1 Department of Nephrology, Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, 201203 Shanghai, China
2 TCM Institute of Kidney Disease of Shanghai University of Traditional Chinese Medicine, 201203 Shanghai, China
3 Key Laboratory of Liver and Kidney Diseases, Ministry of Education, Shanghai Key Laboratory of Traditional Chinese Clinical Medicine, 201203 Shanghai, China
†These authors contributed equally.
Abstract
Background: Salvianolic acid C (SAC) is a natural compound derived from
Salvia miltiorrhiza that can protect against renal diseases. The aims of this
work were to explore the effect of SAC on kidney tubulointerstitial fibrosis and
study the associated mechanism. Methods: Models for unilateral ureteral
obstruction (UUO) and aristolochic acid I (AAI) were established in mice to study
renal tubulointerstitial fibrosis. Rat kidney fibroblasts (NRK-49F) and human
kidney epithelial cells (HK2) were used as cellular models to evaluate the
effects of SAC on kidney fibrosis. Results: Treatment with SAC for two
weeks reduced the level of renal tubulointerstitial fibrosis in UUO- and
AAI-induced fibrotic kidneys, as demonstrated by Masson’s staining and Western
blot. SAC inhibited extracellular matrix protein expression in NRK-49F cells and
TGF-
Keywords
- renal fibrosis
- EMT
- SAC
- CKD
Chronic kidney disease (CKD) is a major health issue worldwide because of its
high prevalence, bad prognosis, and high medical burden [1]. CKD prevalence has
been increasing recently and has now reached 14.3% worldwide [2]. Renal
tubulointerstitial fibrosis occurs in all CKDs that progress to late-stage kidney
disease [3]. This condition is characterized by pathological accumulation of
extracellular matrix (ECM) proteins, including fibronectin and type 1 collagen
[4]. Signaling through the TGF-
During epithelial-mesenchymal transition (EMT), epithelial cells transform into a mesenchymal phenotype. Upregulation of EMT genes, including N-cadherin and vimentin, represent hallmarks of renal tubulointerstitial fibrosis [7]. A major inducer of EMT is Snail, which plays a major part in organ fibrosis, including renal tubulointerstitial fibrosis [8, 9].
Salvianolic acid C (SAC) is a phenolic acid extracted from Salvia miltiorrhiza [10]. The effect of different components of salvianolic acids on renal fibrosis
has recently been studied. Salvianolic acid B exerts an anti-fibrotic effect on
kidney diseases by regulating the HPSE/SDC1 axis and activating sirt1-mediated
autophagy [11, 12]. Salvianolic acid A ameliorates kidney fibrosis in 5/6
nephrectomized rats through inhibition of nuclear factor kappa-B (NF-
The goal of the current work was to study the effect of SAC on kidney tubulointerstitial fibrosis, as well as the associated mechanism.
SPF grade C57BL/6 male mice were obtained from Shanghai Laboratory Animal Company (Shanghai, China) and housed at Shanghai University of Traditional Chinese Medicine in line with their rules and regulations. The animal ethics committee of the Shanghai University of Traditional Chinese Medicine approved this study (PZSHUTCM18111601).
Male mice weighing from 18 to 22 g were anesthetized intraperitoneally with 8 mg/kg of pentobarbital before performing an abdominal incision on the left side. This was followed by either a sham operation or left ureteral ligation. Animals were assigned into 4 groups at random: (a) Sham + dimethyl sulfoxide (DMSO) (n = 6), (b) Sham + SAC (n = 6), (c) UUO + DMSO (n = 6), (d) UUO + SAC (n = 6). Intraperitoneal injections of DMSO or SAC (10 mg/kg body weight, TopScience, T3149, Shanghai, China) were administered after the surgery and for 14 consecutive days. Mice were sacrificed at day 14, with kidney tissue harvested for protein extraction and tissue embedding.
Mice were injected intraperitoneally with 5 mg/kg AAI (A9451, Sigma, St. Louis, MO, USA) in normal saline (NS) or with NS only once per week for 2 weeks (2 injections in total). One week after the second injection of AAI, the DMSO or SAC (10 mg/kg body weight, TopScience, T3149, Shanghai, China) was injected intraperitoneally and daily for two weeks (n = 6). Mice were sacrificed after the treatment, and kidneys were collected for protein extraction and tissue embedding.
