed in humans [35, 36]. It
has significant antibacterial activity against both gram-negative and
gram-positive bacteria. However, LL-37 is lacking in normal skin, but its
secretion is significantly higher in inflamed skin than in non-inflamed skin [37, 38]. One future trend is to consider antimicrobial peptides for treating skin
inflammation [39]. LL-37 can inhibit the release of pro-inflammatory cytokines
and promote the release of anti-inflammatory cytokines through the regulation of
NF-
Here, we used P. acnes-stimulated HaCaT cells to construct an
in vitro inflammatory HaCaT cell model. This study investigated the
effects of S. chinensis fruit oil (SCO), obtained by supercritical
CO
HaCaT cells were purchased from Peking Union Medical College (Beijing, China),
and Dulbecco’s Modified Eagle’s Medium (DMEM), dichloro-dihydro-fluorescein
diacetate (DCFH-DA), and calcium fluorescent probe (Fluo-4, AM ester) were
purchased from BIOAGRIO (Shanghai, China). The methyl thiazole tetrazolium (MTT)
kit was purchased from Shanghai Biyuntian Biotechnology Co., Ltd (Shanghai,
China), and trypsin, fetal bovine serum (FBS), and a double antibody mix were
purchased from Gibco (Grand Island, NY, USA). Phosphate-buffered saline (PBS) was
obtained from HyClone (Logan, UT, USA). COX-2, PGE2, and IL-1
The dried SC was crushed and passed through a 20 mesh sieve to obtain SC powder.
SC powder (10.0 g) was accurately weighed and extracted using a Waters MV-10 ASFE
supercritical fluid extraction system (Waters Corporation, Milford, MA, USA) at a
CO
The inhibition of COX-2 by SCO at 0.50 mg/mL was measured according to the instructions of the COX-2 ELISA kit.
HaCaT cells were cultured in DMEM containing a mixture of 10.0% FBS and 1.0%
double antibodies in an incubator at 37 °C and 5% CO
HaCaT cells in the logarithmic growth phase were prepared at a cell
concentration of 2
HaCaT cells in the logarithmic growth phase were prepared at a concentration of
2
The safe concentration of SCO for HaCaT cells was measured using an MTT assay,
and the appropriate concentration was screened for subsequent experiments based
on the results of cytotoxicity experiments. The blank, model, and sample groups
were set up separately; the blank and model groups were connected to 1.0 mL of
culture medium, and the sample group was connected to 1.0 mL of sample diluted to
a safe concentration with culture medium. Samples were incubated in a cell
incubator for 6 h and then stimulated with P. acnes. P. acnes stimulation was added only to model and sample
group, except blank. The cell supernatant was collected after incubation at 37 °C
and 5% CO
A method based on reversed-phase high-pressure liquid chromatography with a diode array detector (RP-HPLC-DAD) (1260 Infinity; Agilent Technologies, Santa Clara, CA, USA) was developed to determine the contents of five lignins simultaneously.
SCO was prepared at a concentration of 10.0 mg/mL in methanol, and the sample
was separated on a reverse column ZORBAX SB-C18 (4.6 mm
| Time/min | A% | B% | Flow rate (mL/min) |
| 0 | 55 | 45 | 1.0 |
| 10 | 35 | 65 | 1.0 |
| 30 | 35 | 65 | 1.0 |
| 35 | 25 | 75 | 1.0 |
| 40 | 25 | 75 | 1.0 |
To investigate the mechanism underlying the anti-inflammatory effect of SCO,
Ca
After P. acnes stimulation, the cells were incubated for 2 h and then
washed thrice with PBS. Thereafter, 100 µL of Fluo-4 AM was added to the
cells and incubated for 20 min at 37 °C while protected from light. Next, Fluo-4
AM was removed, 200 µL of trypsin was added to each well separately, and
the cells were digested for 1–2 min before 200 µL of the medium was added
to terminate the digestion. Subsequently, the cytosol was removed, and 500
µL of PBS was added to each tube to disperse the cells, after which
Ca
After P. acnes stimulation and 2 h of cell culture, the cells were washed thrice with PBS. Afterward, 500 µL of DCFH-DA working solution was added to each well and incubated for 30 min at 37 °C protected from light. After the same operation described in Section 2.5, PBS (500 µL) was added to each tube, the cells were lysed, and ROS expression was detected by flow cytometry.
The same experimental procedure as that described in Section 2.5 was performed.
