1 Department of Laboratory Medicine, Translational Research Center Karolinska (TRACK), Karolinska Institutet, Karolinska University Hospital, SE-141 86 Stockholm, Sweden
2 Department of Molecular Biosciences, The Wenner-Gren Institute, Stockholm University, SE-106 91 Stockholm, Sweden
3 Food Science Technology, School of Applied Sciences and Mathematics (SASM), University Technology Brunei (UTB), BE1410 Mukim Gadong A, Brunei Darussalam
4 Center for Rare Diseases, Karolinska University Hospital, SE-171 76 Stockholm, Sweden
5 Neuro-ICT Laboratory, Advanced Institute of Information and Communications Technology, 651-2492 Kobe, Japan
Abstract
Background: Bruton’s tyrosine kinase (BTK) is a non-receptor type
tyrosine kinase originally identified as the genetic signature responsible for
X-linked agammaglobulinemia (XLA) when mutated. Its functional form is required
for B lymphocyte maturation in both humans and mice, whereas loss-of-function
causes a different form of developmental defect in the fruit fly,
Drosophila melanogaster. Methods: Ibrutinib and other
therapeutic inhibitors of BTK have been extensively used to successfully treat
various leukemias and lymphomas. Btk29A type 2 is the ortholog of BTK in
the fruit fly. We show that feeding wild-type flies an ibrutinib-containing diet
induces phenocopying of Btk29A mutants, i.e., failure in the fusion of
left and right halves of the dorsal cuticles, partial loss of wing tissues and
dysregulation of germ cell production. Results: We have previously
reported that Btk29A phosphorylates Drosophila Arm (
Keywords
- non-receptor tyrosine kinase
- beta-catenin
- germ cell proliferation
- morphogenesis
- drug screens
- disease model
Bruton tyrosine kinase (BTK) was originally identified as a non-receptor tyrosine kinase whose deficiency is responsible for X-linked agammaglobulinemia (XLA) in humans [1, 2, 3]. Patients with XLA suffer from severe antibody deficiency and recurrent infections due to a differentiation defect causing the absence of mature B lymphocytes [4, 5, 6]. Conversely, inhibition of BTK has been shown to ameliorate various forms of leukemia and lymphoma [7, 8], with the premier example being chronic lymphocytic leukemia (CLL) [9, 10, 11]. Ibrutinib is the first such inhibitor of BTK to be clinically applied [7, 8, 10, 11]. However, there remain uncertainties as to how it alters signaling mediated by BTK in vivo, ultimately affecting development, physiology, and behavior at the organismal level. In addressing these questions, a genetically tractable in vivo animal model would be most useful.
Drosophila melanogaster (hereinafter Drosophila) is an
outstanding model organism due to its amenability to experimental manipulations
at the molecular, cellular, and organismal levels. The Btk29A type 2 isoform
encoded by the gene Btk29A is the sole ortholog of human BTK in
Drosophila: the other isoform, Btk29A type 1, has a unique N-terminus
and distinct expression and is thus structurally and functionally separable from
Btk29A type 2 [12]. Btk29A type 2 has been shown to play an essential role in
dorsal closure in postembryonic development [13], a morphogenetic process to
suture the left and right halves of the exoskeleton. Btk29A type 2 is also
pivotal for stem cell niche functions, e.g., germ cell proliferation and
differentiation, as well as follicle precursor migration in the ovary at the
adult stage [13, 14, 15, 16, 17, 18]. In the ovary, the Btk29A type 2 protein was shown to bind
to
Flies were raised on cornmeal-agar-yeast medium at 25 °C. The
Canton-Special (CS) strain was used to test the drug effects. The
Btk29A
Wings: Wings were removed from thoraces for observation and image acquisition using an Axio microscope (ZEISS, Oberkochen, Germany).
Thoraces: Dorsal thoraces were photographed under an M205FA microscope (Leica, Wetzlar, Germany). The distance between the left and right counterparts of homologous innermost bristles was measured by counting the number of intervening pixels on the computer screen with the aid of Photoshop software. Half of the value of the inter-bristle distance thus measured was defined as the distance between the bristle and the midline and used for comparisons among tested fly groups. Some of the flies fed an ibrutinib-containing diet during the larval stage showed disrupted dorsal structures. The proportion of flies exhibiting the dorsal open phenotype was calculated to quantify the ibrutinib action on the dorsal structure formation.
For the antibody staining, ovaries were dissected in Phosphate-Buffered Saline (PBS) and immersed in 4% paraformaldehyde in PBS for 40 minutes. The ovaries were then washed three times in PBS-T (PBS supplemented with 0.1% Triton-X), and the reaction was blocked for 1.5 h in PBS supplemented with 0.1% Triton and 0.5% Bovine Serum Albumin (BSA). Then, the tissue was incubated with a primary antibody for 3 h at room temperature or at 4 °C overnight. The primary antibodies used in this study were mouse monoclonal 1B1 (1:10; Developmental Studies Hybridoma Bank) and rat anti-Vasa (1:10; Developmental Studies Hybridoma Bank). The fluorescence-conjugated secondary antibodies were from Molecular Probes and were used at a 1:200 dilution. All samples were mounted in 80% glycerol.
