1 Department of Otorhinolaryngology-Head and Neck Surgery, Zhujiang Hospital, Southern Medical University, 510282 Guangzhou, Guangdong, China
†These authors contributed equally.
Abstract
Background: The role of Pin2 telomeric repeat factor 1-interacting telomerase inhibitor 1 (PinX1) in tumorigenesis and development has been
extensively studied. As we previously demonstrated, PinX1 plays an important role
in modulating epithelial-mesenchymal transition (EMT), stemness, cell
proliferation, and apoptosis in nasopharyngeal carcinoma (NPC). However, the
relationship between PinX1, autophagy, and cell function in NPC remains unclear.
This study aimed to investigate the mechanisms by which PinX1 regulates autophagy
in NPC, and to explore its biological role and clinical significance in disease
progression. Methods: The proliferative capacity of NPC cells was
assessed by MTT and xenograft tumorigenicity assays. Autophagic flux was
monitored using a tandem monomeric DAPI–FITC–LC3 reporter assay. The rates of
apoptosis and the cell cycle in NPC cells were analyzed using flow cytometry. The
activation of autophagy and the signaling status of the AKT/mTOR and
NF-
Keywords
- nasopharyngeal carcinoma
- autophagic flux
- migration
- AKT/mTOR pathway
Nasopharyngeal carcinoma (NPC) is a highly malignant tumor, originating from the nasopharyngeal mucous membrane, which metastasizes easily throughout the body via the lymph nodes; it is common in South China and Southeast Asia [1, 2]. Patients with early-stage NPC often lack symptoms or have nonspecific symptoms. Approximately 75% of NPC patients are at an advanced stage when they first seek medical attention, and about 10% exhibit distant organ metastasis [3]. The locoregional control rate of NPC has improved significantly in the past decade following therapeutic improvements, including the development of comprehensive treatment strategies such as intensity-modulated radiotherapy and chemotherapy, concurrent radiotherapy and chemotherapy [4], surgical treatments under nasal endoscopy [5], and PD-1 antibody immunosuppressive therapy [6, 7, 8]. However, the long-term survival rate of patients with NPC remains poor due to recurrence and/or distant metastasis [9]. Further the side-effects of treatments used in NPC can lead to poor outcomes, especially in patients with locoregionally advanced NPC [10, 11]. Current research is therefore focused on clarifying the molecular mechanisms underlying tumor invasiveness and metastasis in NPC, and to investigate new treatment methods for improving prognosis and prolong survival in NPC patients.
Autophagy is a highly conserved cyclical degradation process, regulated by lysosomes, that is stably present in eukaryotes [12]. Abnormal or inhibited autophagy may induce various diseases, including cancer and neurodegeneration [13]. Autophagy can make tumor cells more resistant to apoptosis [14]. In contrast, autophagy and apoptosis can act on cancer cells to promote their death [15]. Autophagy plays important roles in NPC cell proliferation and differentiation, and in chemo- and radioresistance [16]; it promotes both survival and apoptosis [17]. The effects of autophagy on tumor growth in NPC remain to be clarified due to differences in research targets or drugs investigated. This study therefore aimed to clarify the regulation of autophagy in NPC cells, on the basis of our prior investigations.
Pin2 telomeric repeat factor 1-interacting telomerase inhibitor 1 (PinX1) has been demonstrated to be an intrinsic inhibitor of telomerase that directly interacts with the telomerase catalytic component telomerase reverse tranase (TERT) [18]. The unique property of PinX1 in targeting telomerase plays an important role in regulating tumor proliferation and metastasis. Previous evidence showed that the expression of PinX1 is generally inhibited in various types of cancer including glioma [19], lung squamous cell carcinoma [20] and prostate cancer [21], and cancer patients with low PinX1 expression have a poor prognosis and metastatic nature. Preliminary research from our group indicates that PinX1 expression was decreased in CD133+ cancer stem cells isolated from a nasopharyngeal carcinoma cell line, whereas transfecting NPC cells with PinX1 inhibited their telomerase activity and proliferation, and significantly increased their apoptosis rate [22]. Our objective in this study was therefore to examine how PinX1 affects autophagy in NPC cells, and to elucidate the molecular mechanisms involved. Further, we aimed to elucidate the interaction between autophagy and apoptosis. Finally, the effect of PinX1 on the oncogenesis of NPC was evaluated in vivo. This work will provide new and precise treatment strategies for NPC.
The nasopharyngeal cancer CNE1 cell line (Catalogue number: WN-10371, Homo sapiens, epithelioid) derived from a 58-year-old female patient with nasopharyngeal carcinoma and 6-10B cell line (Catalogue number: WN-10197, Homo sapiens, epithelioid) were purchased from Wuhan Warner Bio Co., Ltd (Wuhan, China). Mycoplasma testing of the two cell lines measured by PCR were both negative. The cell lines used in the manuscript have been authenticated using Short Tandem Repeat (STR) analysis as described in 2012 in ANSI Standard (ASN-0002) by the ATCC Standards Development Organization (SDO). The cell lines were cultured in
RPMI-1640 (HyClone, Logan, UT, USA) supplemented with 10% fetal bovine serum
(FBS, Hyclone, Logan, UT, USA), 100 U/mL streptomycin, and 100 U/mL penicillin. All the cell
lines were incubated in a humidified incubator with 5% CO
The PinX1-overexpression plasmid (pcDNA3.0-PinX1) and the empty plasmid were
constructed by Guangzhou Vipotion Biotechnology Co., Ltd (Guangzhou, China).
Before transfection, CNE1 cells (2
The proliferative capacity of the transfected and non-transfected CNE1 cells was
measured via MTT Assay Kit (ab211091, Abcam, Cambridge, UK). Briefly, the cells
were seeded onto 96-well plates at 1
Migration and invasion by transfected and non-transfected CNE1 cells were
determined using Transwell assays. Migration analysis was performed with DMEM
containing 10% FBS (Hyclone) in the lower chamber and
2
Using an Annexin V/propidium iodide (PI) apoptosis detection kit (Beyotime, Shanghai, China), flow cytometry was conducted to analyse the cell-cycle phases and apoptosis rates of the transfected and non-transfected CNE1 cells following the manufacturer’s instructions. Briefly, after 48 h of incubation in a 96-well plate, the CNE1 cells (blank, Vector, Over-PinX1, and Over-PinX1 + 3-MA) were collected and stained with Annexin V-fluorescein isothiocyanate (FITC) and PI, then incubated for 15–20 min in the dark at room temperature. Flow cytometry data were then acquired using a FACSCalibur HG flow cytometer (BD Biosciences) and analyzed by FlowJo 10 (Tree Star Software, San Carlos, CA, USA).
Total RNA of cultured cells was extracted using TRIzol reagent (Invitrogen), and
cDNA was prepared using total RNA as a template based on the Bestar qPCR RT Kit
(Applied Biosystems, Grand Island, NY, USA). RT-qPCR with
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as internal reference
was carried out on the Agilent Stratagene Mx3000 real-time qPCR Thermocycle
Instrument (Agilent Stratagene, CA, USA). Thermal cycling conditions of PCR
amplifications were degeneration at 95 °C for
2 min followed by 40 cycles of 30 s
at 94 °C , 20 s at
58 °C, and 20 s at
72 °C, and extension at 72 °C for
10 min. Primer sequences used for qRT-PCR assays were synthesized
by Shanghai Sangon Biotechnology Co., Ltd. (Shanghai, China), as follows:
PinX1 (forward: 5
Total protein of cultured cells was extracted using radioimmunoprecipitation
assay (Kettner, #378) lysate (Beyotime, Nanjing, China). Protein samples (each
well ca. 20
Female nude mice (4-week-old, a body weight of 17 g) provided by the Animal
Laboratory of Southern Medical University were used to establish NPC mouse model.