After removal of the supernatant, the cytoplasmic protein extraction reagent was
added to the collected cells to lyse them sufficiently and the cytoplasmic
protein samples were harvested by centrifuging the supernatant. To the
precipitate, cellular nucleoprotein extraction reagent was added and the
supernatant was centrifuged to obtain a sample of cellular nucleoprotein. The
concentration of bands on the membrane was analysed by gel preparation, loading,
electrophoresis, transfer, blocking, incubation with antibodies and stain
development to calculate the expression of I
All experiments were repeated thrice, and the results are expressed as mean
In the CO
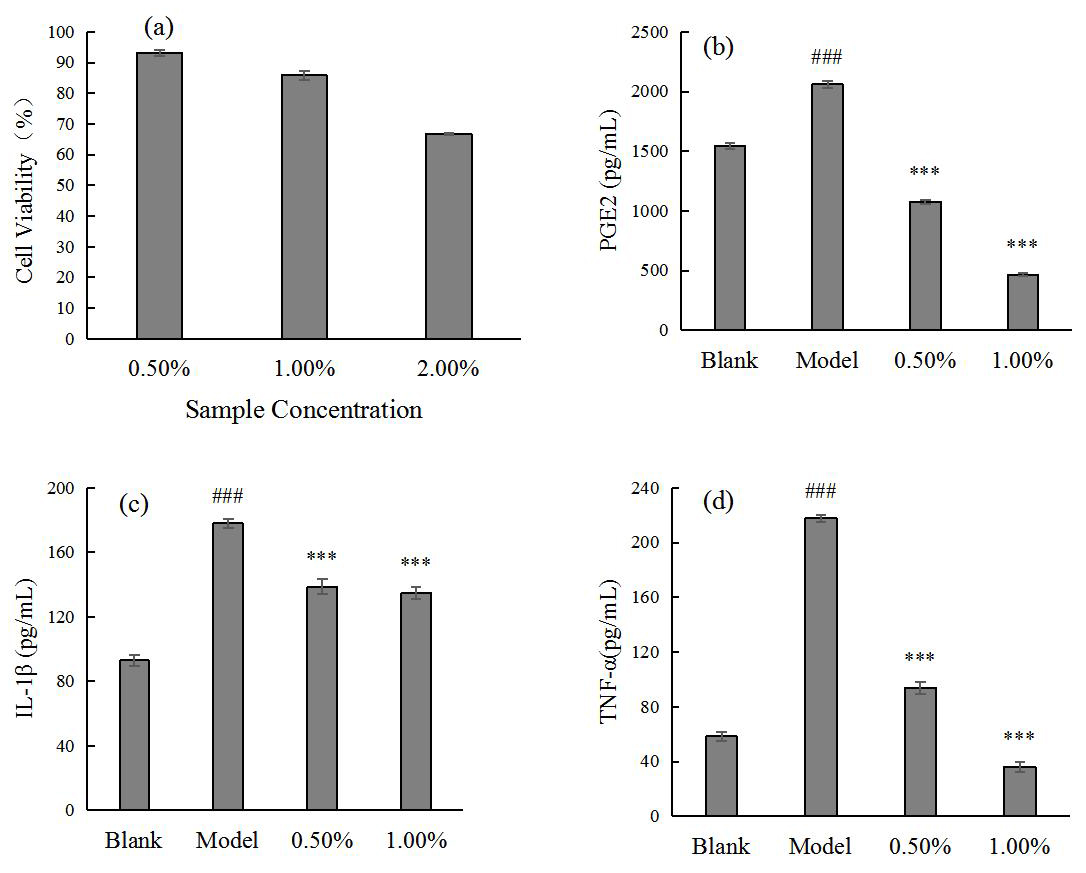
COX-2 is an inducible enzyme with cyclooxygenase and peroxidase activities and is a key enzyme in converting arachidonic acid to PGE2. COX-2 is expressed at very low levels under normal conditions but is significantly expressed in response to stimuli such as inflammation, pain, and tumors. Therefore, it can be used as an indicator of anti-inflammatory effects. The COX-2 inhibition experiments showed that SCO could inhibit COX-2 by up to 93.84% at 0.50 mg/mL, indicating that SCO has some anti-inflammatory potential.
