1 Yunnan Key Laboratory of Stomatology, 650106 Kunming, Yunnan, China
2 Department of Preventive Dentistry, Kunming Medical University School and Hospital of Stomatology, 650106 Kunming, Yunnan, China
3 Department of Pediatric Dentistry, Kunming Medical University School and Hospital of Stomatology, 650106 Kunming, Yunnan, China
4 Department of Prosthodontics Dentistry, Kunming Medical University School and Hospital of Stomatology, 650106 Kunming, Yunnan, China
Abstract
Background: Sappanwood is widely used in the prevention and treatment
in diseases due to its ability to seal blood vessels, dissipate stasis, and
relieve pain. Important monomer components of sappanwood, Protosappanin A (PA)
and Protosappanin B (PB) have anti-tumour and antimicrobial medicinal properties.
This study investigated the anti-inflammatory and osteogenic differentiation
effects of a crude extract of Sappanwood (ESP), PA and PB against periodontitis
in periodontal ligament stem cells (PDLSCs). Methods: Oil Red O staining
was used to assess the ability of adipocytes to differentiate. Alizarin Red
staining was used to assess the capacity to differentiate into osteoblasts.
Third-passage PDLSCs were grown in either basic medium alone or basic media with
varying doses of ESP (0.0625 mg/mL, 0.03125 mg/mL and 0.125 mg/mL), PA and PB
(2.5 µM, 5 µM, 10 µM). The CCK-8 assay was used to measure cell
proliferation. Real Time PCR (RT-qPCR) and Enzyme-Linked Immunosorbnent Assay
(ELISA) assay were used to measure gene expression. The capacity to differentiate
into osteoblasts was evaluated using Alizarin Red staining, and Alkaline
Phosphatase (ALP) staining and activity. Results: The development of
lipid droplets and mineralized nodules was examined using Oil Red O staining and
Alizarin Red staining. Flow cytometry revealed that PDLSCs were CD29 (98.23%)
and CD44 (98.81%) positive, but CD34 (0.16%) and CD45 (0.09%) negative. CCK-8
assay showed that ESP at three concentrations (0.03125 mg/mL, 0.0625 mg/mL and
0.125 mg/mL) and 2.5 µM, 5 µM and 10 µM PA and PB had no
cytotoxicity at 5 and 7 days (p
Keywords
- periodontitis
- periodontal membrane stem cells
- sappanwood
- Protosappanin A and Protosappanin B
- osteogenic differentiation
Periodontitis is defined by periodontal tissue destruction and may lead to tooth loss. Currently, engineering and life sciences research in tissue engineering have provided methods to replace missing alveolar bone, periodontal ligament, and root cementum [1].
The periodontal ligament is rich in periodontal ligament stem cells (PDLSCs). These cells are capable of multinomial differentiation and self-renewal [2]. Studies have shown that PDLSCs maintain dynamic periodontal balance and regulate periodontium regeneration. The interaction between PDLSCs and the peripheral periodontitis niche is important for periodontal tissue repair. Injured PDLSCs may disrupt the microenvironment by exacerbating the host immune response, resulting in abnormal angiogenesis, and promoting osteoclast activity [2]. The periodontal regenerative capacity of PDLSCs is impaired in the inflammatory microenvironment [3]. Therefore, regulation of the inflammatory response for PDLSCs is crucial for the generation of periodontal tissue. A vital component of Sappanwood, Protosappanin A (PA), may prevent atherosclerosis by inhibiting NF-B signalling, hyperlipidaemia, and inflammation in hyperlipidaemic rabbits.
Sappanwood, a red dye in ancient China, has the advantages of bright colours and
can easily be produced. Studies have documented the antitumor effects and
regulatory apoptotic effects of Sappanwood [4, 5]. Sappanwood also plays a
neuroprotective role through multitarget pharmacological mechanisms and prevents
brain injury caused by ischemia/reperfusion [6]. Sappanwood has the advantages of
low price, convenient extraction and preparation, and a wide range of materials
and sources. This dye also has more research value because of its good efficacy
and low cytotoxicity as a natural medicine [7, 8]. As a vital component of
Sappanwood. Protosappanin A (PA) may prevent atherosclerosis by inhibiting NF-
ESP was purchased from Yunnan Hongxiang Yitang Pharmaceutical (Yunnan, Kunming, China) in China and dissolved it in pure sterile water for cell treatment. We extracted medicinal materials by heating them at 100 °C and boiling them for 24 hours in water as a solvent. PA and PB were purchased from Yunnan Xili Biotechnology (Yunnan, Kunming, China) and dissolved in DMSO (Solarbio, Beijing, China) for cell treatment. We used 10 µg/mL lipopolysaccharides (LPS) from Porphyromonas gingivalis (Invitrogen, Carlsbad, CA, USA).
Permission to conduct this study was obtained from the Kunming Medical
University School’s ethical committee (no. KYKQ2021MEC025). PDLSCs were removed
from premolars without caries and periodontitis. These samples were obtained from
healthy individuals who voluntarily agreed to be part of this study (donors’ age:
14–18 years of age). The extracted teeth were submerged in a phosphate-buffered
saline (PBS) solution consisting of 10% antibiotics (Solarbio, Beijing, China).