Cos7 cells (American Type Culture Collection (ATCC), Washington, DC, USA) were
seeded overnight at 50% confluence in 6-well plates. Mycoplasma testing has been
done for the cell lines used. Transfections were carried out by using
polyethylenimine (PEI) (Polyscience, Warrington, PA, USA). Cells at 48 h
post-transfection were harvested, washed twice with PBS in Eppendorf tubes, and
re-suspended in 1 mL PBS. Cells were collected by low-speed centrifugation of
1250 rpm (500 g) at 4 °C. The collected cells were re-suspended in 100
µL of lysis–Radioimmunoprecipitation assay (RIPA) buffer containing cocktails of protease inhibitor
and phosphatase inhibitors, and the sample was then boiled at 65 °C for
2–3 min, vortexed, and centrifuged. The protein was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
probed with the respective antibody. The anti-pY142 (ECM Biosciences, Versailles,
KY, USA) antibody was diluted 1:1000, the anti-HA (Sigma-Aldrich, Burlington,
MA, USA) antibody was diluted 1:2000, and the anti-
Pharmacological inhibition was achieved with ibrutinib (PCI-32765; Pharmacyclics, Sunnyvale, CA, USA). The ibrutinib kept at –20 °C was dissolved in dimethyl sulfoxide (DMSO) to produce a 10 mM solution, which was added to PBS to attain a final concentration of 10 µM for application onto cells. For each experiment, fresh dilutions were prepared. At 36–48 h post-transfection, cells were starved under serum-free conditions for 4 h, and inhibitors were added during the last 2 h period of starvation.
The band intensity was quantified with the aid of the ImageJ (version 1.53t,
NIH, Bethesda, MD, USA) software run on a MacIntosh computer, and obtained data
were transferred to Microsoft Excel (version 16, Microsoft, Redmond, WA, USA) and presented as column histograms. The bars
represent the means and standard errors of two independent experiments. Data
analysis was carried out using statistically available software Graph Prism