P. acnes causes skin inflammation, leading to the overexpression of
inflammatory factors, such as PGE2, IL-1
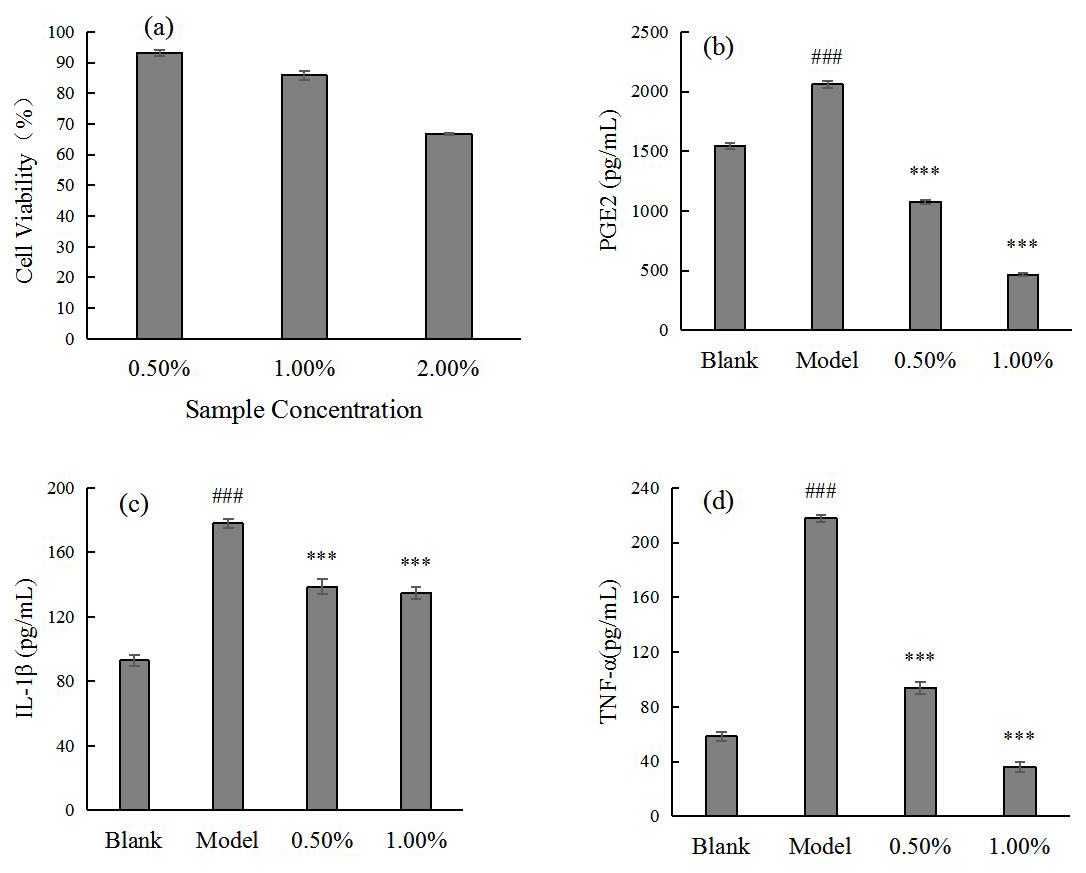 Fig. 1.
Fig. 1.SCO safe concentration for HaCaT (a) and the effect of SCO on
PGE2 (b), IL-1
Thus, the effect of SCO on the expression levels of inflammatory factors induced
by P. acnes in HaCaT cells was demonstrated using the above cellular
model. The results showed that the cell survival rate could reach more than 80%
at a concentration of 1.00% or less; therefore, a sample concentration of 1.00%
or less was chosen for the experiment. The results in Fig. 1 show that SCO
significantly reduced the expression of inflammatory factors PGE2, IL-1
Lignans as the main anti-inflammatory active component in SC [4, 43, 44]. The
contents of schisanol A, schisanol B, schisanin A, schisanin B, and schisanin C