A total of 1
CNE1 cells in each group were grown on 6-well plates and fixed at 4 °C overnight using 4% paraformaldehyde (PFA) after washing with phosphate buffered saline (PBS). The cells were then blocked with 10% goat serum for 15 min at room temperature, and incubated with LC3B antibodies (1:200) for 1 h at 4 °C followed by incubation with Alexa Fluor 488 conjugated secondary antibodies (1:1000) for 1 h at room temperature. The cells were washed three times with PBS (5 min per wash) before each incubation step. Finally, the cells were mounted with DAPI staining solution and incubated for 10 min at room temperature in the dark, and analyzed under a Bx51 inverted florescence microscope (Olympus Corporation, Shinjuku, Japan).
Changes in tumor tissue morphology were observed via Hematoxylin-Eosin (H&E) Staining. The tumor tissue in each group was dehydrated by exposure to decreasing concentrations of ethanol, embedded in paraffin wax, and cut into sections 5 mm thick. Paraffin-embedded sections of tumor tissue were deparaffinized and rehydrated in decreasing concentrations of ethanol, then stained with hematoxylin and eosin (both from Servicebio, Wuhan, China), following the manufacturer’s protocols.
Immunohistochemistry assays were carried out to detect protein expression level
of PinX1 in tumor tissues. Paraffin sections prepared from the in vivo
experiments and the indirect streptavidin–peroxidase method were used following
the manufacturer’s instructions. Paraffin sections were rehydrated using
Histo-Clear (National Diagnostics, Atlanta, GA, USA) followed by a 100% to 70% ethanol gradient.
Endogenous peroxidase activity was quenched using H
Statistical analyses were carried out using SPSS 24.0 (SPSS Inc., Chicago, IL,
USA). Data are recorded as the mean
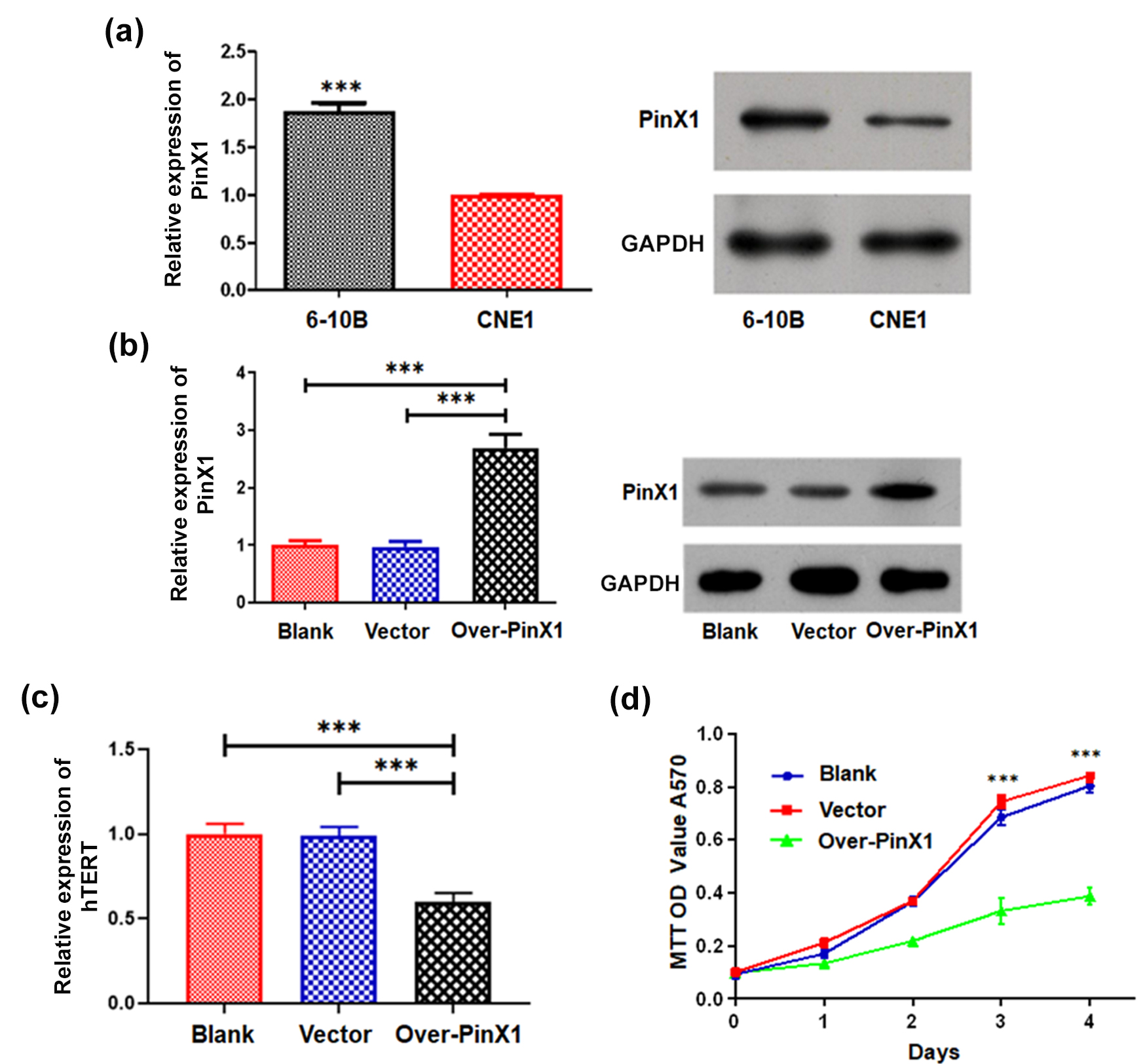
We first examined the expression of PinX1 in 6-10B and CNE1 cells to determine
its role in NPC development. Based on RT-qPCR and western blot analysis, PinX1
was strongly expressed in 6-10B cells, but weakly expressed in CNE1 cells (Fig. 1a). Therefore, the PinX1-overexpression plasmid (pcDNA3.0-PinX1) was introduced
