- Academic Editor
†These authors contributed equally.
§These authors contributed equally.
While increasing numbers of studies have established that adipose tissue plays a vital role in balancing energy intake and energy expenditure as both an energy and an endocrine organ, the detailed functions of adipose tissue remain unclear. Adipose tissues are complex, with multiple resident cell populations that communicate to diverse cells and organs via local and systemic metabolic, thermal, and inflammatory signaling. In normal physiology, adipose tissue-derived extracellular vesicles mediate the regulation of energy storage/consumption in adipose tissue, liver, and muscle. In a pathological sense, fat-derived extracellular vesicles can promote the progression of obesity, endocrine diseases, cancer, and reproductive system disorders. In this review, we demonstrate that adipocyte-derived extracellular vesicles function not only in physiological balance but also in the pathological process. We aim to illustrate the impact of adipocyte-derived extracellular vesicles and their value in understanding both homeostasis and disorders.
Obesity is a significant health problem worldwide as it is currently viewed as a major driving factor in circulation disorders [1, 2], insulin resistance, metabolic syndrome [3], and even some malignancies such as liver and breast cancer [4]. Adipose tissue is an endocrine organ with high metabolic activity [5]. With a better appreciation of adipose tissue comes an increased understanding of adipose tissue-secreted adipokines and lipoxins, as well as peptides and extracellular vesicles with biological activity [6]. By delivering encapsulated cargos, extracellular vesicles mediate communication between diverse cell, tissue, and organ types [7]. Extracellular vesicles derived from adipose tissues have been studied by several groups in recent years; however, the function of adipose tissue-derived extracellular vesicles, as well as the detailed mechanisms they control, remain unclear. In the present review, we explore these relationships, focusing on some of the newest studies that link adipose tissue-derived extracellular vesicles to both normal physiological balance and pathological processes.
Adipose tissue can be classified into three major types: (i) white adipose tissue [8] (WAT); (ii) brown adipose tissue [9] (BAT); and (iii) beige adipose tissue [10]. In general, white adipose tissue represents more than 95% of adipose tissue mass, while brown adipose tissue represents just 1% to 2% of total adipose mass [11]. Beige adipose tissue, which is not clearly quantifiable, is able to switch into brown-like adipocyte tissue under cold exposure or adrenergic stimulation.
Over the past 20 years, it has become well-acknowledged that homeostasis depends not only on a balance between energy intake [12] and consumption [13] but also depends on a balance between white fat, the primary site of energy storage, and brown adipose tissue, the site for energy consumption. In general, white adipose tissue is the most variable and dynamic tissue in the human body. The percentage of white adipose tissue ranges from less than 10% to more than 65% of body weight [14]. Adipocyte hypertrophy through lipid accumulation [15], in addition to adipocyte hyperplasia through preadipocyte proliferation, leads to the increase of fat mass in obesity. Numerous studies report that the total number of fat cells is established during childhood and remains in balance into adulthood [16]. For example, Zhou et al. [6] reported that adipocyte-derived extracellular vesicles contribute to both the menstrual cycle and fertilization. However, overfeeding or other non-healthy lifestyles might induce an increase in white adipose tissue [17]. White adipocytes contain a large unilocular lipid droplet in the cytoplasm for the storage of energy [18]. In contrast, brown [18] and beige adipocytes contain multilocular droplets and high mitochondrial density for the consumption of energy [19], which can inhibit the development of obesity.
Recently, several studies reported that adipose tissue, beyond its role as an energy storage organ, also functions as an endocrine organ [20]. Adipose tissues secrete a variety of adipokines, including leptin [21] and adiponectin [22]. Leptin [23] is a 16 kDa protein produced by white adipocytes and binds to leptin receptors in the hypothalamus resulting in the inhibition of feeding behavior. Adiponectin [24] is a 30 kDa protein produced in both white and brown adipocytes and is associated with insulin resistance as adiponectin levels increase in patients with severe insulin resistance due to anti-insulin receptor antibodies or insulin receptor mutations. In addition to leptin and adiponectin, adipose tissue also produces numerous hormones that regulate insulin balance and metabolic homeostasis.