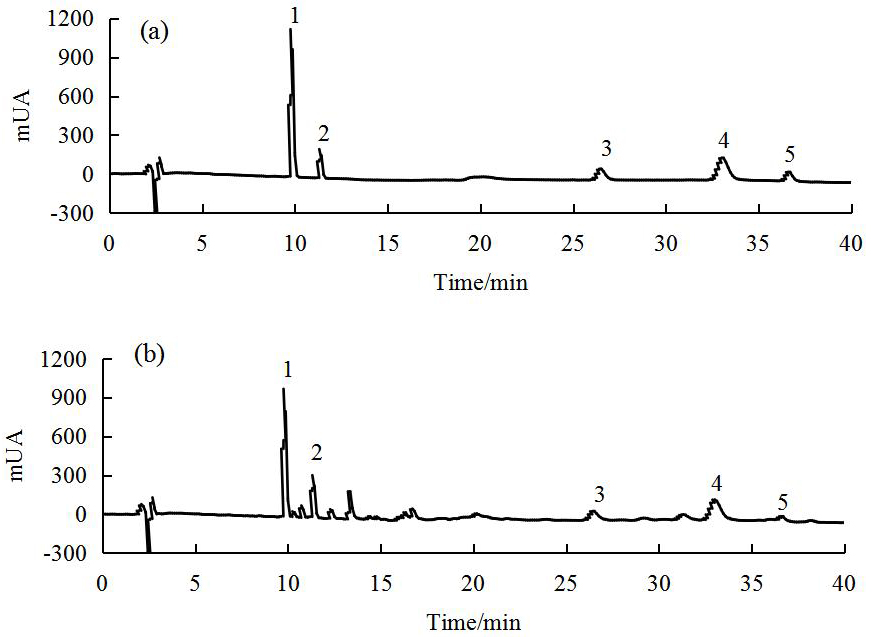
are important indicators of SCO quality. Fig. 2 shows the RP-HPLC-DAD profiles of
the mixed standards and SCO samples. The linear ranges and correlation
coefficients (R
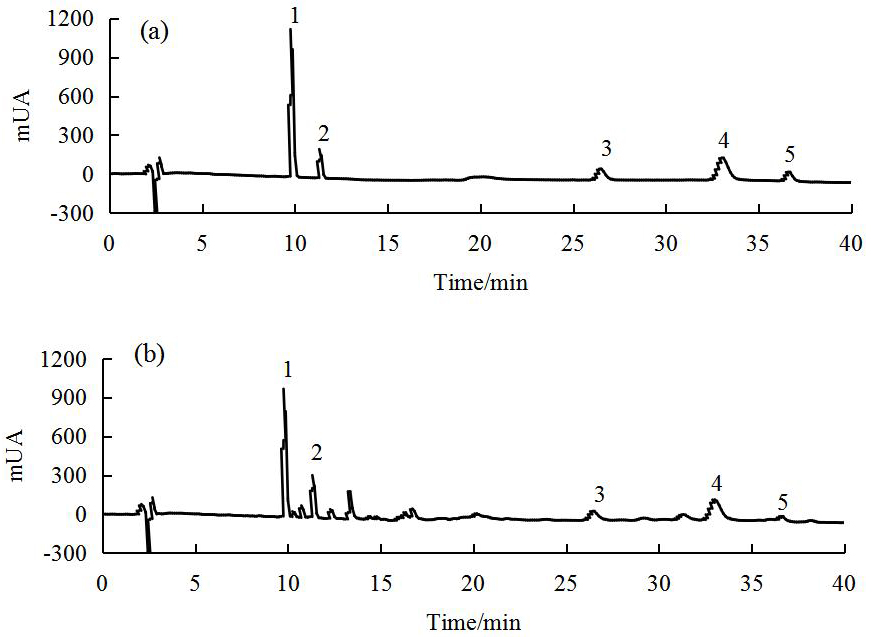 Fig. 2.
Fig. 2.RP-HPLC-DAD profiles of mixed standards (a) and SCO (b) (The numbers in the profiles: 1. schisanol A, 2. schisanol B, 3. schisanin A, 4. schisanin B, 5. schisanin C).
| No. | Components | CAS# | Time/min | R |
Linear range (µg/mL) |
| 1 | schisanol A | 7432-28-2 | 9.841 | 0.9984 | 40–400 |
| 2 | schisanol B | 58546-54-6 | 11.385 | 0.9992 | 10–100 |
| 3 | schisanin A | 61281-38-7 | 26.495 | 0.9987 | 10–100 |
| 4 | schisanin B | 61281-37-6 | 33.055 | 0.9992 | 30–300 |
| 5 | schisanin C | 61301-33-5 | 36.643 | 0.9962 | 10–100 |