into the CNE1 cell line, to further explore its biological role in NPC. PinX1
expression was more than twofold greater in CNE1 cells treated with
pcDNA3.0-PinX1 than those of the blank and vector groups, based on RT-qPCR and
western blot analysis (Student’s t-tests, p
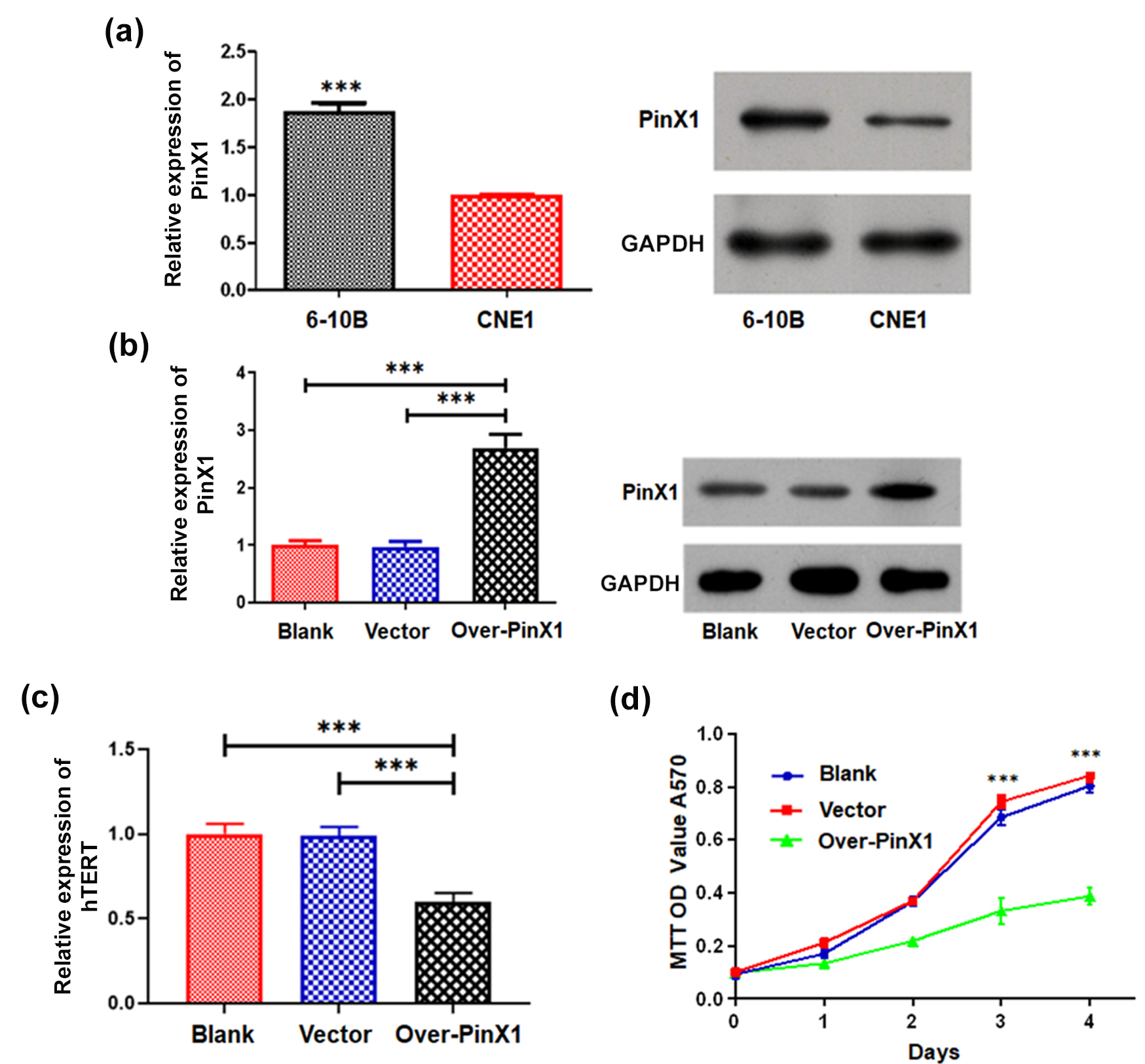 Fig. 1.
Fig. 1.PinX1 inhibits the growth of NPC cells by targeting
telomerase. (a) The expressions of PinX1 in the 6-10B and CNE1 cell lines as
determined by RT-qPCR and western blot analysis. (b) The expression of PinX1 in
CNE1 cells and those transfected with empty vector and pcDNA3.0-PinX1 as
determined by RT-qPCR and western blot analysis. (c) The mRNA expression of hTERT
in CNE1 cells and those transfected with empty vector and pcDNA3.0-PinX1 as
determined by RT-qPCR. (d) The cell proliferation curves of CNE1 cells and cells
transfected with empty vector and pcDNA3.0-PinX1 were measured via MTT Assay. ***
p
Afterward, we examined in vitro the effects of PinX1 expression on
hTERT expression and NPC cell growth. hTERT expression in CNE1 cells treated with
pcDNA3.0-PinX1 was significantly suppressed relative to those of the blank and
vector groups, based on RT-qPCR (Student’s t-tests, p
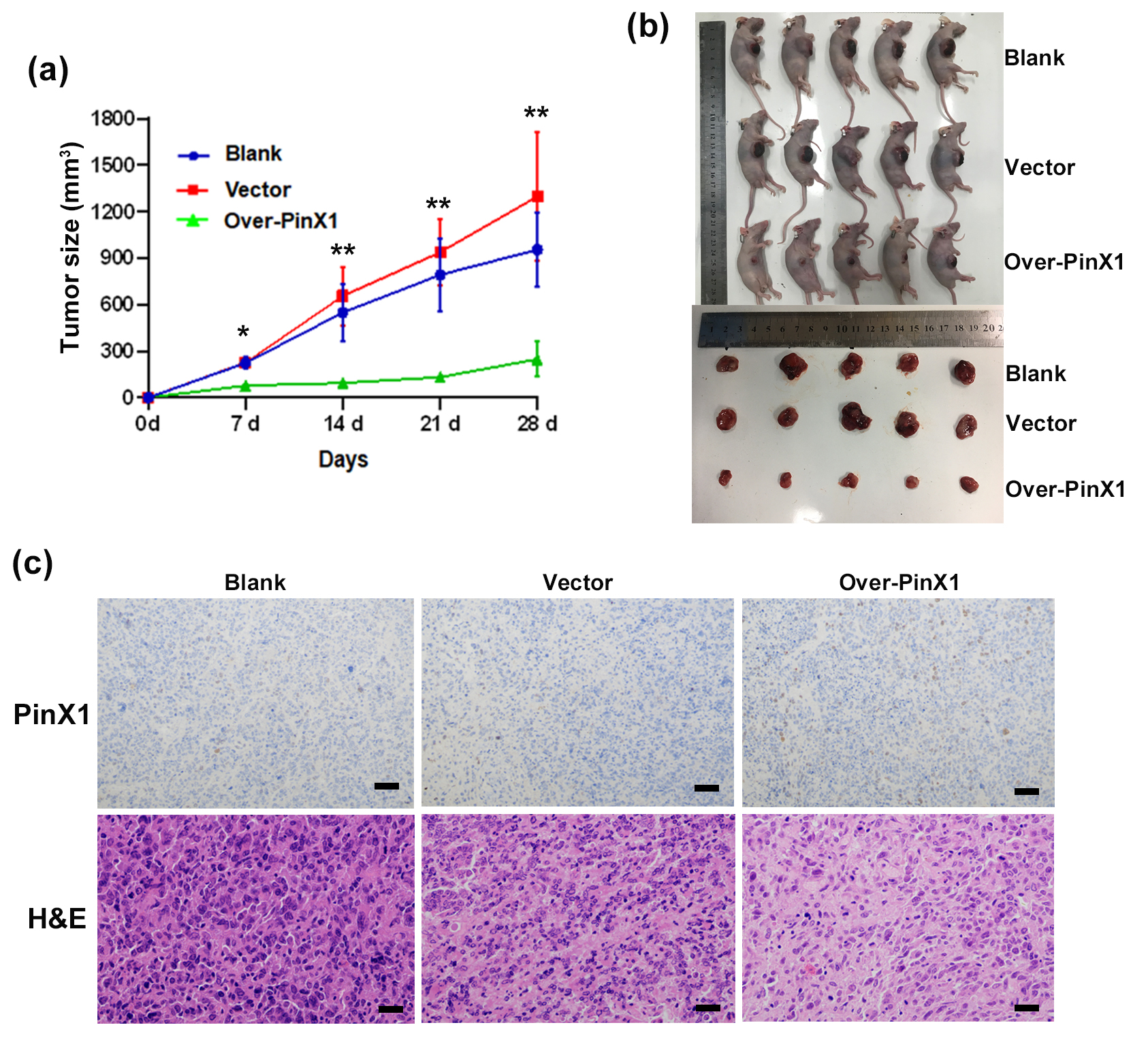
Using CNE1 cells (untreated, treated with the empty vector, or pcDNA3.0-PinX1-transfected cells) subcutaneously injected, we performed an in vivo tumor-formation experiment. The tumor growth curve was obtained by calculating the volume of tumors in each group at 7, 14, 21, and 28 days after inoculation: the tumor growth rate in the PinX1-overexpressing mice was significantly lower than that in the blank and vector groups (Fig. 2a). At 28 d after implantation, the PinX1-overexpressing mice had smaller tumor burdens (Fig. 2b) and displayed higher PinX1 expression in tumor tissues than the controls, and their transplanted tumor tissues showed fewer obviously pathological mitotic cell nuclei and cellular atypia than the control groups (Fig. 2c). These results suggest that PinX1 significantly inhibits tumorigenesis in vivo.