Extracellular vehicles (EVs) are small, membrane-bound vesicular structures derived from diverse cell types [25] and include exosomes, macrovesicles (MVs), and apoptotic bodies. EVs are released during cell activation, senescence, or programmed cell death, including apoptosis, necroptosis, and pyroptosis.
Extracellular vesicles can deliver cargos, including miRNAs, long non-coding RNAs, proteins, DNA fragments, lipids, and mRNAs, from producing cells to recipient cells/tissues [26]. Recently, increasing numbers of studies have focused on the emerging roles of adipocyte-derived extracellular vesicles in regulating obesity and metabolic homeostasis [27]. Extracellular vesicles have historically been viewed as conveyors of cellular waste; however, increasing evidence indicates that extracellular vesicles can, in addition to cargo delivery, convey information between diverse cells and tissues [28]. Extracellular vesicles derived from cancer cells, or cancer-associated cells, can mediate cancer progression [28, 29], wound healing [30], and macrophage polarization. Extracellular vesicles derived from a primary site could dictate cell behavior at distant sites, including immunosuppression. Furthermore, extracellular vesicles have been viewed as potential disease biomarker candidates [31], as well as potential therapeutic carriers for drug delivery [32].
Adipose tissue can secrete extracellular vesicles into the microenvironment and circulation, resulting in disbursement throughout the body. As depicted in Fig. 1, adipose tissue-derived extracellular vesicles play important roles in regulating energy consumption, circulation homeostasis, and metabolic balance.
 Fig. 1.
Fig. 1.Schematic diagram showing the genesis and targets of extracellular vesicles derived from adipocytes. EV, extracellular vesicles; IR, insulin resistance.
Considering that adipose tissue represents the largest energy storage and secretory organ in the human body, adipose tissue-derived extracellular vesicles play important roles in regulating energy consumption by communicating with adipose tissue, liver [33], and skeletal muscle cells [34]. As adipose tissues contain diverse types of cells, the crosstalk between extracellular vesicles and adipose tissue depends on cell heterogeneity within adipose tissue [35]. Adipose tissue-extracellular vesicles derived from obese mice can promote the activation of adipose tissue macrophages. This, in turn, can lead to increased expression of pro-inflammatory cytokines, which further promote the development of insulin resistance. The adipocyte-derived extracellular vesicle miRNA-34a was reported to inhibit M2 macrophage polarization [36] and subsequent induction of fat-associated inflammation [37]. Furthermore, adipose tissue macrophage-derived extracellular vesicles contain diverse miRNA species that can mediate metabolic homeostasis through interplay with adipocytes, hepatocytes, and skeletal muscle cells [38]. For example, EVs released by brown adipose tissue (BAT) containing miR-99b can target Fgf21 in the liver, and EVs released by brown adipose tissue containing miR-92a are a potential biomarker for BAT [39].
Extracellular vesicles derived from adipose tissue are tightly associated with vascular health and circulatory balance. In general, adipose tissue-derived extracellular vesicles can influence the regulation of vascular homeostasis through neovascularization and angiogenesis. Human adipose stem cell (ADSC)-derived extracellular vesicles are rich in miRNA-125a and miRNA-31, which can be transferred to vascular endothelial cells to stimulate proliferation and promote angiogenesis [40]. ADSC-derived extracellular vesicles can induce vascular endothelial cell migration and vascular endothelial cell proliferation [41] and mediate neo-vessel formation. Previous reports indicated that extracellular vesicles released by adipocytes express high levels of glycosylphosphatidylinositol (GPI)-anchored proteins [22] and CD73 [42]. These proteins are viewed as mediators of triacylglycerol esterification as well as lipolysis—both processes involved in vascular health. In addition, other studies reported that adipocyte-derived extracellular vesicles could carry various miRNAs, including miR-221 [43], miR-26 [6], and miR-143 to regulate cell proliferation and apoptosis, as well as to mediate inflammation and control angiogenesis in vascular tissues. Recent studies indicate that extracellular vesicles can transport mitochondria from energetically stressed adipocytes to protect cardiomyocytes from acute oxidative stress by inhibiting the release of Reactive Oxygen Species (ROS) production [44]. Adipocytes can also release extracellular vesicles during mitochondrial stress, and these adipocyte EVs would promote protection from ischemic stresses that result from obesity.