software (version 6 for MacOS, Boston, MA, USA). Comparisons between groups were
made using one-way analysis of variance (ANOVA). p values
Fly food was softened by heating and then kept for 3 min to cool it down to ~40 °C before mixing with concentrations of BTK inhibitors, followed by cooling again to room temperature. DMSO (the final concentration: 0.38%) as a control was similarly added to food following the same protocol. Parental flies were transferred to food containing DMSO or a BTK inhibitor for 5 days so that their offspring would develop in food containing DMSO or the BTK inhibitor. Adult offspring were transferred 5 days after emergence to analyze their phenotypes. To determine the concentrations of ibrutinib for oral administrations in phenotypic analyses, female flies were allowed to lay eggs on the culture medium (2.6 mL) supplemented with either 200, 100, 75, 50, 25, 20, 15, or 10 µL of the 50 µM drug solution and the offspring were subjected to preliminary analysis: eggs deposited on the medium containing 200 or 100 µL of the drug solution failed in hatching; larvae were unable to complete development in the medium with 75 or 50 µL of the drug solution; adults emerged when grown on the media containing 15 or 10 µL of the drug solution. Based on this observation, we applied 10 µL of 50 µM ibrutinib onto fly media for subsequent phenotypic analyses. Larvae consume only small portions of the drug-supplemented medium during the entire larval stage, which spans about 7 days, whereas 400–500 mg ibrutinib is administered daily to adult human patients.
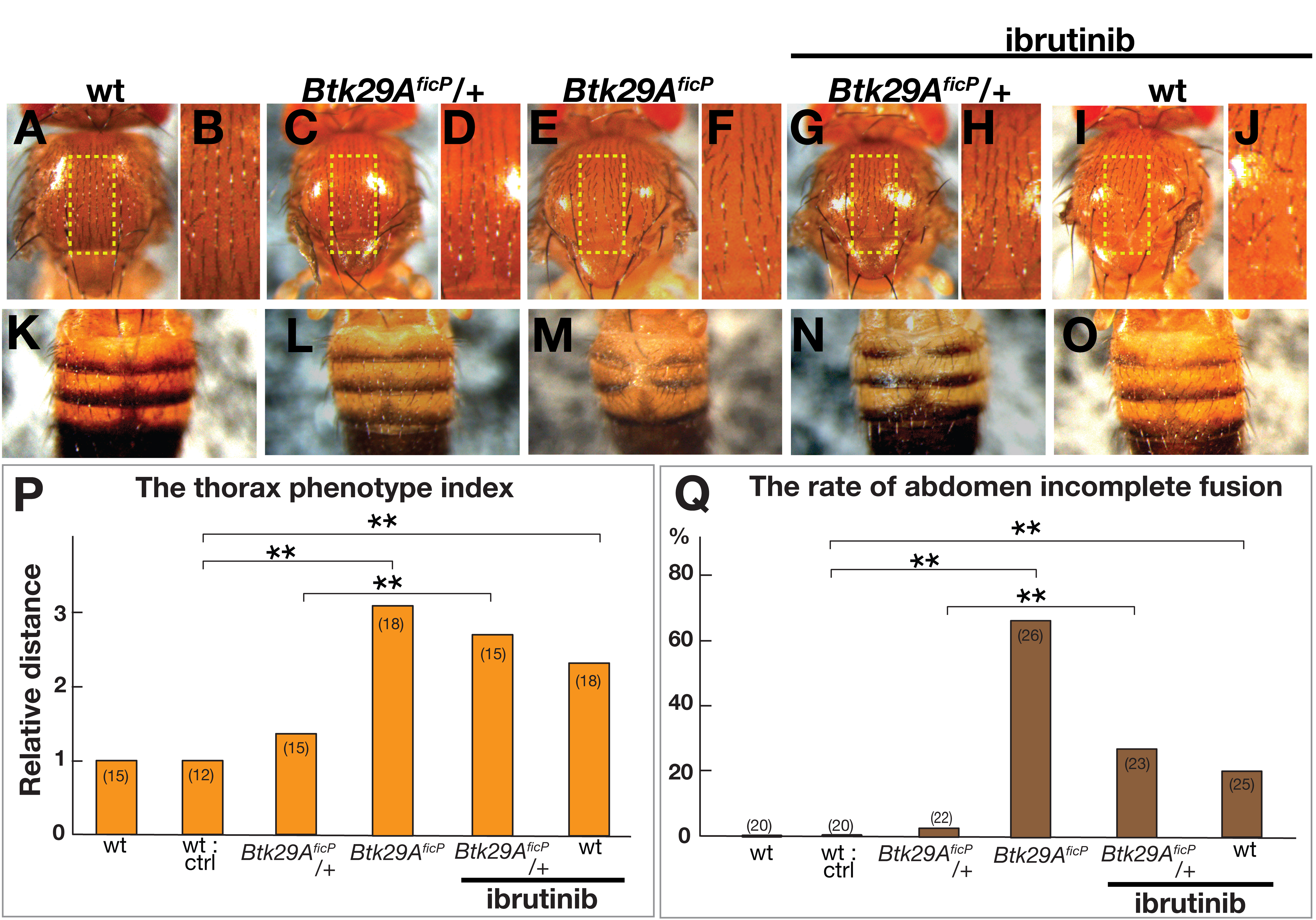
Visual inspection of the notum of adult flies suggested that feeding ibrutinib
throughout the larval stage results in the widening of the distance between left
and right homologous bristles along the midline (Fig. 1A–D,G–J), a phenotype
also observed in Btk29A
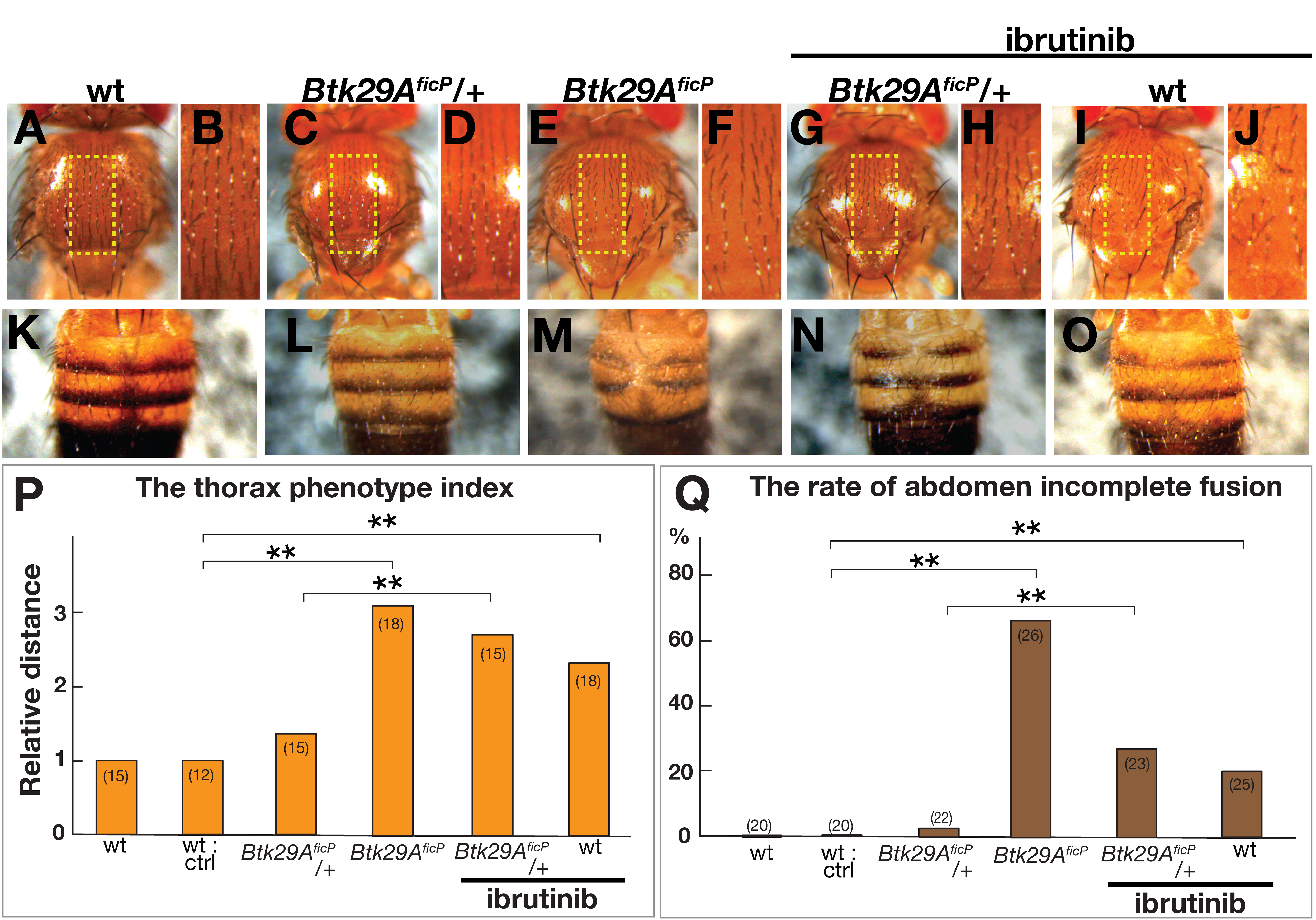 Fig. 1.
Fig. 1.Dorsal nota and tergites of Drosophila melanogaster.