After three PBS washes, the periodontal membrane was scraped off one-third of the
root under aseptic conditions. The small pieces of periodontal tissue were
digested with collagenase I (3 mg/mL, Gibco, Grand Island, NE, USA) and Dispase II (4
mg/mL, Sigma Chemical Co. St. Louis, MO, USA) for 30 min. The cells were
maintained in basic medium with 89% alpha minimal essential medium
(
Markers on the cellular surfaces of the PDLSCs were examined utilizing the flow cytometry approach. We followed the guidelines provided by the manufacturer (Agilent NovoCyte, Santa Clara, CA, USA). We used PBS to wash the trypsinised cells and cultured them with the markers CD44, CD105, CD34 and CD45 (Abcam, Wales, UK) for 20 min at 4 °C in complete darkness. The outcomes were examined using the NovoExpress program (version1.4.1, Santa Clara, CA, USA).
We changed the medium to adipogenic or osteogenic medium from the wells after
2–3 weeks to assess adipogenic or osteogenic differentiation. Cells were treated
in osteogenic medium with vitamin C (50 µg/mL, Sigma, Chemical Co. St. Louis, MO, USA) and
We determined the cytotoxicity of ESP, PA and PB to PDLSCs. According to the
standard of 2000 cells per well, we transferred cells into a 96-well plate
containing various concentrations of ESP, PA and PB with refreshment every other
day. Consistent with the guidelines provided by the manufacturer, 90 µL of
The Alkaline Phosphatase (ALP) level in PDLSCs was assessed by an ALP activity kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) and an ALP staining kit (Beyotime Institute of Biotechnology, Shanghai,China). Osteogenic medium was used to cultivate third passage cells in six-well plates for one week. Using ALP staining, we conducted PBS staining three times and fixed the sample in 4% paraformaldehyde. Then, we viewed the sample under an inverted microscope (Leica, Weztlar, Germany). Similarly, after osteogenic differentiation, we lysed the cells using RIPA buffer and three PBS washes. We introduced a liquid phase containing ALP into a 96-well plate for 15 min at 37 °C and measured the sample’s optical densities using a spectrophotometer set to 520 nm.
Cells were incubated in osteogenic medium for 14 days with the medium replaced every three days. The cells were fixed for 30 min in a 2 mL solution of 4% formaldehyde after being rinsed with PBS. After three washes with PBS, 1 mL of Alizarin Red dye solution (Cyagen, Guangzhou, China) was instilled into one well for 3–5 min to stain the cells. We used Alizarin Red staining to detect lipid droplets examined by an inverted microscope (Leica, Weztlar, Germany).
After the stimulation of 10 µg/mL lipopolysaccharides (LPS) from the
Porphyromonas gingivalis (Invitrogen, Carlsbad, California, USA) for 24 h.
PDLSCs were cultured at a density of 5
| Gene | Primer | |
| Interleukin 8 (IL-8) | Forward | 5 |
| Reverse | 5 | |
| Interleukin 6 (IL-6) | Forward | 5 |
| Reverse | 5 | |
| Interleukin 10 (IL-10) | Forward | 5 |
| Reverse | 5 | |
| Interleukin 4 (IL-4) | Forward | 5 |
| Reverse | 5 | |
| Interleukin |
Forward | 5 |
| Reverse | 5 | |
| Osteocalcin (OCN) | Forward | 5 |
| Reverse | 5 | |
| Osterix (OSX) | Forward | 5 |
| Reverse | 5 | |
| Runt-related transcription factor 2 (RUNX2) | Forward | 5 |
| Reverse | 5 | |
| Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | Forward | 5 |
| Reverse | 5 | |
We used RIPA buffer (Solarbio, Beijing, China) containing protease inhibitors to
extract proteins from cells in six-well plates and centrifuged the samples at
12,000 rpm for 30 minutes. The protein concentration was measured by a BCA
protein assay kit (Beyotime Institute of Biotechnology, Shanghai, China). The
extracted proteins were denatured at 100 °C for 5 minutes, separated in
5
The supernatants of PDLSCs were collected and centrifuged at 4 °C
(300
For each experiment, all results from at least three replicates were reported as
the mean
PDLSCs were isolated from the periodontal tissue of healthy individuals. They were plastic adherent with a fibroblast-like morphology when incubated (Fig. 1a). When stimulated in an osteogenic and lipid-forming inductive medium, these cells could be converted into adipocytes and osteoblasts (Fig. 1b,c). Flow cytometry revealed that PDLSCs were CD29 (98.23%) and CD44 (98.81%) positive, and negative for the markers CD34 (0.16%) and CD45 (0.09%) (Fig. 1d) (Supplementary Fig. 1). These results confirmed that the isolated PDLSCs were stem cells with powerful multipotency.