The endocrine function of adipose tissue is partially mediated by adipose
tissue-derived extracellular vesicles by affecting various aspects of metabolic
homeostasis, including insulin signaling, lipolysis [45], and inflammation.
Increasing evidence has shown that the composition of adipose-derived
extracellular vesicles fluctuates during microenvironmental changes. For example,
when the proteomic profiles of adipocyte-derived extracellular vesicles from
obese diabetic rats and obese non-diabetic rats are analyzed, different results
are obtained. Levels of caveolin 1, lipoprotein lipase, and aquaporin 7 were
significantly higher in exosomes and cells of Otsuka Long-Evans Tokushima
Fatty (OLETF) rats than in those of Long-Evans Tokushima Otsuka (LETO) rats. In
contrast, AK2, catalase, and liver carboxylesterase were expressed at lower
levels in OLETF rats when compared to LETO rats [46]. Brown adipose tissue (BAT)
generates heat during adaptive thermogenesis in response to cold temperatures and
in profound hypothyroidism [47]. Circulating TGF-beta 1 might be available to
monitor type 2 diabetes status in obese patients, and EV may be useful to track
adiposity [48], according to studies that qualitatively and quantitatively
characterized EV subpopulations secreted by fat cells, large extracellular
vesicles (lEVs) were found to possess high levels of externalized
phosphatidylserine. Exposure to a chronic low-grade inflammation state associated
with obesity could increase the secretion of lEVs and small EVs (sEVs) [49]. In
obesity, adipose tissue and placenta-derived EVs are related to the development
of gestational diabetes mellitus (GDM) [50]. Extracellular vesicles derived from
the obese diabetic group expressed high levels of proteins and enzymes involved
in lipolysis and glycerol export, which can lead to the subsequent development of
insulin resistance. Moreover, it was reported that adipocyte-derived
extracellular vesicles could mediate the survival and function of pancreatic
Increasing evidence has demonstrated that adipose tissue can secrete diverse types of extracellular vesicles that contain numerous cargos and hormone-like functions into the microenvironment and circulation. These EVs can, in turn, communicate with cells or tissues to regulate metabolic homeostasis and energy balance. When these system balances are disturbed, adipose tissue-derived extracellular vesicles could participate in pathophysiological processes such as obesity development, metabolic syndrome, cancer progression, and other diverse disease types.