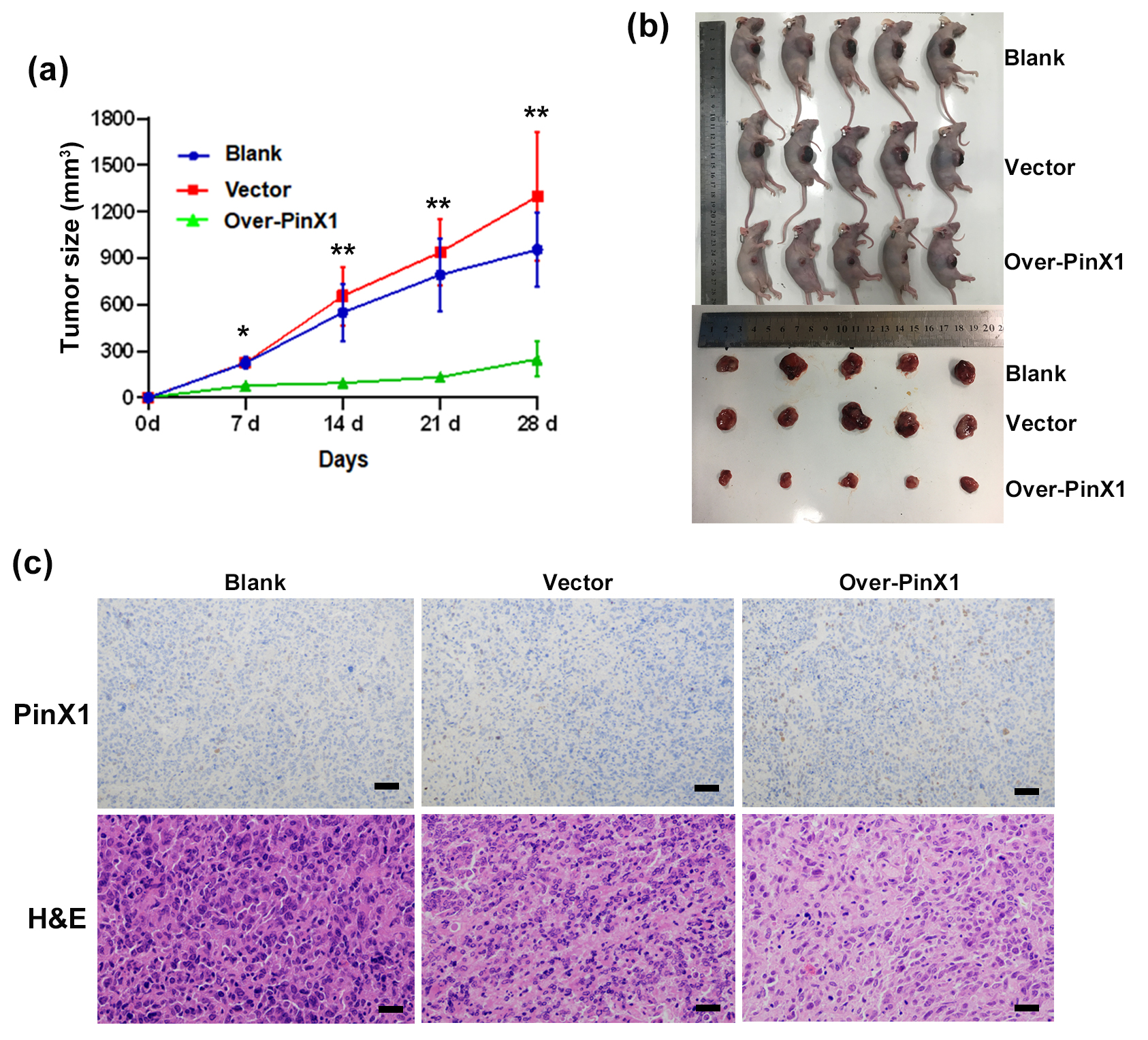 Fig. 2.