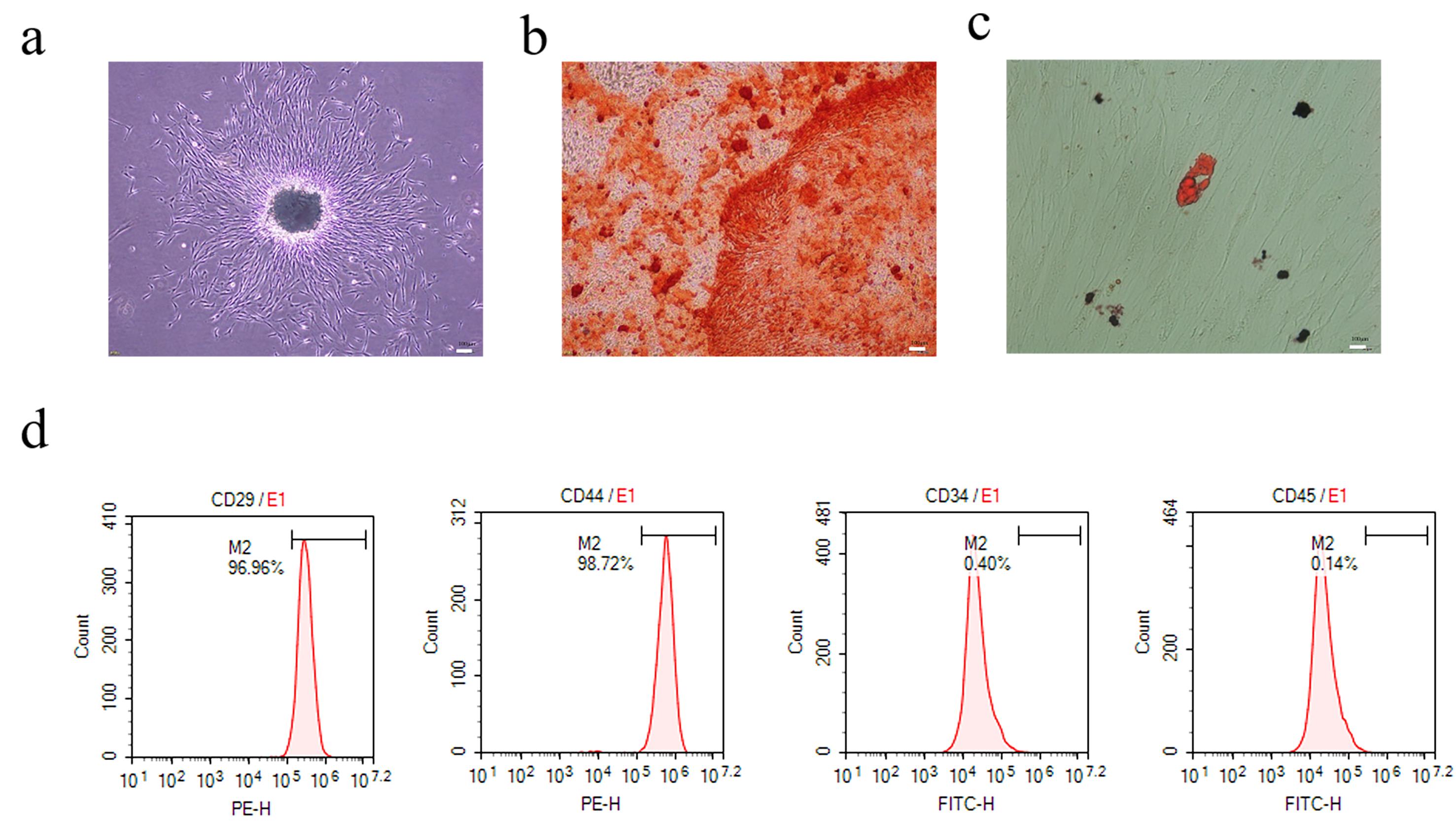 Fig. 1.
Fig. 1.Morphology and identification of periodontal ligament stem cells (PDLSCs). (a) PDLSCs (P3) formed a spiral arrangement in a fusiform shape (scale 200 µm). (b) PDLSCs were stained with Alizarin Red to assess osteogenic differentiation, showing red mineralized nodules (scale bar 50 µm). (c) Lipid differentiation of PDLSCs showed red oil droplets (scale bar 20 µm) by Oil-Red O staining. (d) PDLSCs expressed positive markers (CD29, CD44) but not negative markers expresse (CD45, CD34).
CCK-8 assay were used to determine the cytotoxicity of ESP, PA and PB to PDLSCs. Cells treated with 0.0625 mg/mL, 0.03125 mg/mL and 0.125 mg/mL ESP showed no cytotoxicity at 1, 3, 5 and 7 days (Fig. 2a). We established a concentration gradient of PA and PB and found that 2.5 µM, 5 µM and 10 µM PA and PB had no cytotoxicity at 5 and 7 days (Fig. 2b,c). Finally, we chose 0.03125 mg/mL, 0.0625 mg/mL and 0.125 mg/mL ESP, and 2.5 µM, 5 µM and 10 µM PA and PB for the follow-up experiments.