It has been demonstrated that adipose tissue-extracellular vesicles contribute
to diabetes, nonalcoholic fatty liver disease, and insulin resistance-related
cardiovascular disease. For example, adipocyte-derived extracellular vesicles may
mediate crosstalk between adipose tissue and insulin-sensitive organs such as the
liver and skeletal muscle. A consensus view has developed that adipose
tissue-derived extracellular vesicles are linked to insulin resistance. Studies
have reported that adipocyte-derived extracellular vesicles containing miR-27a
may induce insulin resistance in skeletal muscle by suppressing PPAR
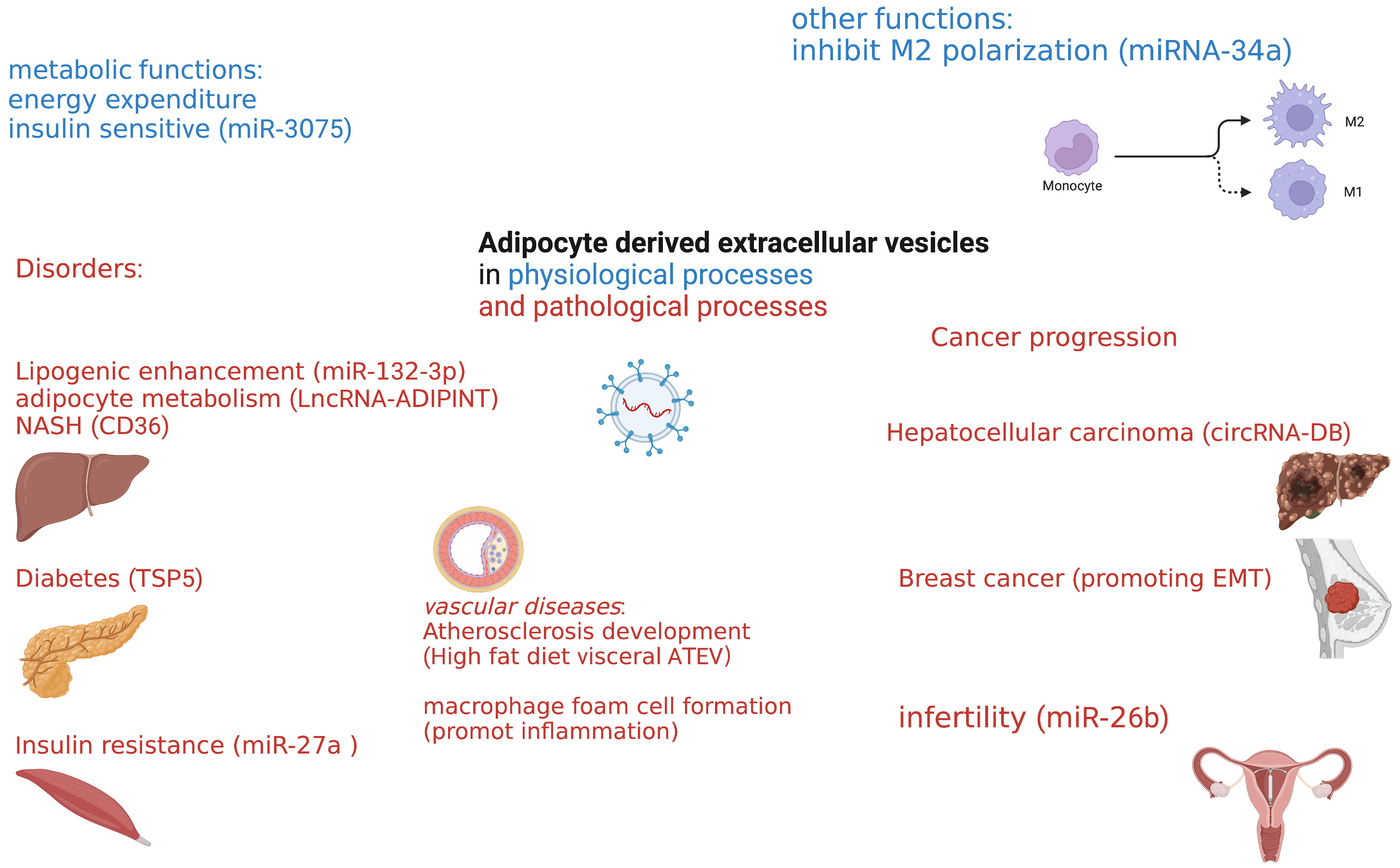 Fig. 2.
Fig. 2.Overview of adipose-derived extracellular vesicles in health and disease.