Fig. 2.PinX1 inhibits NPC tumorigenesis in vivo. (a)
Tumour growth curves were plotted in CNE1 cells and those transfected with empty
vector and pcDNA3.0-PinX1. (b) The tumour volume was measured periodically for
each of the xenograft mice models bearing tumours originating from CNE1 cells and
those transfected with empty vector or pcDNA3.0-PinX1 (n = 5/group). (c)
Immunohistochemistry detection of PinX1 in xenografts derived from CNE1 cells and
those transfected with empty vector and pcDNA3.0-PinX1 as well as Representative
H&E staining of primary cancer tissues are shown. Magnification
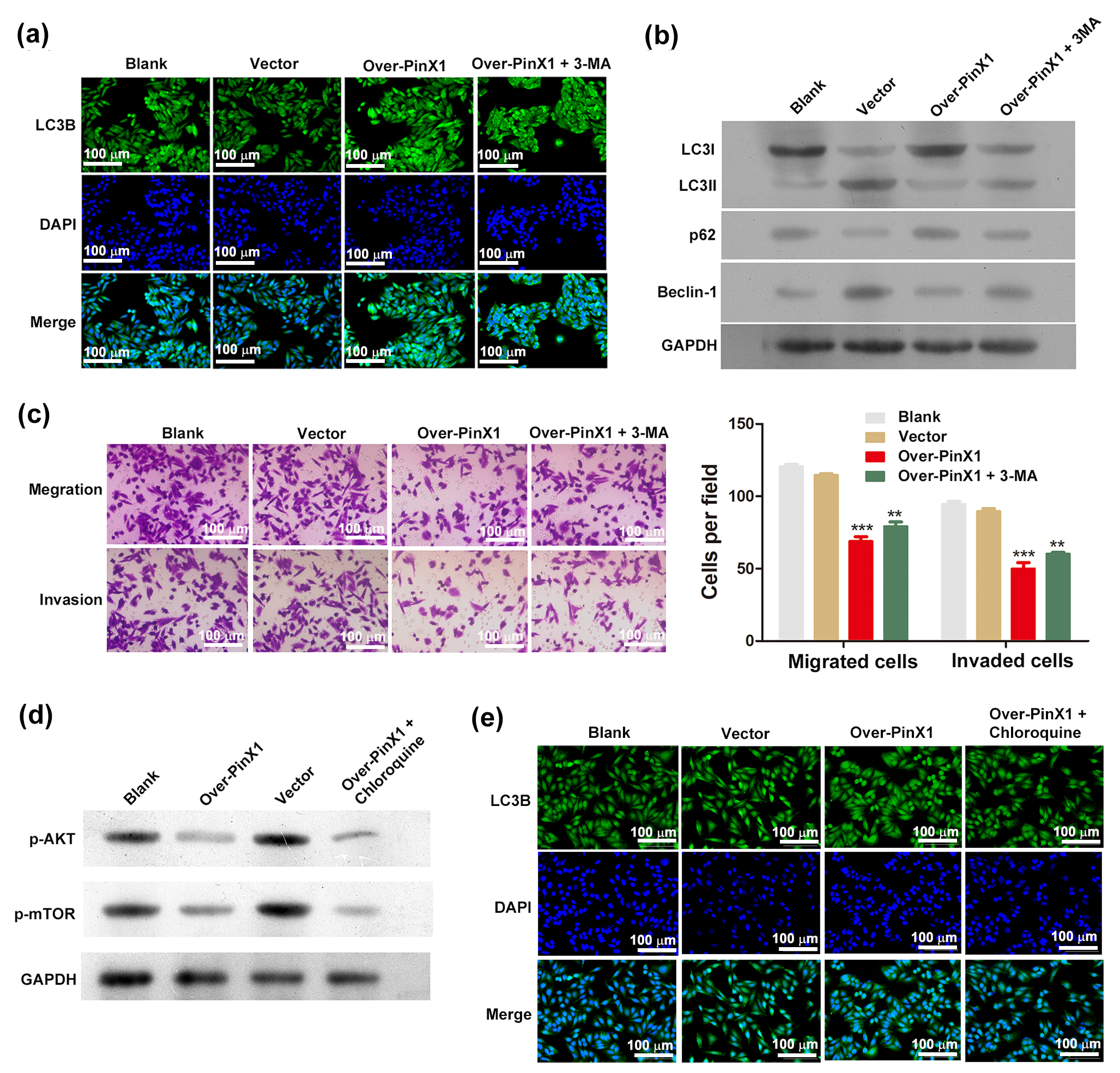
To investigate the impact of PinX1 overexpression on autophagy, the DAPI–FITC–LC3 reporter was used to monitor the autophagic flux. PinX1-overexpressing CNE1 cells contained more blue-green puncta than the control groups, suggesting that PinX1-overexpression activated autophagy (Fig. 3a). By measuring the level of the autophagy marker, Beclin-1, we established whether PinX1-overexpressing cells were actually undergoing autophagy. Beclin-1 protein levels were markedly elevated in PinX1-overexpressing cells, which also exhibited an elevated LC3-II/LC3-I protein ratio. In addition, p62 protein levels were reduced in PinX1-overexpressing cells, relative to the controls (Fig. 3b). As a result of these observations, PinX1 overexpression induces autophagy. We further examined PinX1 overexpression’s role in cell invasion and migration through pharmacological inhibition of autophagy using 3-MA, a widely used specific inhibitor of autophagy [23], and monitored its effects on autophagy. By monitoring autophagic flux using the DAPI–FITC–LC3 reporter, we found that 3-MA treatment of PinX1-overexpressing cells reduced the number of blue-green puncta (Fig. 3a). Western blot analysis revealed that 3-MA reduced the LC3-II/LC3-I ratio, and abolished the PinX1-overexpression-induced reduction of p62 expression (Fig. 3b). These results indicate that 3-MA is a potent inhibitor of PinX1-overexpression-induced autophagy in CNE1 cells. Using the Transwell assay, we then determined the number of migrating and invading cells. A significant reduction in migration and invasion was found in PinX1-overexpressing cells compared with controls, while treatment with 3-MA markedly increased migration and invasion (Fig. 3c).
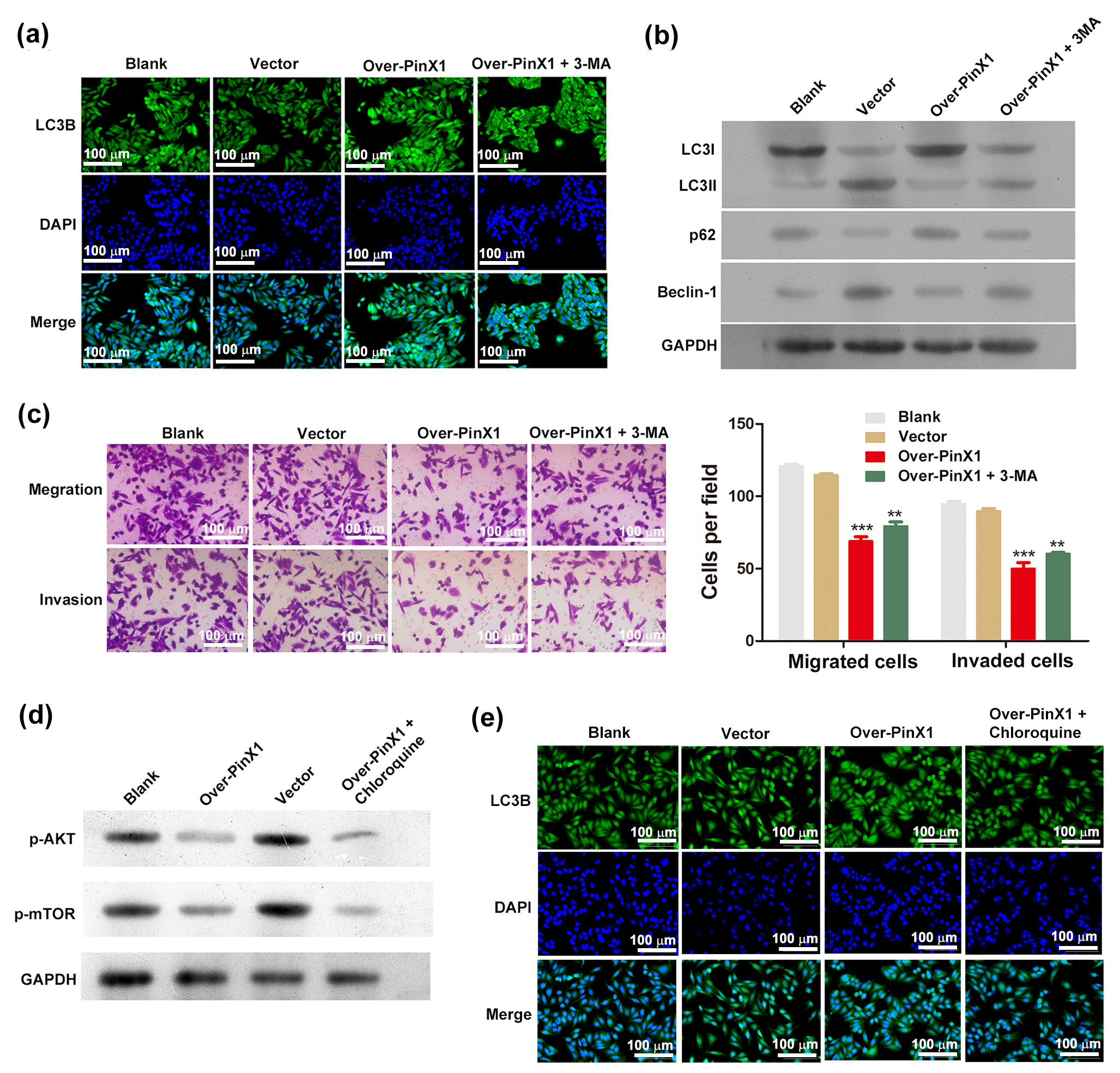 Fig. 3.