(A–O) Dorsal nota (A–J) and tergites (K–O) of male wild-type (A, B, K and I,
J, O), Btk29A
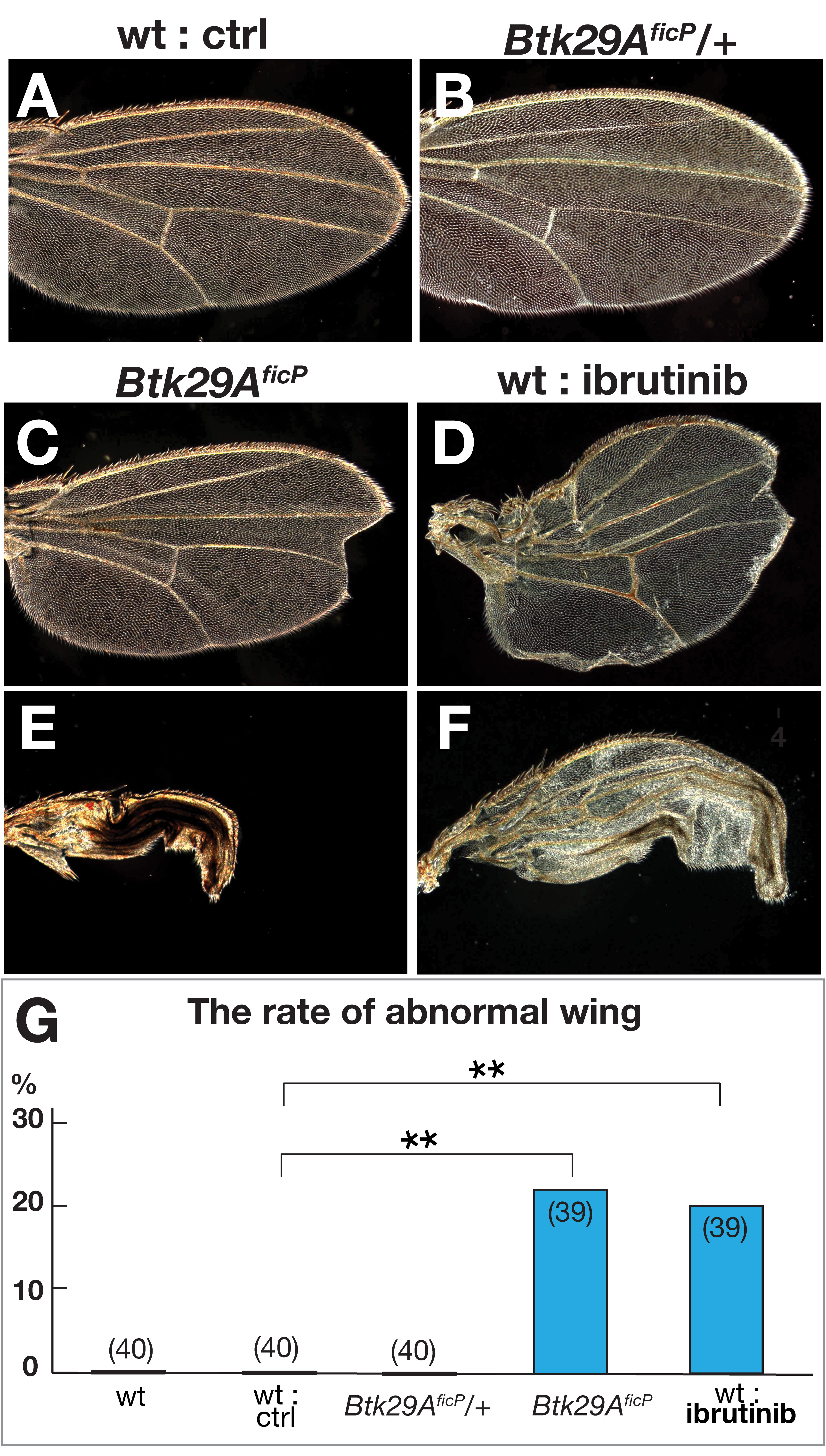
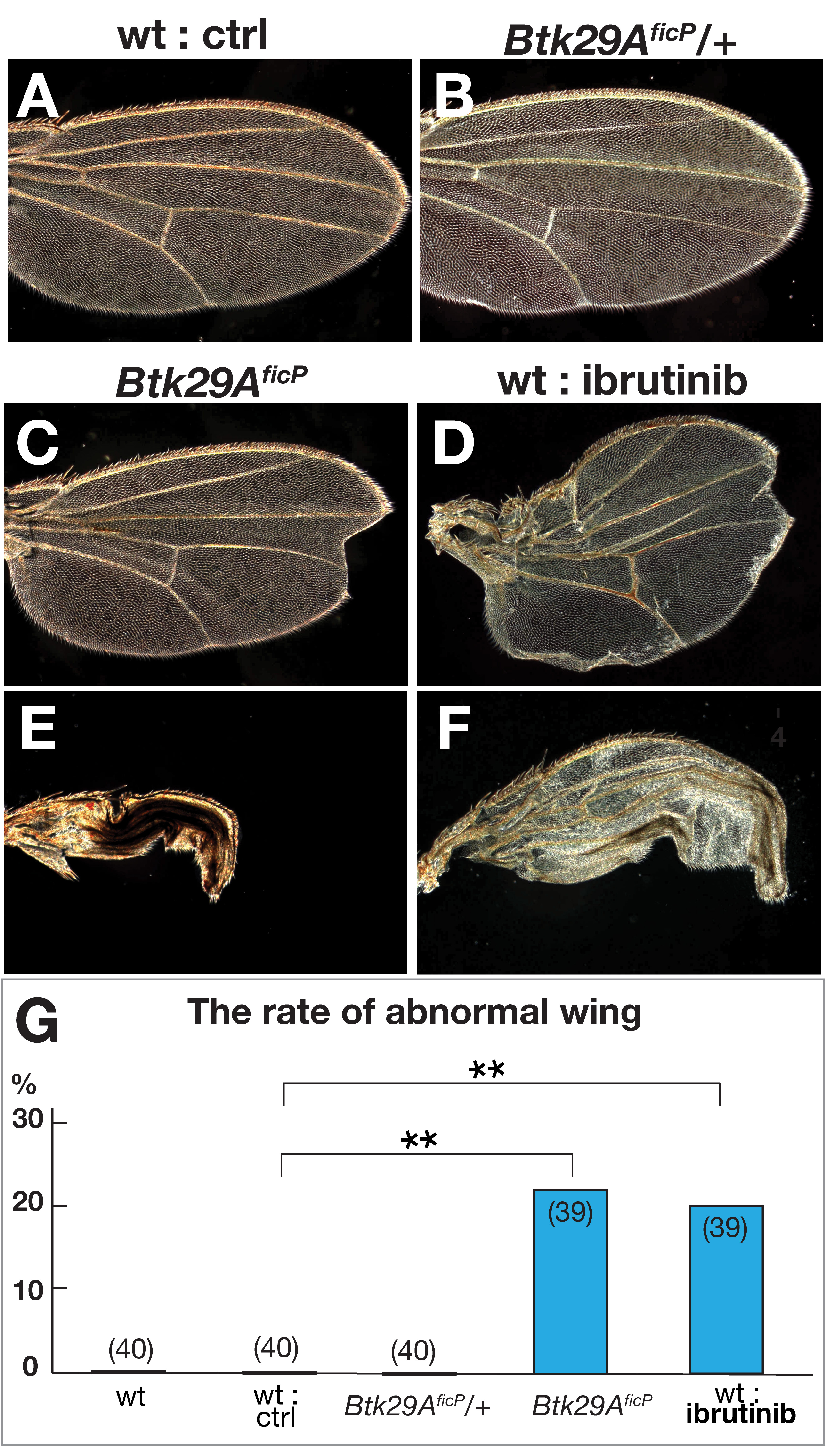 Fig. 2.
Fig. 2.Wing phenotypes in flies deficient in functional Btk29A type 2.
(A–F) Wings of wild-type (A, D, and F) flies without (A) or with ibrutinib (D
and F) and Btk29A
We then examined, in wild-type flies, the possible effects of ibrutinib feeding