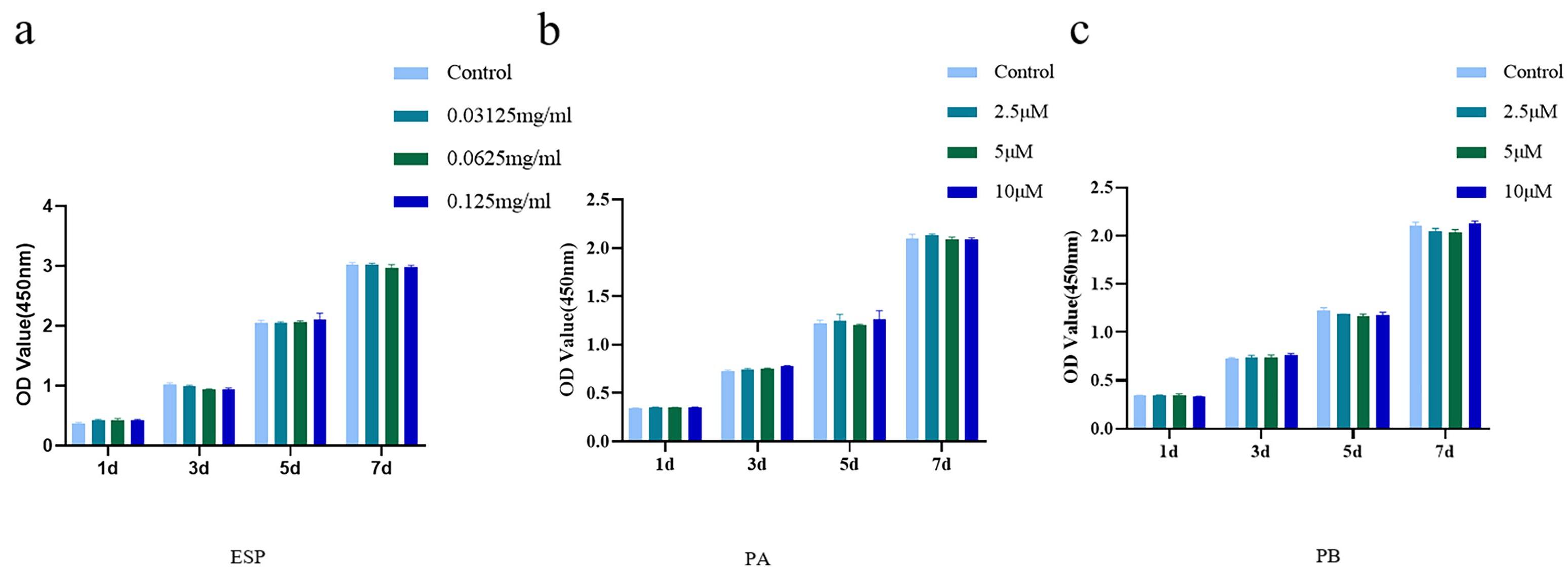 Fig. 2.
Fig. 2.Cytotoxicity of extract of Sappanwood (ESP), Protosappanin A (PA) and Protosappanin B (PB) to PDLSCs. (a) The cytotoxicity of
of ESP at 0.03125 mg/mL, 0.0625 mg/mL and 0.125 mg/mL to PDLSCs of the control
groups on days 1, 3, 5 and 7. (b,c) The cytotoxicity of PA and PB at 2.5 µM,
5 µM and 10 µM to PDLSCs contrasted with that of control groups on
days 1, 3, 5 and 7. Each bar represents the mean
LPS (10 µg/mL) significantly increased IL-8, IL-6, IL-1
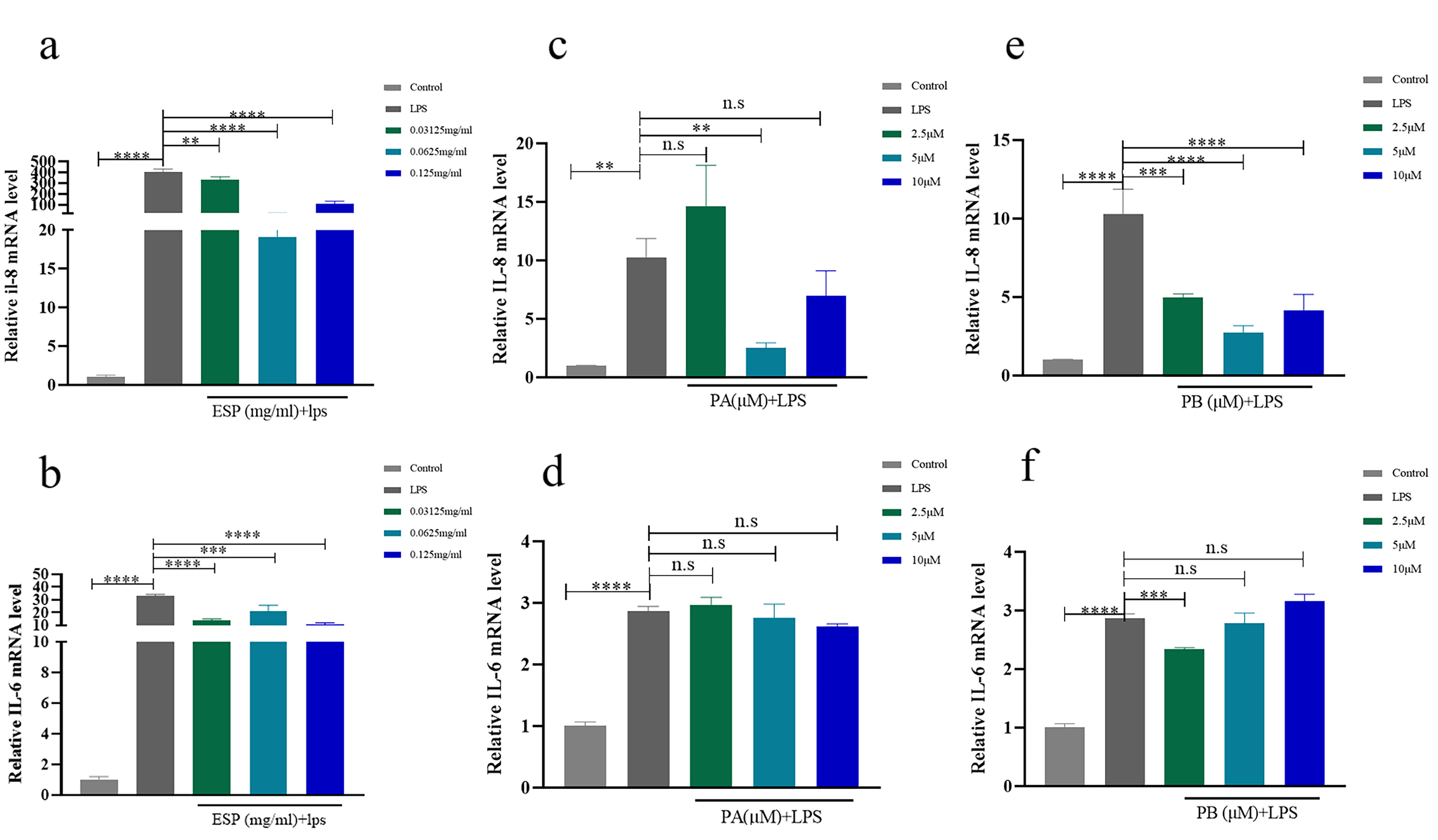 Fig. 3.
Fig. 3.Effects of ESP, PA and PB on the inflammatory response of
PDLSCs. (a,b) The RNA expression of IL-8 and IL-6 with ESP at 0.03125 mg/mL, 0.0625
mg/mL and 0.125 mg/mL in PDLSCs contrasted with that of the control groups. (c–f)
The mRNA expression of IL-8 and IL-6 with PA and PB at 2.5 µM, 5 µM
and 10 µM in PDLSCs compared to the controls. Each bar represents
the mean
Under the condition of osteogenic induction, the mRNA levels of RUNX2 and OSX in PDLSCs were increased compared with those under normal conditions. The expression of RUNX2 was also increased in the PDLSCS treated with three concentrations of ESP (Fig. 4a). Treatment with 0.0625 mg/mL and 0.125 mg/mL ESP increased the expression of OSX at the RNA level in PDLSCs in comparison to that in the control groups over 14 days (Fig. 4b). 0.0625 mg/mL and 0.125 mg/mL ESP increased the expression of OCN at the RNA level in PDLSCs and 0.125 mg/mL ESP increased the expression of OCN at the protein level (Supplementary Fig. 4a–c) in comparison to that in the control groups over 14 days. Similarly, PB at the three concentrations upregulated the mRNA expression of OSX (Fig. 4f). The expression of OSX was also increased in the PDLSCS treated with 10 µM PA (Fig. 4d). Treatment with 2.5 µM and 10 µM PA and PB increased the expression of RUNX2 at the mRNA level in PDLSCs compared with that in the control groups for 14 days (Fig. 4c,e).