The method was validated in terms of repeatability, precision, stability, and
recovery. Five batches of samples were injected, and the relative standard
deviation (RSD) values of each component were
The results showed that SCO comprises a wealth of lignin-like active
ingredients, including schisanol A, schisanol B, schisanin A, schisanin B, and
schisanin C at 33.89
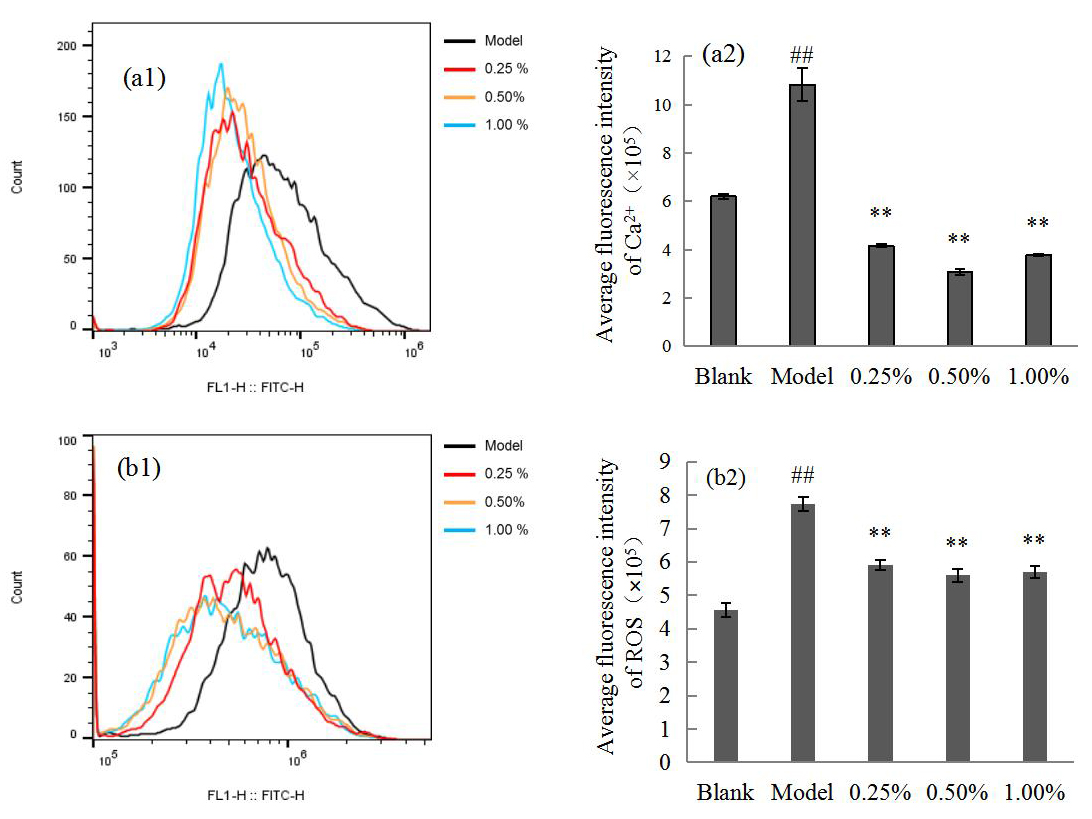
When the skin is exposed to harmful stimuli, oxidants, such as ROS, are produced excessively, and the body’s balance between resistance and promotion of oxidation is disturbed. Oxidative stress is generated by the excessive accumulation of free radicals in the body, which can also open Ca channels and promote inflammation and apoptosis [46, 47].
Fig. 3 shows that Ca
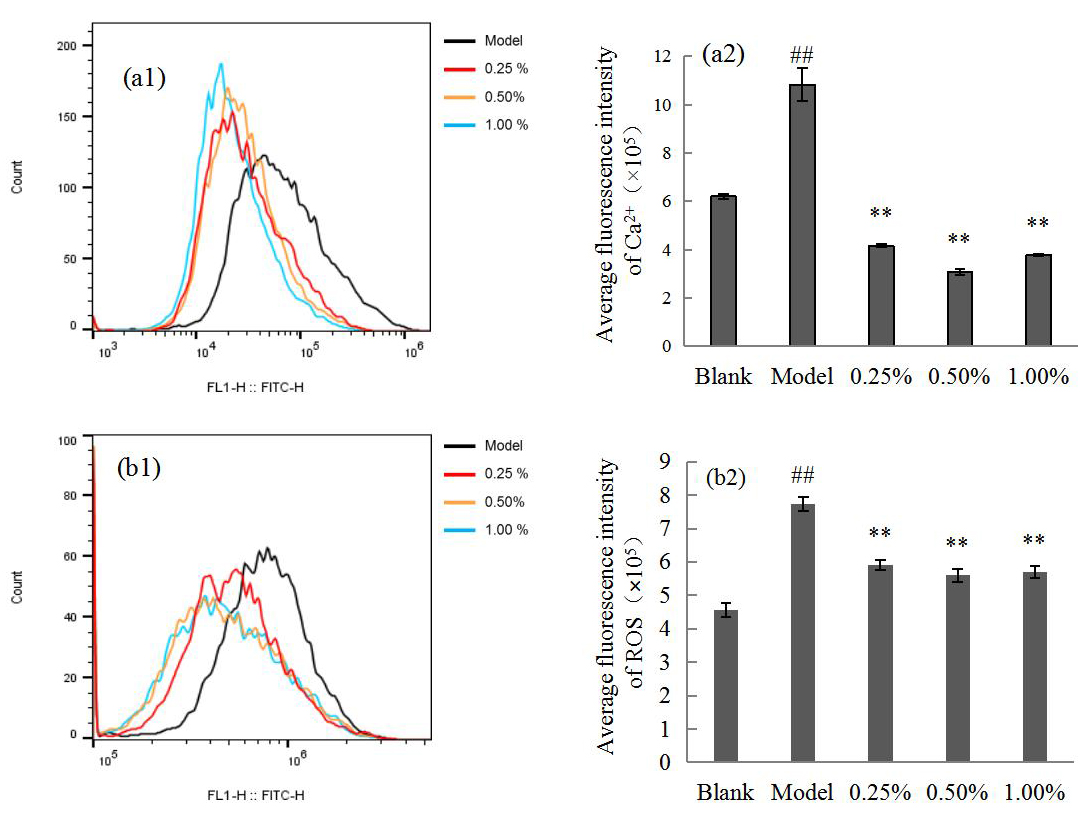 Fig. 3.