on oogenesis, during which Btk29A mutations are known to induce
overproduction of cystoblasts (see below), which are directly derived from germ
stem cells (GSCs); a cystoblast differentiates into cystocytes that transform
into an oocyte and nurse cells after four rounds of divisions [21, 22]. This
process of germ cell proliferation takes place in the anterior tip of an ovariole
called the germarium, which contains one to three GSCs, several cystoblasts, and
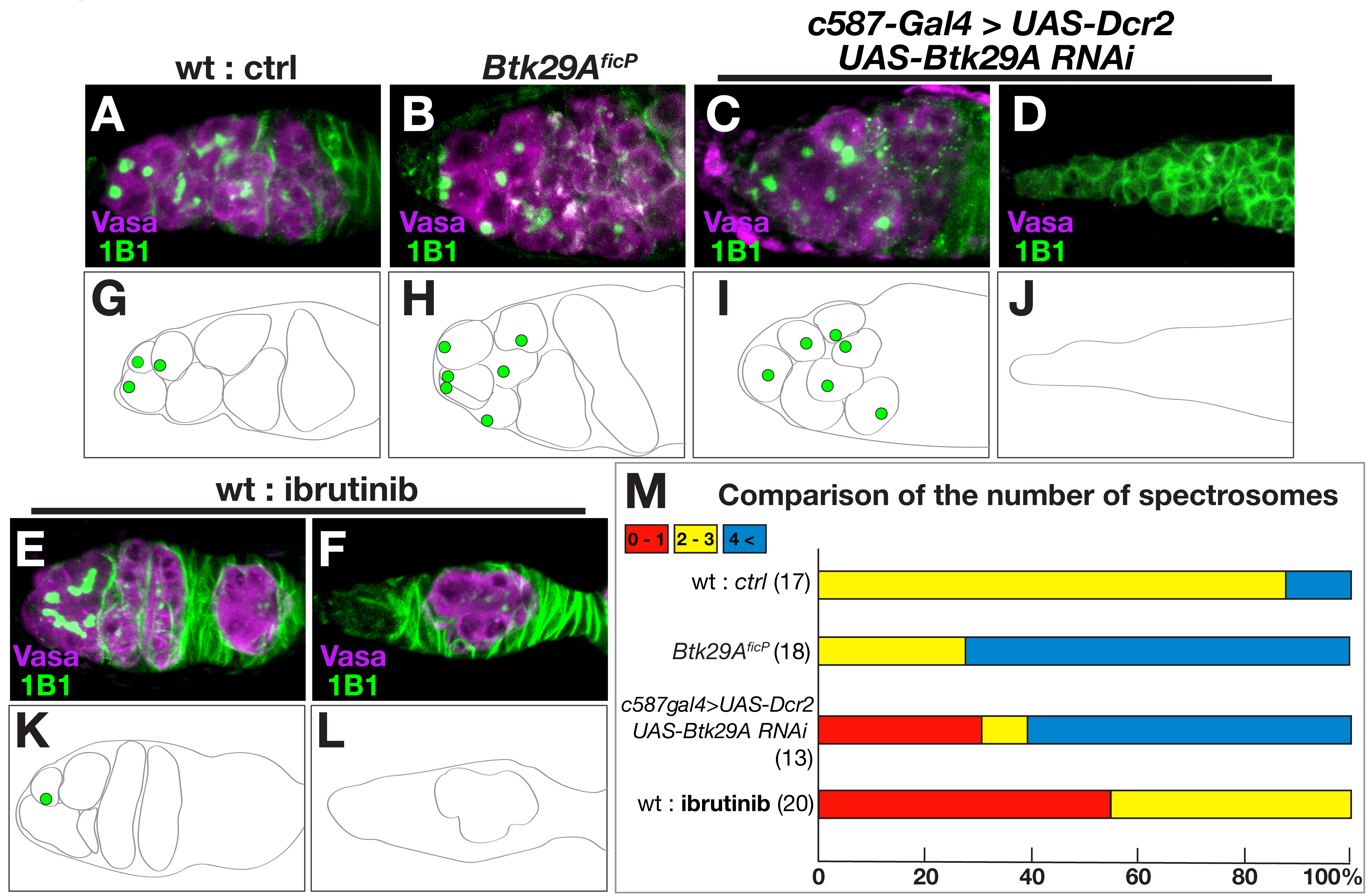
tens of cystocytes in wild-type flies [21]. Feeding ibrutinib caused
underproduction of cystoblasts (Fig. 3E,F,K and L cf. Fig. 3A,G), as
identified by the presence of spectrosomes (recognized as round structures rich
in actin), often leading to a complete lack of cystoblasts in a substantial
proportion of germaria (Fig. 3M). This is in sharp contrast to the germaria of
Btk29A
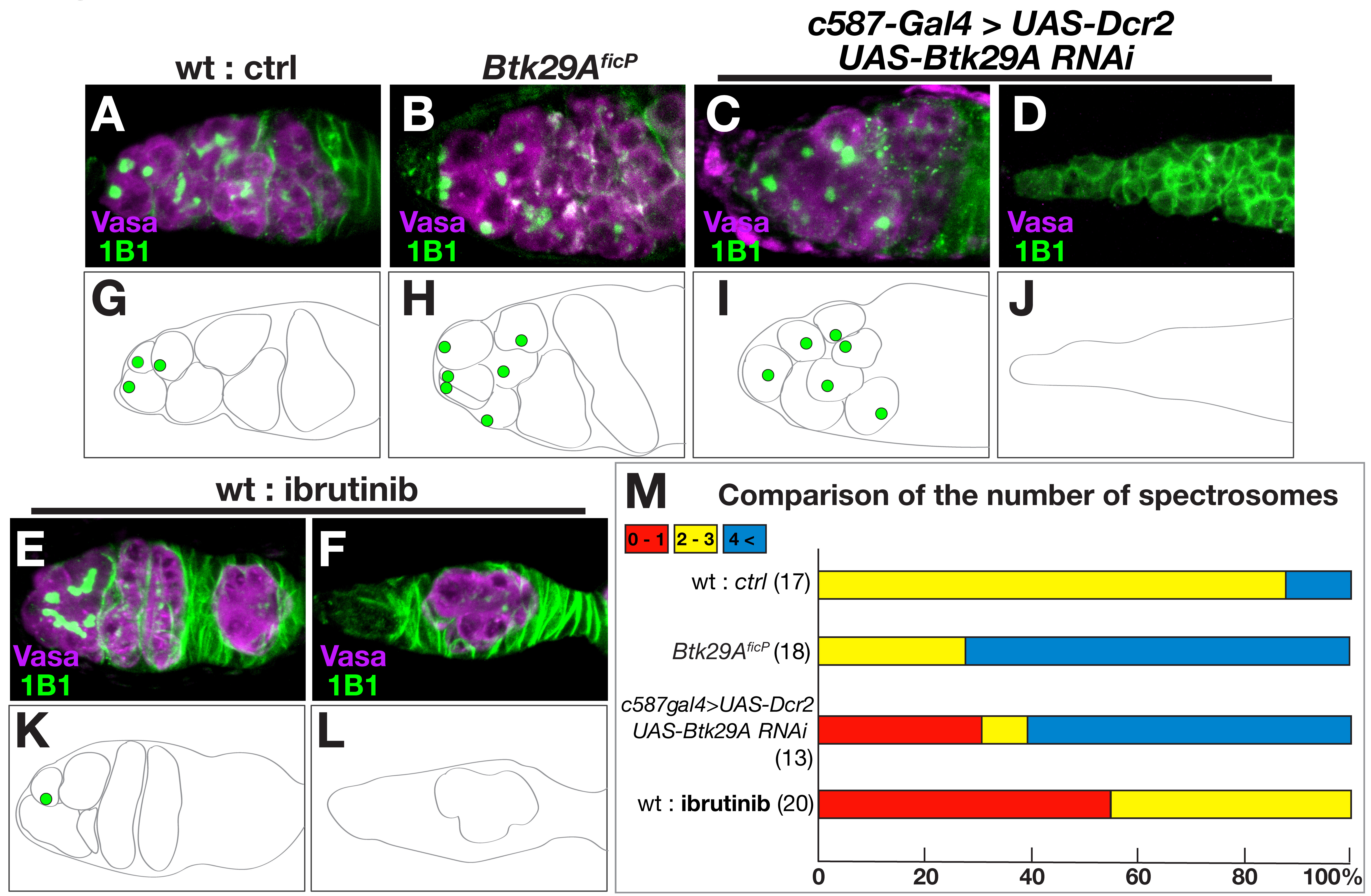 Fig. 3.