Fig. 3.Overexpression of PinX1 induces autophagy in NPC cells via the
AKT/mTOR signaling pathway. (a) Comparison of autophagy flux and (b) the protein
levels of LC3-II, LC3-I, p62 and Beclin-1 in CNE1 cells and those transfected
with empty vector and pcDNA3.0-PinX1 as well as pcDNA3.0-PinX1 + 3-methyladenine.
(c) Transwell assay for measuring cell migration and invasion following
transfection. (d) The protein levels of p-AKT and p-mTOR and (e) Comparison of
autophagy flux in CNE1 cells and those transfected with empty vector and
pcDNA3.0-PinX1 as well as pcDNA3.0-PinX1 + Chloroquine. ** p
We next investigated the mechanism whereby PinX1 overexpression activates autophagy in NPC cells. Autophagy can be potently induced by inhibiting the AKT/mTOR signaling pathway. Therefore, we determined the state of AKT/mTOR signaling in CNE1 cells by measuring the changes in the levels of phosphorylated AKT and mTOR. Significantly lower expression levels were observed in PinX1-overexpressing CNE1 cells than those in the controls (Fig. 3d), indicating suppressed AKT/mTOR signaling in these cells. Adding chloroquine to PinX1-overexpressing cells did not cause significant differences in phosphorylated AKT and mTOR levels relative to the untreated PinX1-overexpressing cells, although it did inhibit autophagic flux, as revealed by the immunofluorescence assay (Fig. 3e), indicating that PinX1 might directly modulate AKT phosphorylation. Together, these results suggest a mechanism whereby PinX1 overexpression inhibits AKT/mTOR signaling in NPC cells, thereby activating autophagy.
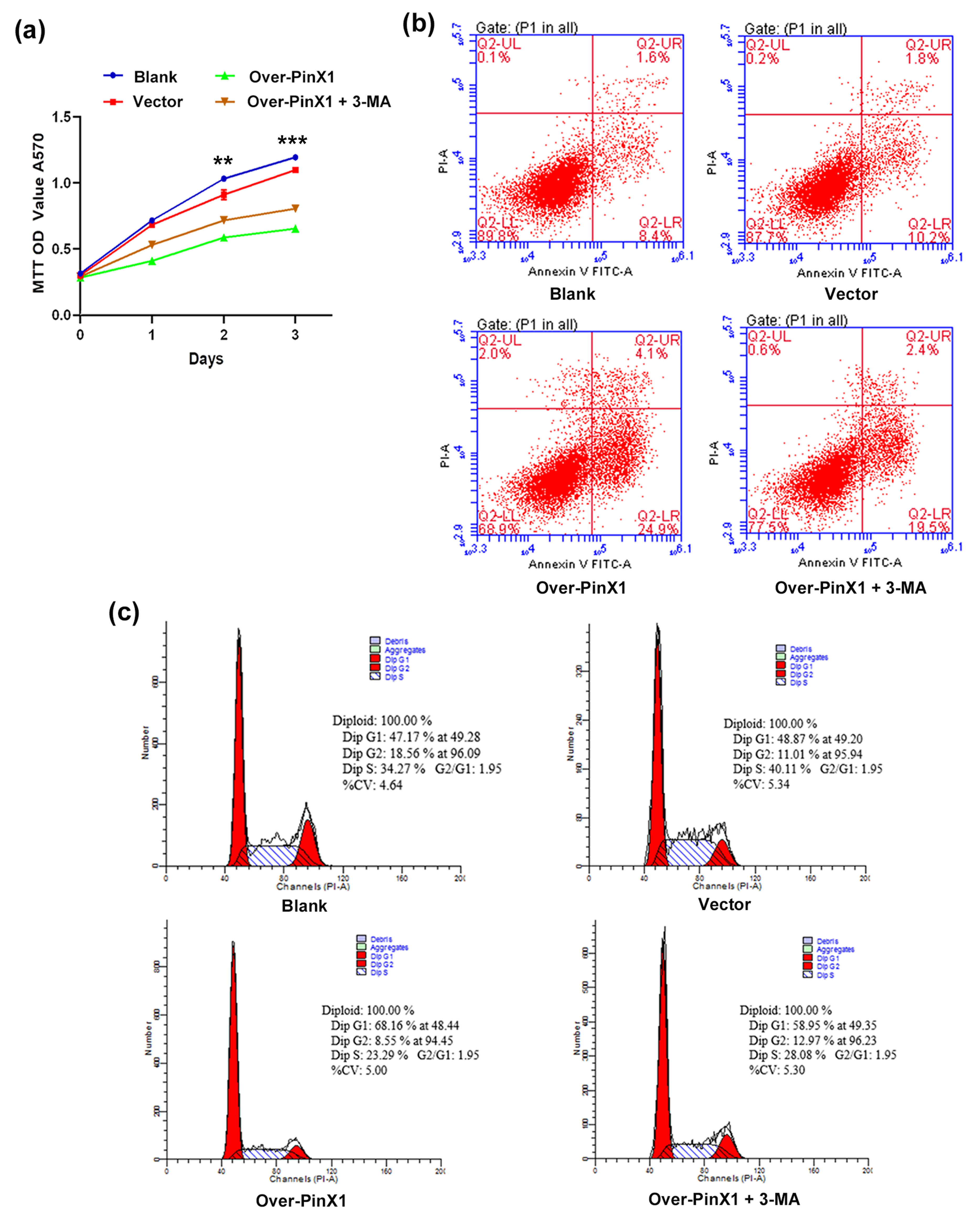
To determine whether the inhibition of cell proliferation and apoptosis induced by PinX1 overexpression is caused by the induction of autophagy, the effect of 3-MA on these processes in PinX1-overexpressing cells using a MTT assay was investigated. Compared with the controls, the CNE1 cell proliferation was remarkably inhibited by PinX1 overexpression, and this effect was reversed by treating PinX1-overexpressing cells with 3-MA (Fig. 4a). We next investigated how inhibiting autophagy affected apoptosis in PinX1-overexpressing cells. Flow cytometry analysis revealed an obviously higher apoptosis rate in PinX1-overexpressing cells than in the control groups; further, treating PinX1-overexpressing cells with 3-MA markedly reduced the rate of apoptosis, relative to the untreated PinX1-overexpressing cells (Fig. 4b). These results indicate that inhibiting autophagy reverses the inhibition of cell proliferation and induction of apoptosis, that resulted from PinX1 overexpression. Furthermore, we monitored the effect of inhibiting autophagy on the cell cycle in PinX1-overexpressing cells. The percentage of cells in the G0/G1 phase was higher, and that of cells in G2/M phase was lower, in PinX1-overexpressing cells than in the control groups (Fig. 4c). Further, treating PinX1-overexpressing cells with 3-MA significantly reduced the percentage of cells in the G0/G1 phase and increased that of cells in the G2/M phase. These findings indicate that inhibiting autophagy reverses the deceleration of cell-cycle progression caused by PinX1 overexpression in CNE1 cells.