Fig. 3.Flow cytometry (a1,b1) showing the Ca
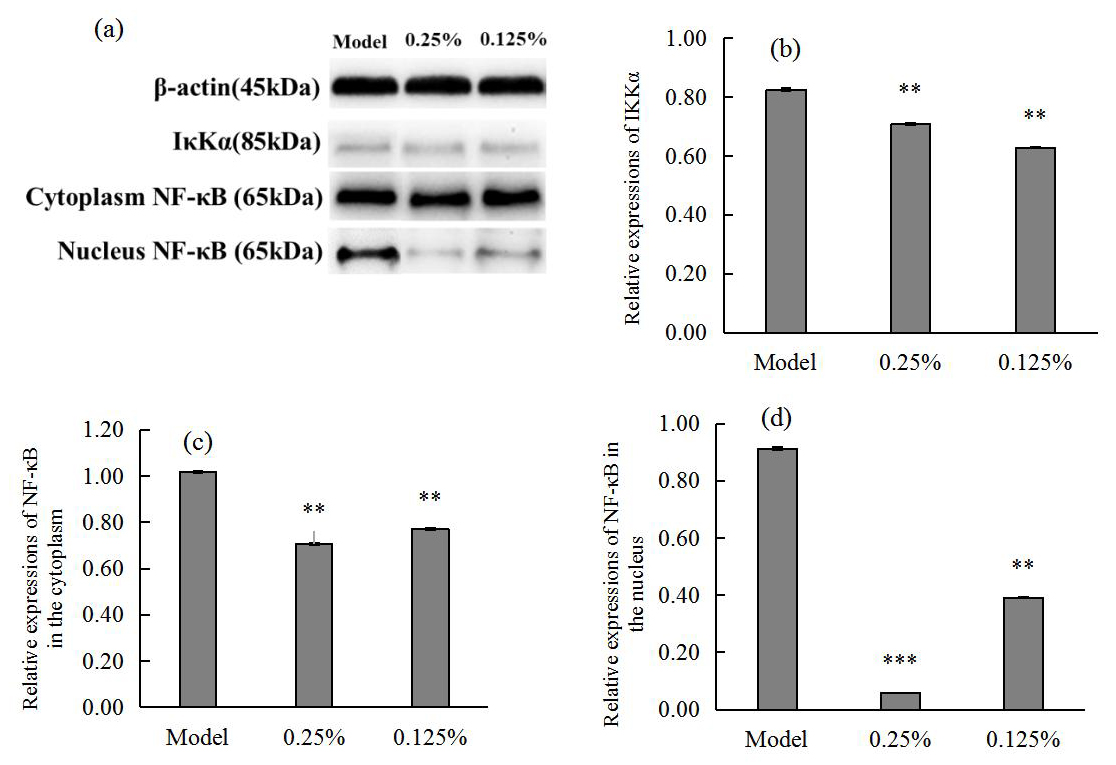
Quantitative analysis of western blot bands and grey scale values in Fig. 4
showed that SCO could significantly reduce the secretion of
I
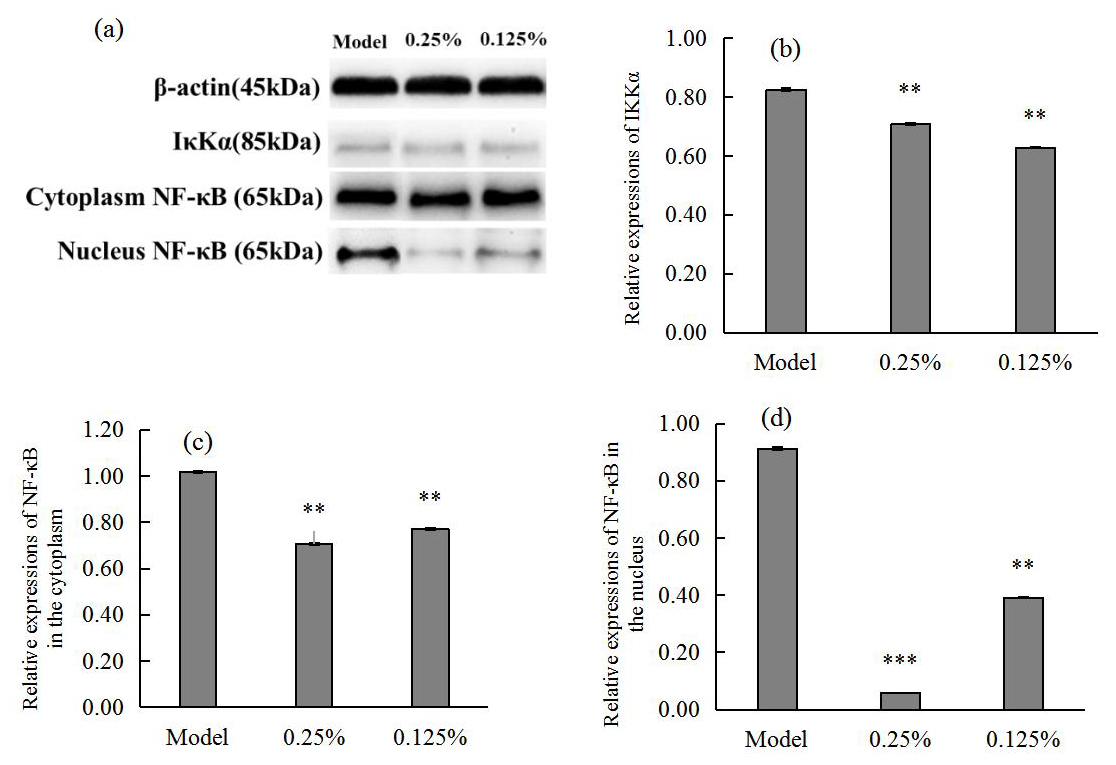 Fig. 4.