Obesity is causally associated with atherosclerosis, and adipose tissue-derived
extracellular vesicles may be implicated in metabolic complications of
obesity beyond their role in regulating whole-body energy metabolism through its
storage function in white adipocytes and its energy dissipating function in brown
and beige adipocytes. High-fat diet visceral adipose tissue-derived extracellular
vesicles could significantly induce M1 phenotype transition and
pro-inflammatory cytokines such as tumor necrosis factor
It is acknowledged that obesity is associated with some malignancies, such as
liver and breast cancer. Adipocytes within the cancer microenvironment not only
surround the tumor but also communicate with cancer cells through both local and
systemic effects [64]. Increasing evidence indicates that extracellular vesicles
derived from adipose tissue function in the growth and migration of liver,
ovarian, breast, and other cancer types. Communication between adipocytes and
cancer cells can occur directly through the microenvironment or systemically
through EV-mediated cell-to-cell communication [65]. Adipose tissue-derived
extracellular vesicle circRNA-DB (circular RNA related to deubiquitylation) can
promote the growth of hepatocellular carcinoma by suppressing miR-34a and
activating the USP7/Cyclin A2 signaling pathway [66]. Extracellular vesicles
derived from human adipose tissue can promote the migration of breast cancer
through epithelial-mesenchymal transition (EMT) signaling [67]. Furthermore,
breast cancer-derived extracellular vesicles induce adipose tissue-derived
mesenchymal stem cells to adopt a tumor-associated myofibroblast phenotype with
high levels of
Infertility has drawn the attention of many doctors and scientists as increased infertility affects more than 10 percent of reproductive-age couples. It was reported that obesity is associated with dysregulation of the reproductive system, not only through polycystic ovary syndrome and paramenia but also asthenospermia. It has been demonstrated that a high-fat diet can enhance the expression of phosphatidylcholine in extracellular vesicle-derived adipose tissue [69], and adipose tissue-derived extracellular vesicles can lead to insulin resistance and polycystic ovary syndrome [25]. Hepatocyte-derived extracellular vesicles from early-onset obese mice promote insulin sensitivity through miR-3075. Additionally, a previous study from our group showed that extracellular vesicles derived from adipocytes could deliver encapsulated miR-26b into cumulus cells and promote their apoptosis. This had the effect of further inducing ovulation failure and subsequent polycystic ovary syndrome [32]. Mechanistically, increasing findings suggest that adipose tissue-derived extracellular vesicle-mediated insulin resistance may be a prominent reason for subsequent infertility.
Herein, we reviewed the literature concerning adipose tissue-derived extracellular vesicles and their function in maintaining physiological balance as well as promoting pathophysiological processes (Table 1). Adipose tissue function not only in energy storage but also play a prominent role in endocrine function through extracellular vesicle activity. EVs may influence energy consumption in adipose tissues, liver, and muscle, and through insulin sensitivity, circulation, and effects on the reproductive system. Although various functions of adipose tissue-derived extracellular vesicles have been reported, more studies are required to fully understand the underlying mechanisms and signaling pathways that control response to these response mediators. It is well acknowledged that adipose tissue-derived extracellular vesicles are of significance and act as small vesicles with a big impact.
| Disorders | EV or EV-related cargoes | |
| Metabolic disorders: | ||
| a. Insulin resistance | EV miR-27a | |
| b. Diabetes | EV-TSP5 | |
| c. NASH | EV-CD36 | |
| Vascular disorders: | ||
| d. Atherosclerosis | High-fat diet AT-EV | |
| e. Macrophage foam cell formation | Pro-inflammation EV | |
| Cancer progression: | ||
| f. Hepatoma carcinoma cell | EV circRNA-DB | |
| g. Breast cancer progression | Promoting EMT EV | |
| Other disorders: | ||
| h. Infertility | miR-26b | |
NASH, non-alcoholic fatty liver disease; AT, adipose tissues; EV, extracellular Vesicles; EMT, epithelial-mesenchymal transition.
YL: Substantial contributions to the conception of the manuscript, and literature research, manuscript writing, as well as reviewing. XT: Analysis of data, interpretation of data and manuscript polishing. YG: Analysis of data, drafting the figures and reviewing the manuscript. GZ: Design of the conception, interpretation of data, drafting the manuscript and final approval of the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors contributed to the article and approved the submitted version.
Not applicable.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