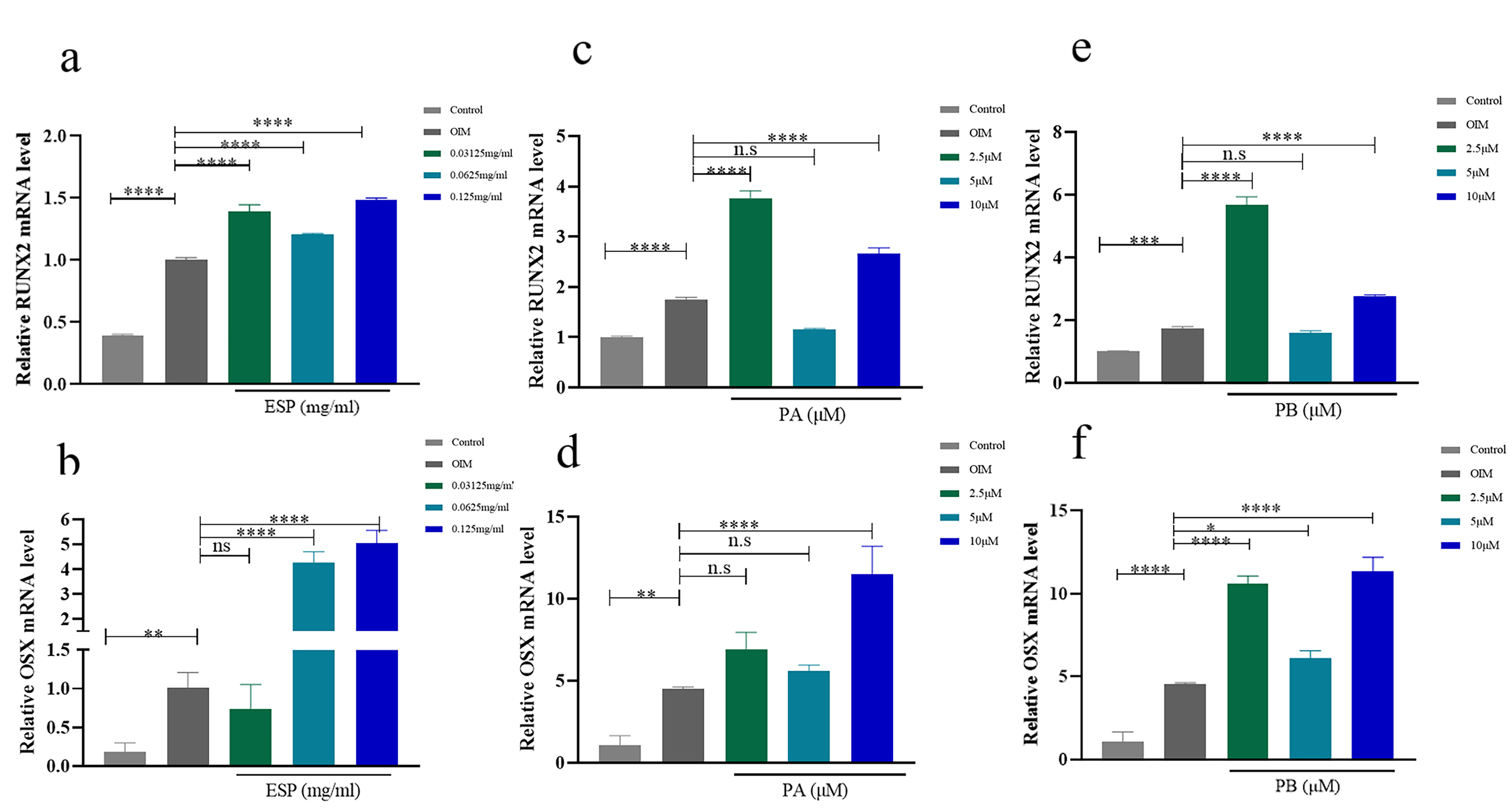 Fig. 4.
Fig. 4.Effects of ESP, PA and PB on osteogenesis of PDLSCs. (a,b) The mRNA
expression of RUNX2 and OSX with ESP at 0.03125 mg/mL, 0.0625 mg/mL and 0.125
mg/mL in PDLSCs vs. the control cells after osteogenic induction for 14 days. (c–f) The mRNA expression of RUNX2 and OSX with PA and PB at 2.5 µM, 5
µM and 10 µM in PDLSCs compared to the controls after osteogenic
induction for 14 days. Each bar represents the mean
5 µM and 10 µM PB increased the expression of OCN at the mRNA level in PDLSCs compared with that in the control groups for 14 days. 0.125 mg/mL ESP and 5 µM PB increased the expression of OCN at the protein level (Supplementary Fig. 4a–c). To further determine the osteogenic effect on ESP, PA and PB, we performed Alizarin Red staining, ALP staining and activity assay. The content of ALP and mineralized nodules of PDLSCs were increased in a dose-dependent manner at three concentrations of ESP (Fig. 5a,c). The activity and content of ALP in the PDLSCs treated with 5 µM PA and 2.5 µM and 5 µM PB were significantly higher than those in the control group (Fig. 5b,d,e). In addition, Alizarin Red staining demonstrated that 2.5 µM PA and 2.5 µM and 5 µM PB generated a significant increase in mineralization (Fig. 5b). The quantity of ALP staining in the PDLSCs treated with 0.0625 mg/mL and 0.125 mg/mL ESP and 2.5 µM and 5 µM PB were significantly higher than those in the control group (Supplementary Fig. 5b). The quantity of Alizarin Red staining demonstrated that 0.0625 mg/mL ESP, 2.5 µM PA and 2.5 µM PB generated a considerable increase in mineralization (Supplementary Fig. 5a). These assays showed that ESP, PA and PB can promote the osteogenesis of PDLSCs.