Fig. 3.Btk29A deficiency impairs germ cell production in the
ovary. (A–F) Enlarged views of the germarium at the anterior tip of the ovary,
which contains the niche where germ stem cells (GSCs) divide into cystoblasts
while reproducing GSCs themselves. (G–L) Schematic drawings of the germarium are
shown below the photographs to highlight germline cells containing spectrosomes.
Compared with the wild-type germarium (A and G), the Btk29A
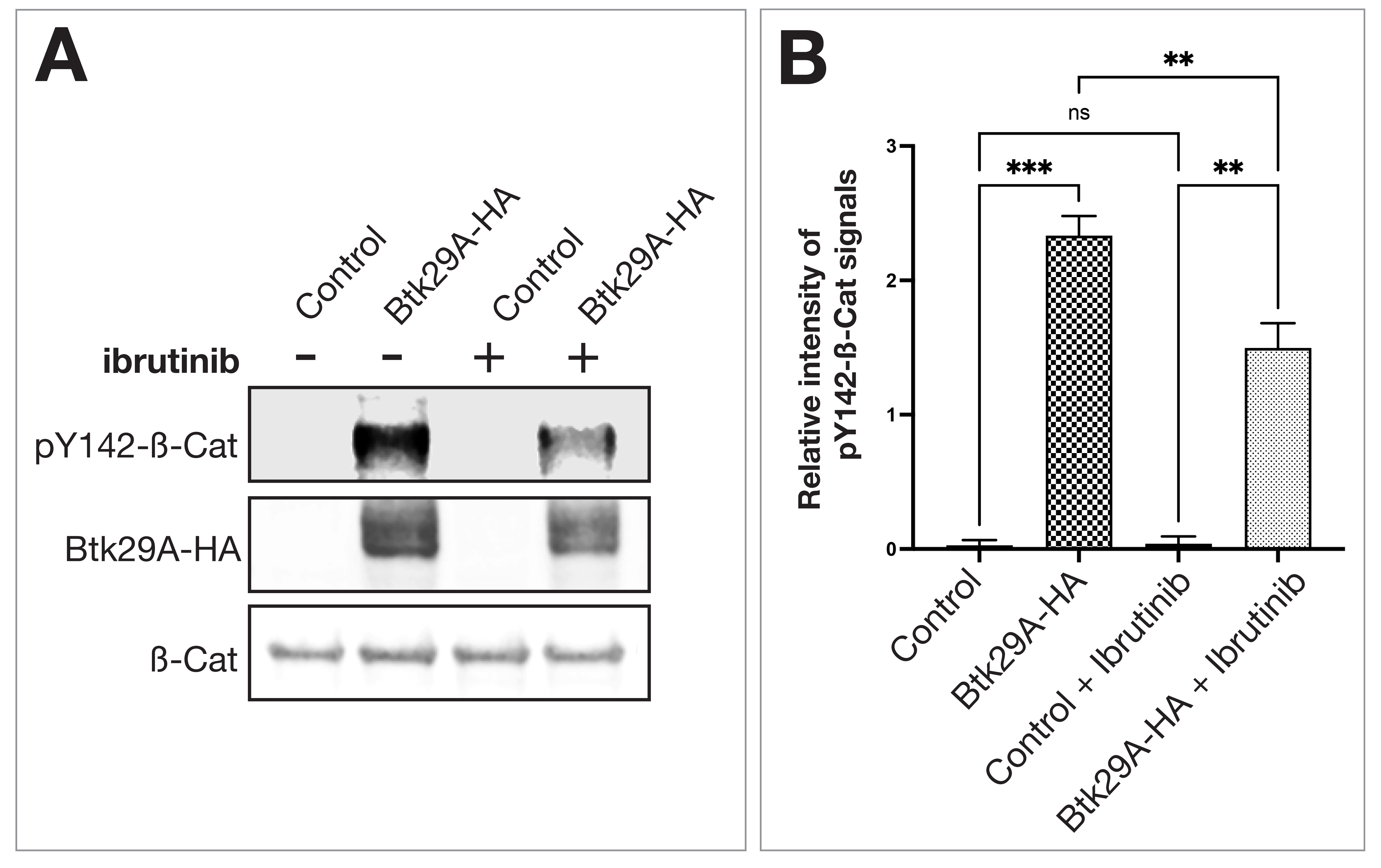
Finally, we examined whether ibrutinib affects molecular interactions between
Btk29A and
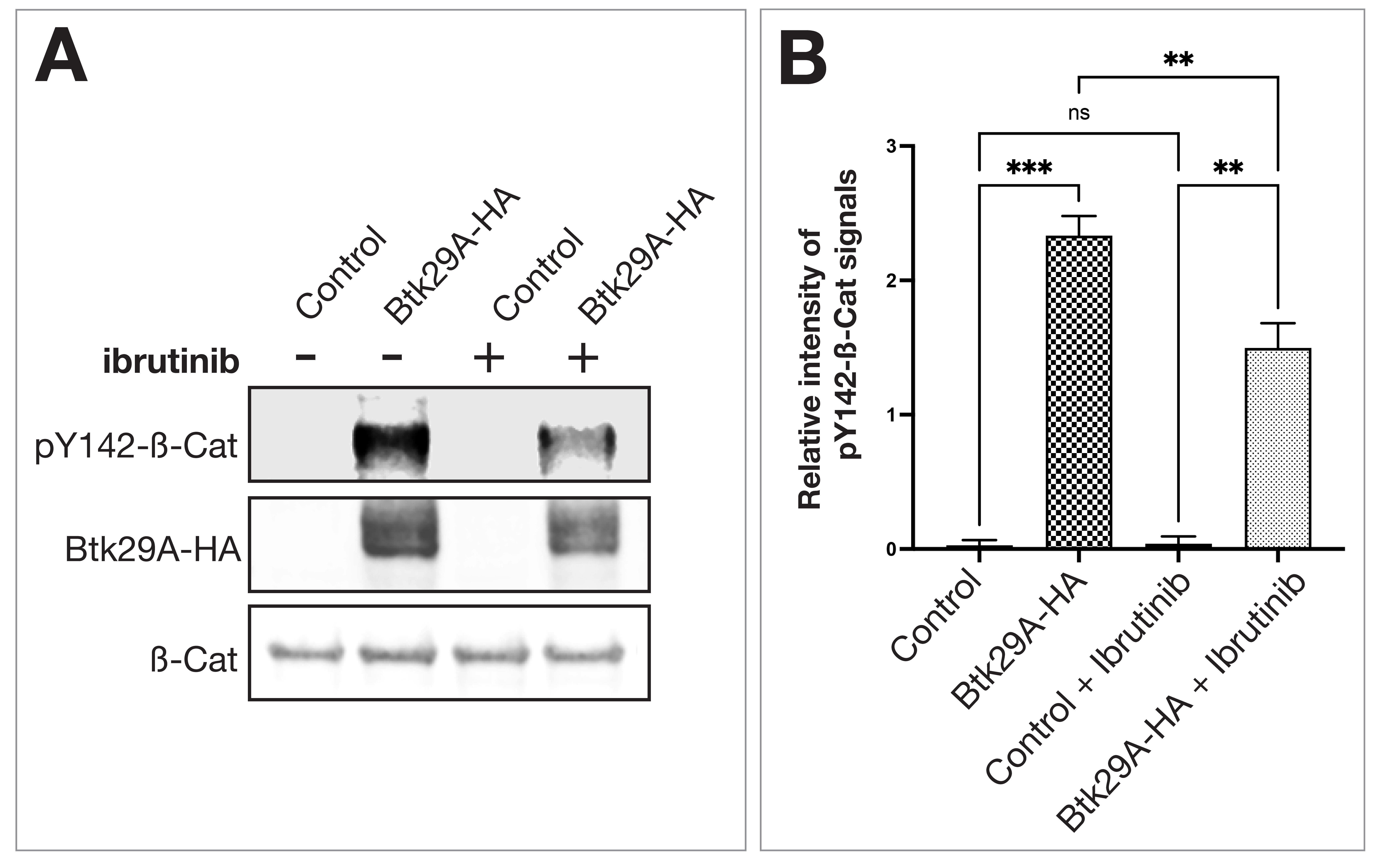 Fig. 4.
Fig. 4.Ibrutinib reduces phosphorylation of Y142
In the present work, we showed that ibrutinib faithfully reproduced some of the Btk29A mutant phenotypes in Drosophila when orally administered during the larval and adult stages, an observation consistent with the view that BTK and its ortholog are the major targets for ibrutinib in both humans and flies. It is also reminded that ibrutinib does not distinguish between the two Btk29A isoforms because they share the same kinase domain. Our results also point to the possibility that Drosophila, with its genetic tractability and rich collections of molecular resources, may provide an ideal in vivo experimental system for elucidating the mode of action of therapeutic drugs that target BTK. Drosophila will be particularly effective when used to screen chemicals that potentially act on BTK for the development of novel therapeutic drugs to control CLL because a large number of flies can be easily obtained for rounds of assays, and due to their short life cycle, life-long chronic effects of compounds can be evaluated in only a few weeks. Visible phenotypes such as a collapse in the dorsal closure and wing formation and other external structures [23, 24] will help to promptly reveal the effects of compounds without dissection of tissues or any other experimental handling, making it possible to enrich promising candidate molecules within a short period of time.