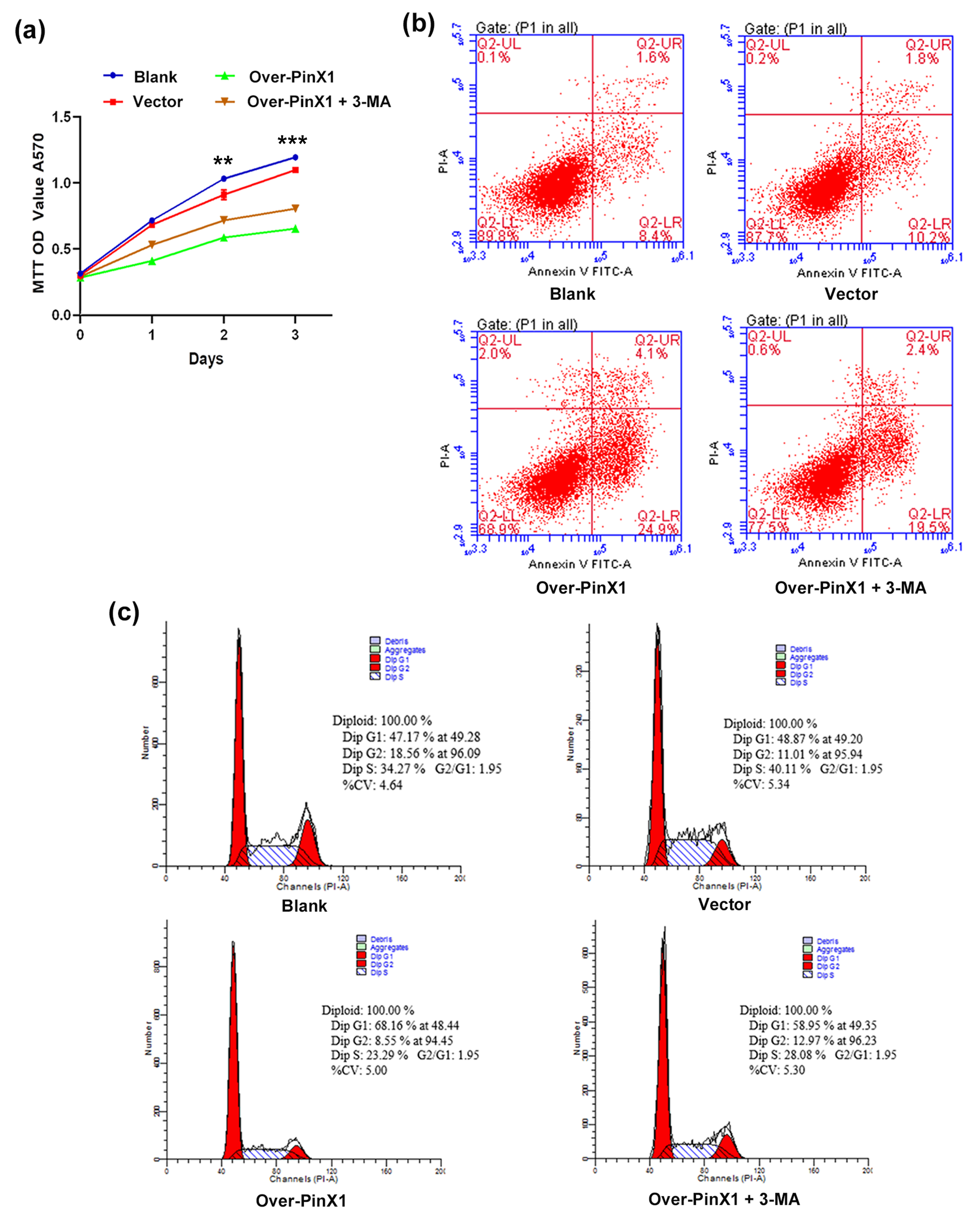 Fig. 4.
Fig. 4.Autophagy inhibitor 3-MA reverses the effects of PinX1
overexpression on cell proliferation, apoptosis, and the cell cycle in NPC
cells. (a) The cell proliferation curves, (b) the apoptosis rate and (c) the
cell cycle of CNE1 cells and those transfected with empty vector and
pcDNA3.0-PinX1 as well as pcDNA3.0-PinX1 + 3-methyladenine. ** p
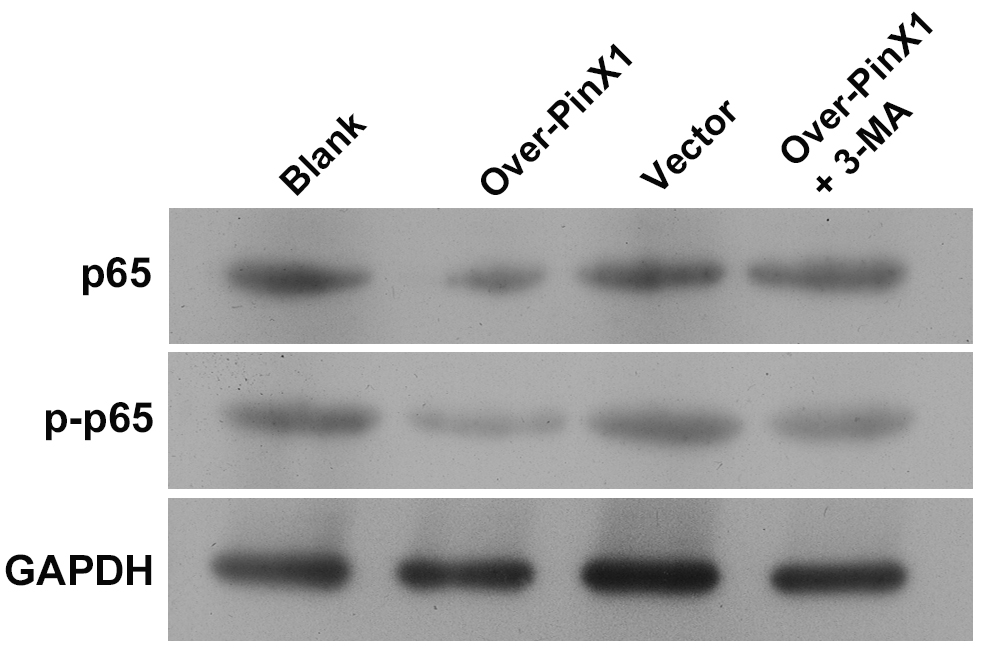
To further elucidate the signaling pathway involved in
PinX1-overexpression-induced apoptosis, we assessed NF-
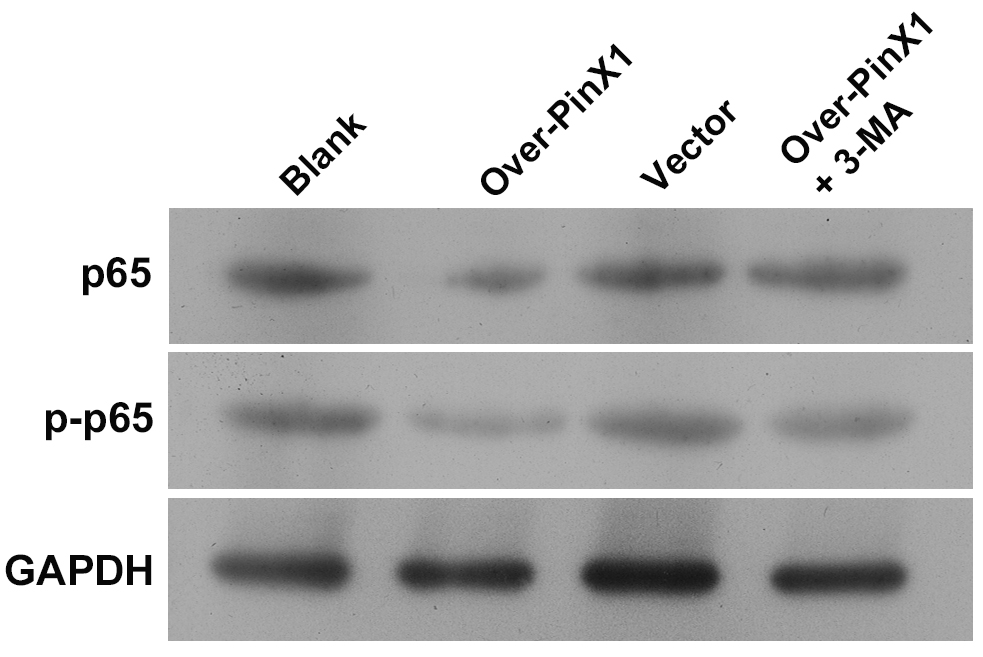 Fig. 5.