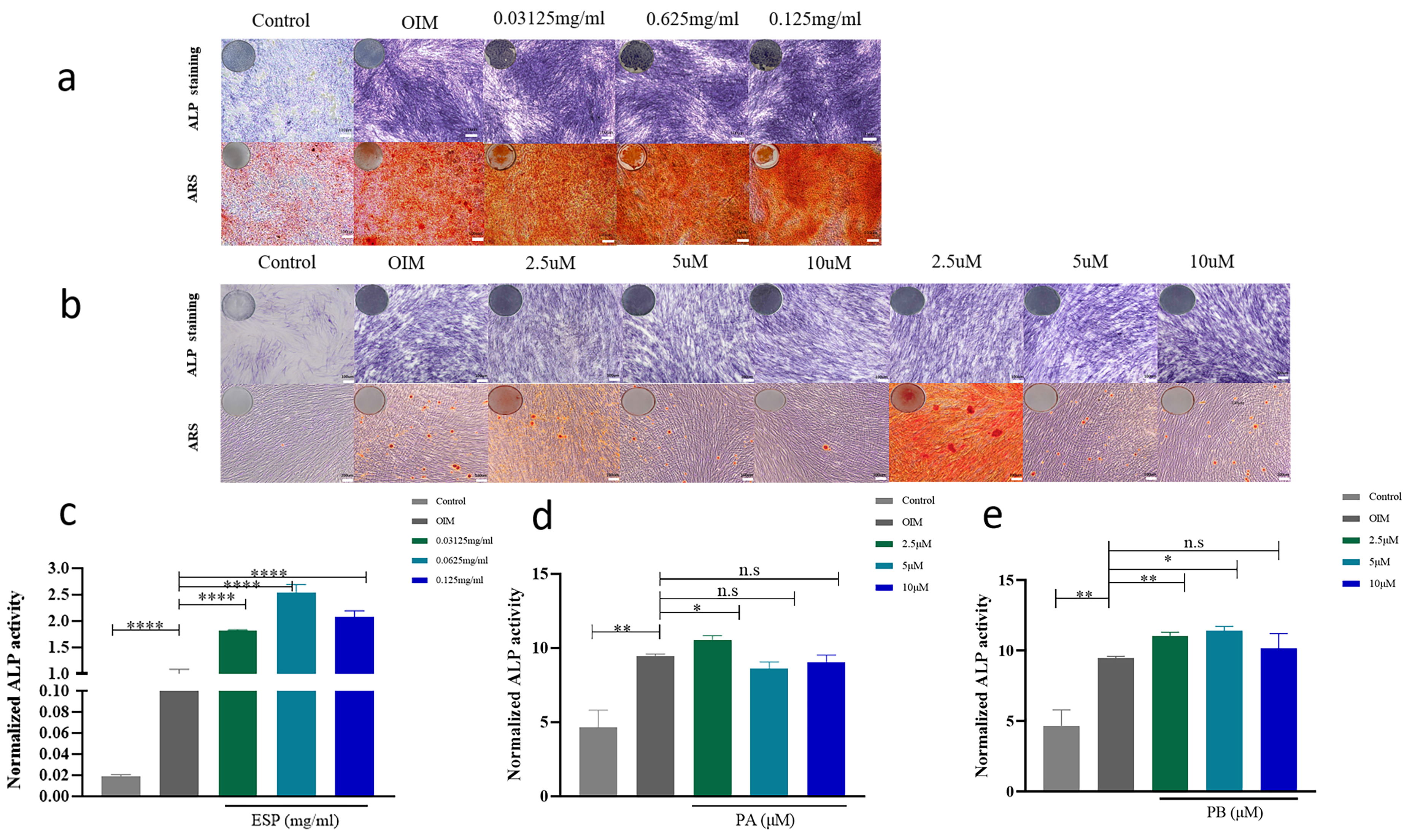 Fig. 5.
Fig. 5.Effects of ESP, PA and PB to on PDLSCs osteogenesis. The result
of the Alkaline Phosphatase (ALP) activity assays after osteogenic induction for 7 days. Representative images of ARS (Alizarin Red staining) after osteogenic induction for 14 days. ALP staining and Alizarin Red staining with ESP at 0.03125 mg/mL, 0.0625 mg/mL and 0.125 mg/mL compared to that of the control groups (scale bar 200 µM). (a) ALP staining and Alizarin Red staining of the PDLSCs treated with PA and PB at the concentrations of 2.5 µM, 5 µM and 10 µM compared to that of the control groups (scale bar 200 µM). (b) The ALP content of PDLSCs with ESP at 0.03125 mg/mL, 0.0625 mg/mL and 0.125 mg/mL compared to that of the control groups. (c) The ALP content of PDLSCs with PA and PB at 2.5 µM, 5 µM and 10 µM of PDLSCs compared to that of the control groups (d,e). Each bar represents the mean
The treatment of periodontitis is mainly nonsurgical by mechanical subgingival instruments, but this method is palliative, unable to repair periodontal tissue, and cannot meet the needs of all patients. Although MSC transplantation can effectively restore periodontal tissue, it is still expensive and has disadvantages such as immune rejection [12, 13]. Therefore, improving the inflammatory microenvironment of MSCs and promoting the proliferation and differentiation of MSCs in the human body has clinical value for the repair and regeneration of the periodontium
PDLSCs are ideal for tissue engineering research because of their strong self-differentiation and renewal ability [14]. In this study, we identified PDLSCs by osteogenesis and lipid differentiation and surface markers. Our experimental results were consistent with previous studies [15]. We successfully isolated PDLSCs with a typical fibroblastic appearance and detected the expression of stem cell surface markers on PDLSCs using flow cytometry. The findings demonstrated that PDLSCs could be used in the ensuing experiments.