While our phenotypic and biochemical data presented in this and other works suggest functional homology between human BTK and fly Btk29A [25, 26], one caveat is that we have no direct evidence that ibrutinib exerts its effects on BTK/Btk29A through the same mechanism in humans and flies. In inhibiting human BTK, irreversible inhibitors, including ibrutinib, bind to the ATP pocket, in which Cystine481 plays an important role in inhibitor–BTK interactions [11, 27]. However, fly Btk29A harbors a cystine-to-serine replacement at the corresponding position. Irreversible inhibitors, including ibrutinib, were found to be less effective for patients with CLL carrying the cystine-to-serine substitution in BTK. It remains to be clarified how ibrutinib modulates the Btk29A functions in flies. It might be envisaged that fly Btk29A has a higher order structure, distinct from that of human BTK, which compensates for the effect of cystine-to-serine replacement that reduces the binding of ibrutinib and other irreversible inhibitors.
Feeding wild-type flies an ibrutinib-containing diet induces phenocopying of Btk29A mutants. Thus, Drosophila is suitable for screens of novel BTK inhibitor candidates and offers a unique in vivo system in which the mode of action of BTK inhibitors can be examined at the molecular, cellular, and organismal levels.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
NHK—Investigation, writing, reviewing, editing; CIES, BFN, YE, RZ—Conceptualization, reviewing; DY—Writing, reviewing, editing, conceptualization, and interpretation of data for the work. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Not applicable.
We thank Yoshimi Takamura for secretarial assistance. The authors are grateful to the Bloomington Drosophila Stock Center (BDSC) and Vienna Drosophila Resource Center (VDRC) for providing fly strains.
This work was supported in part by a MEXT Grant-in-Aid to DY (21H04790), a Grant-in-Aid for JSPS Fellows to NHK, and grants from the Center for Innovative Medicine to CIES, the Swedish Cancer Society (22 2361 Pj 01 H) to CIES, the Stockholm County Council (ALF-project) to CIES, and The Swedish Cancer Society (201044 PjF) to YE.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.