Fig. 5.The protein levels of p65 and p-p65 in CNE1 cells and cells transfected with empty vector and pcDNA3.0-PinX1 as well as pcDNA3.0-PinX1 + 3-methyladenine as determined by Western blot.
In our previous study, the roles of PinX1 in modulating EMT, stemness, proliferation, and apoptosis in NPC cells have been described [24]. Here, we investigated the mechanisms whereby PinX1 regulates proliferation, apoptosis, and autophagy in NPC cells.
There is substantial evidence that PinX1, which has crucial roles in carcinogenesis, is a potential novel human cancer diagnostic biomarker and therapeutic target [25, 26]. Previous studies have shown that PinX1 can specifically inhibit telomerase activity and induce tumor-cell apoptosis [22, 27]. Here, we examined PinX1 expression in two NPC cell lines, CNE1 and 6-10B, using RT-qPCR and western blot analysis. Our findings showed that PinX1 was strongly expressed in 6-10B cells but weakly expressed in CNE1 cells. We therefore chose the CNE1 cell line for subsequent experiments, to clarify the roles and mechanisms of action of PinX1 in cell proliferation and tumorigenesis. Not only did PinX1 significantly inhibit NPC cell proliferation in vitro, it also suppressed tumorigenicity in vivo. These results confirmed that PinX1 is a potential tumor suppressor in NPC.
Several studies have demonstrated that autophagy is involved in tumor growth, proliferation, and apoptosis [28, 29, 30]. However, it remains unclear how autophagy affects carcinogenesis in NPC; this may be related to differences in research targets. For example, TIPE1 promotes NPC progression via AMPK/mTOR signaling to induce cell proliferation and inhibit autophagy [31]. Zhu et al. [32] demonstrated that Annexin A1 promotes NPC cell invasion and metastasis by suppressing autophagy via activating PI3K/AKT signaling. Here, we found that PinX1 overexpression significantly increased the density of characteristic autophagosomes and increased LC3B expression in NPC cells. Further, the LC3-II/LC3-I ratio and Beclin-1 expression were higher in PinX1-overexpressing cells than in the controls. Conversely, PinX1 overexpression suppressed the expression level of p62. In addition, pharmacological inhibition of autophagy using 3-MA significantly reversed these outcomes in PinX1-overexpressing cells, which significantly increased migration and invasion, enhanced their proliferative capacity, and reduced apoptosis. Our data strongly suggest that PinX1 inhibits NPC cell proliferation and induces cell apoptosis by inducing autophagy.
AKT/mTOR signaling is an important pathway for regulating autophagy, which plays a vital role in tumorigenesis [33, 34, 35]. It is known that p-AKT and p-mTOR are highly expressed in various NPC cell lines, and activation of the AKT/mTOR signaling pathway is closely related to poor prognosis [36, 37]. Here, PinX1 overexpression produced significantly lower levels of phosphorylated AKT and mTOR than those in the control groups, suggesting that PinX1 inhibits AKT and its downstream target, mTOR. Further, by adding chloroquine to PinX1-overexpressing cells, autophagic flux was inhibited, but phosphorylated AKT and mTOR levels were not significantly affected relative to the untreated PinX1-overexpressing cells. Chloroquine inhibits autophagy mainly by reducing autophagosome–lysosome fusion, rather than by altering the acidity or degradation activity of organelles [38]. Therefore, chloroquine did not affect the signaling molecules that induce autophagosome formation. Our results confirm that PinX1 overexpression inhibits the activation of the AKT/mTOR pathway, thereby activating autophagy in NPC cells.
Tumor-cell proliferation and apoptosis are primarily influenced by cell-cycle
progression. Therefore, we examined the effects of PinX1 on the cell cycle. PinX1
overexpression decelerated cell-cycle progression and induced apoptosis by
activating autophagy in CNE1 cells. Notably, activation of the NF-
There are several limitations in this research. First, MTT assay was used for the assessment of cell proliferation and viability by evaluating cellular mitochondrial activity of NPC cells, which may not be indicative of the redox state of the cells. Second, the analysis of apoposis by Annexin-V/PI staining may not show the drop of mitochondrial membrane potential and also the associated ROS production, resulting in the inability to distinguish the number of cells in apoptosis and in necrosis. Nevertheless, the methods used in our study are reliable and sufficient, which presents new and interesting findings to the relationship between PinX1, autophagy, and cell function in NPC.
Our findings show that PinX1 inhibits the AKT/mTOR signaling pathway to promote
autophagy. The NF-
The data used to support the findings of this study are available from the corresponding author upon request.
CS, ZW and FC made substantial contributions to the conception or design of the work. MY, FC, CY, ZC, QZ, JF and GL made substantial contributions to the acquisition, analysis, or interpretation of data for the work. FC and MY wrote the manuscript. All authors contributed to revising the manuscript critically for important intellectual content and final approval of the version to be published. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The in vivo experiments were approved by the Laboratory Animal Committee of Southern Medical University(Approval No: LAEC-2019-011) and were conducted in accordance with the National Laboratory Animal Care and Maintenance Guide.
Not applicable.
This research was funded by the grants from National Natural Science Foundation of China Youth Fund (81402456) and Natural Science Foundation of Guangdong Province (2015A030313255).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.