Fig. 4.Western blot images (a) showing I
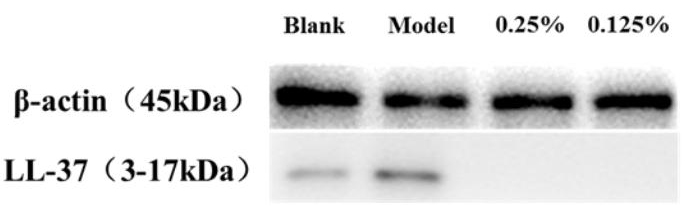
Fig. 5 shows that LL-37 secretion is significantly higher in the model group after induction, indicating that stimulation by P. acnes can activate the inflammatory response and promote the secretion of antimicrobial peptides. In contrast to that in the model group, SCO significantly inhibited the secretion of LL-37, indicating that SCO has a regulatory effect on antimicrobial peptides.
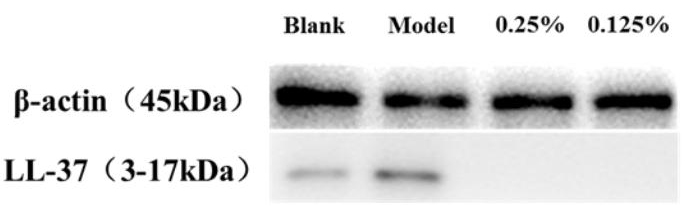 Fig. 5.
Fig. 5.Western blot showing the LL-37 levels in HaCaT.
This study aimed to determine the potential of SCO in regulating P.
acnes-induced inflammatory factors in HaCaT cells. We focused on SCO, as it has
been shown in various studies to have good anti-inflammatory and antioxidant
properties, and its main active ingredients are lignans (schisanol A, schisanol
B, schisanin A, schisanin B, and schisanin C). The lignans content of SCO was
found to be 91.31
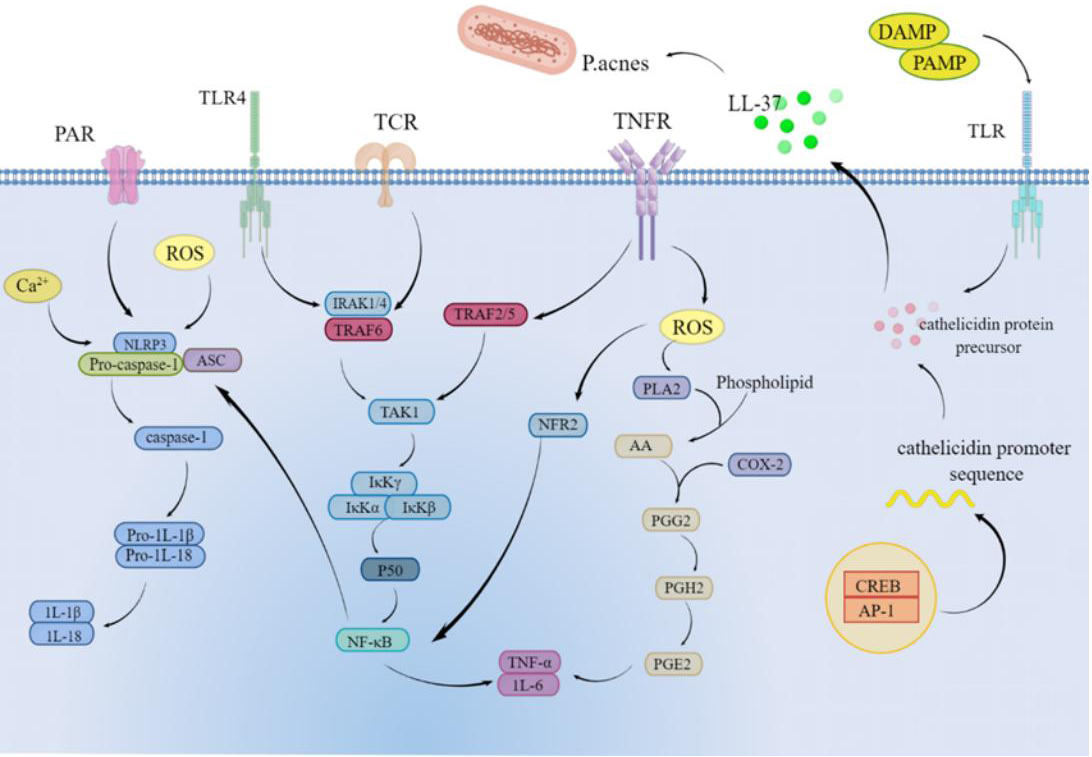
As shown in Fig. 6, in the present study, we found that P. acnes causes
increased levels of inflammatory factor secretion in HaCaT cells; therefore, we
used this cellular model to assess the effect of SCO on inflammation and to
understand the potential mechanisms by which SCO protects against inflammatory
stress. IL-1
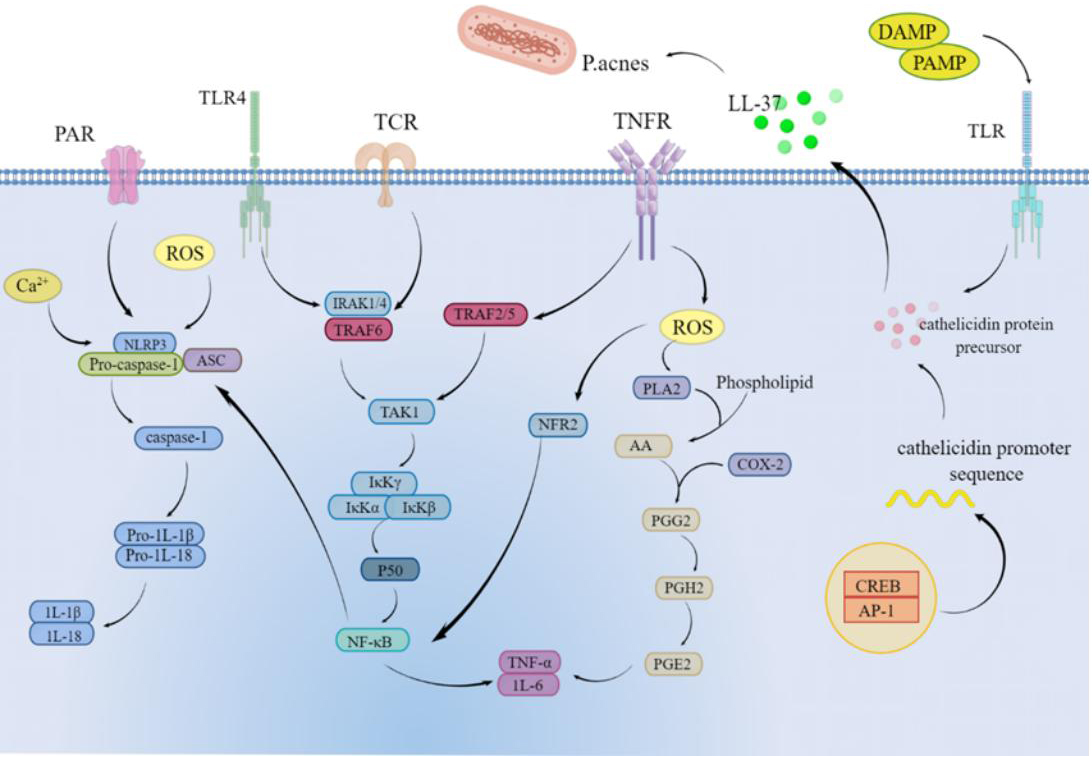 Fig. 6.
Fig. 6.Mechanism of action of SCO on Propionibacterium acnes-induced inflammation in HaCaT cells.
Ca