Due to the effectiveness and relative safety of natural plant ingredients, many
plant ingredients combined with PDLSCs have become a new strategy for promoting
periodontal tissue regeneration and prevention and treatment of periodontitis
[15, 16]. The anti-inflammatory effect of the extract of sappanwood has been
shown previously [17, 18]. PA affects cell anti-inflammatory activity and bone
metabolism [9, 10]. However, no studies have shown that ESP, PA and PB have
anti-inflammatory activity and osteogenic effects on PDLSCs. Here we studied the
anti-inflammatory and osteogenic differentiation effects on these three
components in PDLSCs. In our previous study, we found that ESP had strong
inhibitory effects on Porphyromonas gingivalis, gingivalis,
Clostridium nucleus, Prevotella intermedia, and other core
pathogenic microorganisms of periodontitis (MIC
Inflammation has been implicated in the etiology of periodontal disease [19].
Porphyromonas gingivalis is a major etiological agent in the onset and
progression of severe forms of periodontal disease [20]. It can initiate the
production of various cytokines, such as interleukin-8 (IL-8) [21, 22].
Periodontitis patients have higher salivary IL-8 levels, and IL-8 is an important
parameter in gingival crevicular fluid reflecting the resting and active stages
of periodontitis [23, 24]. Moreover, interleukin 6 (IL-6) has recently been
associated with worsening periodontal disease and enhancing a cascade of tissue
destruction [25, 26]. Most cross-sectional studies have shown that serum
IL-1
Reconstruction of periodontal tissue defects is a serious challenge. When
cultured in vitro under inductive circumstances, PDLSCs display
osteogenic, adipogenic, and chondrogenic traits [49]. In injured periodontal
tissues in animal models, PDLSCs transplantation may increase the formation of
new bone and new cement [50, 51, 52, 53]. Accumulating evidence suggests that natural
products can support the osteogenic differentiation of PDLSCs. Previous studies
found that rutin fosters osteogenic differentiation of PDLSCs [15], and
ipriflavone stimulates PDLSC proliferation and osteogenic differentiation [54].
Sappanwood mainly includes protohaematoxylin, braziloxylin, high isoflavone,
haematoxylin, and other components. Previous studies have shown that it inhibits
osteoclastogenesis and bone resorption [55, 56]. However, there are few studies
on osteogenic differentiation. Therefore, we investigated the effect of ESP, PA
and PB on periodontal regeneration from osteogenic differentiation. Osteoblast
transcription factor 2 (RUNX2) plays a vital role in both osteogenesis and
chondrogenesis [57, 58, 59]. RUNX2 requires initiation of prechondroblast mesenchymal
separation into a precursor osteoblast lineage, whereas OSX subsequently involves
the completion of the osteoblast differentiation pathway [60]. The important role
of OSX is attributed to its regulation of osteoblast markers, such as Dkk1, an
important antagonist of WNT/
Our study demonstrated that ESP, PA and PB could reduce the inflammatory response and facilitate osteogenic differentiation of PDLSCs. The data indicated that ESP, PA and PB have anti-inflammatory and osteogenic effects. Thus, ESP, PA and PB may be used for bone regeneration and periodontal tissue engineering.
Datasets used and/or analyzed for this study are available from the corresponding author upon appropriate request.
XZ, JC, JZ and YL conceived the study, directed the project, designed the experiments; JZ, YL, XZ and JC interpreted the results and wrote the manuscript; NR, CY, JL provide experimental help; JZ, YL, XZ and JC analyzed the data. JZ and YL revised the manuscript. NR substantial contributions to the conception and design of the work. CY and JL acquisition, analysis, and interpretation of data for the work. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work to take public responsibility for appropriate portions of the content and agreed to be accountable for all aspects of the work in ensuring that questions related to its accuracy or integrity.
Permission to conduct this study was obtained from the Kunming Medical University School’s ethical committee (no. KYKQ2021MEC025).
Not applicable.
This study was supported by the National Natural Science Foundation of China, Grant/Award Number: 82160179, Academic Leader Project of Yunnan Province (D2019-007). Natural Science Foundation of Yunnan Province (grant No. 2022GZ006, 202101AY070001-164, and 202001AY070001-151).
The authors declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/j.fbl2808172.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.