LL-37 is the only cathelicidin AMP family expressed in humans and has been shown to play an important role in innate immunity [35, 36]. LL-37 is lacking in normal skin, but its secretion is significantly higher in inflamed skin. Our results show that SCO can modulate LL-37 levels to alleviate skin inflammation.
The research results of Zhang et al. [3, 6] showed that the extract of Schisandra chinensis and its active components played an anti-inflammatory role by acting on immune cells, reducing the secretion of pro-inflammatory cytokines or acting on inflammation-related signaling pathways. Consistent with these results, this study suggests that Schisandra chinensis fruit oil can also play an anti-inflammatory role by reducing the secretion of pro-inflammatory cytokines and acting on the NF-
In this study, we found that SCO reduced the secretion of inflammatory factors
and Ca
Datasets used and/or analyzed for this study are available from the corresponding author upon appropriate request.
LL, MT, YZ and MG designed the research study. HZ, HL and XM performed the research. PL, BC and DY provided research ideas and experimental guidance. YZ analyzed the data. HZ and HL wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Not applicable.
Not applicable.
This research received no external funding.
Author Pingping Lv, Meiling Tai, Biao Che was employed by the Infinitus (China) Co., Ltd. Author Dan Yu were employed by the company Beijing Lan Divine Technology Co.LTD. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.