Cells were grown as previously described by our group [18]. Normal rat kidney
interstitial fibroblasts (NRK-49F) rat kidney interstitial fibroblast cells were
obtained from the National Infrastructure Cell Line Resource, Chinese Academy of
Medical Sciences (cat. 3111C0001CCC000413), which has been identified for the species by PCR and was negative for mycoplasma contamination. The cell line has been tested for the mycoplasma and been authenticated from the Chinese Academy of Medical Sciences. The cell line is orginated from American Type Culture Collection, VA, USA (Cat. No. CRL1570). The culture medium for NRK-49F cells was Dulbecco’s Modified
Eagle Medium/Nutrient Mixture F-12 (DMEM/F12) with 10% fetal bovine serum (FBS)
and 0.5% penicillin/streptomycin. NRK-49F cells were grown in 6-well plates
until 60–70% confluence was reached, then starved in medium containing just
0.5% FBS for 12 h. After overnight starvation, fresh medium with 0.5% FBS was
added and the cells treated with DMSO or with various concentrations of SAC
(TopScience, T3149, Shanghai, China). The kidney proximal tubular epithelial cell
line Human Kidney 2 (HK2, cat. SCSP-511) was purchased from the Cell Bank, Shanghai Institute of
Biological Sciences (Chinese Academy of Science), which has been validated by short tandem repeat profiling and was negative for mycoplasma contamination. The cells were grown in 6-well
plates to 40–50% confluence, then starved overnight with DMEM/F12 containing
just 0.5% FBS. The following day, fresh DMEM/F12 with 0.5% FBS was added,
together with 2.5 ng/mL TGF-
Staining with Masson’s trichrome was carried out as described earlier [18]. Briefly, paraffin-embedded renal tissue sections (4 µm) were cut and then stained using hematoxylin and ponceau, followed by incubation with phospho-molybdic acid. Kidney tissue was then stained using aniline blue and acetic acid and photographed using a Nikon 80i microscope (Nikon Eclipse 80i, Tokyo, Kanto Plain, Japan).
Proteins were obtained from the kidney tissue of mice as previously described
[20]. The Bradford method was employed to measure protein concentrations. Samples
were diluted with 5
Immunofluorescence staining of renal tissue was carried out as described previously [21]. In short, paraffin-embedded tissue was cut into 4 µm sections, blocked with 3% BSA, incubated overnight with anti-collagen I antibody (1:500) at 4 °C, rinsed in PBS, then incubated for 60 min with secondary antibody. Cell nuclei were stained with 4′,6-Diamidino-2-phenylindole (DAPI) solution for 10 min and photographed using a Nikon 80i microscope (Tokyo, Japan). Mean fluorescence intensity for each slide was analyzed using Image J and normalized to the control.
TRIzol (15596-018, Invitrogen, Carlsbad, CA, USA) was used to obtain total RNA
from NRK-49F cells. This was reverse transcribed into cDNA using a Takara
PrimeScript RT kit (RR0036A, Kyoto, Japan). Primer sequences for qPCR and
specific for rat were: Fibronectin (F, forward),
5′-CCGAATCACAGTAGTTGCGG-3′; Fibronectin (R, reverse), 5′-GCATAGTGTCCGGACCGATA-3′; Collagen I (F), 5′-TCAAGATGGTGGCCGTTACT-3′;
Collagen I (R), 5′-CATCTTGAGGTCACGGCATG-3′; Collagen III (F), 5′-ATGAATTGGGATGCAACTAC-3′; Collagen III (R),
5′-TCTAGTGGCTCATCATCACA-3′; GAPDH (F),
5′-TAAAGGGCATCCTGGGCTACACT-3′; GAPDH (R),
5′-TTACTCCTTGGAGGCCATGTAGG-3′. qPCR was performed using FastStart
Universal SYBR Green Master (Rox) (Cat. 4913850001; Merck, Darmstadt, Germany)
and StepOne Plus Sequence Detection System (Applied Biosystems, Foster City, CA,
United States). Relative expression levels for each gene were analyzed using
2
Results are given as mean
The effect of SAC on kidney tubulointerstitial fibrosis was first investigated in vivo. UUO is a classic mouse model for studying kidney fibrosis and was established here for this purpose. UUO mice received DMSO or SAC treatment for two weeks. Compared with sham operated mice, significant deposition of collagen was detected using Masson’s trichrome staining in UUO kidneys. However, this was attenuated by two weeks of treatment with SAC (Fig. 1A). Western blot experiments showed that expression of two ECM proteins, fibronectin and collagen I, was up-regulated in mouse kidneys after UUO surgery, but was lowered by treatment with SAC (Fig. 1B). Elevated levels of collagen I expression in the interstitial areas of UUO kidneys relative to sham kidneys was also shown by immunofluorescent staining. Again, this was significantly attenuated by SAC treatment (Fig. 1C).
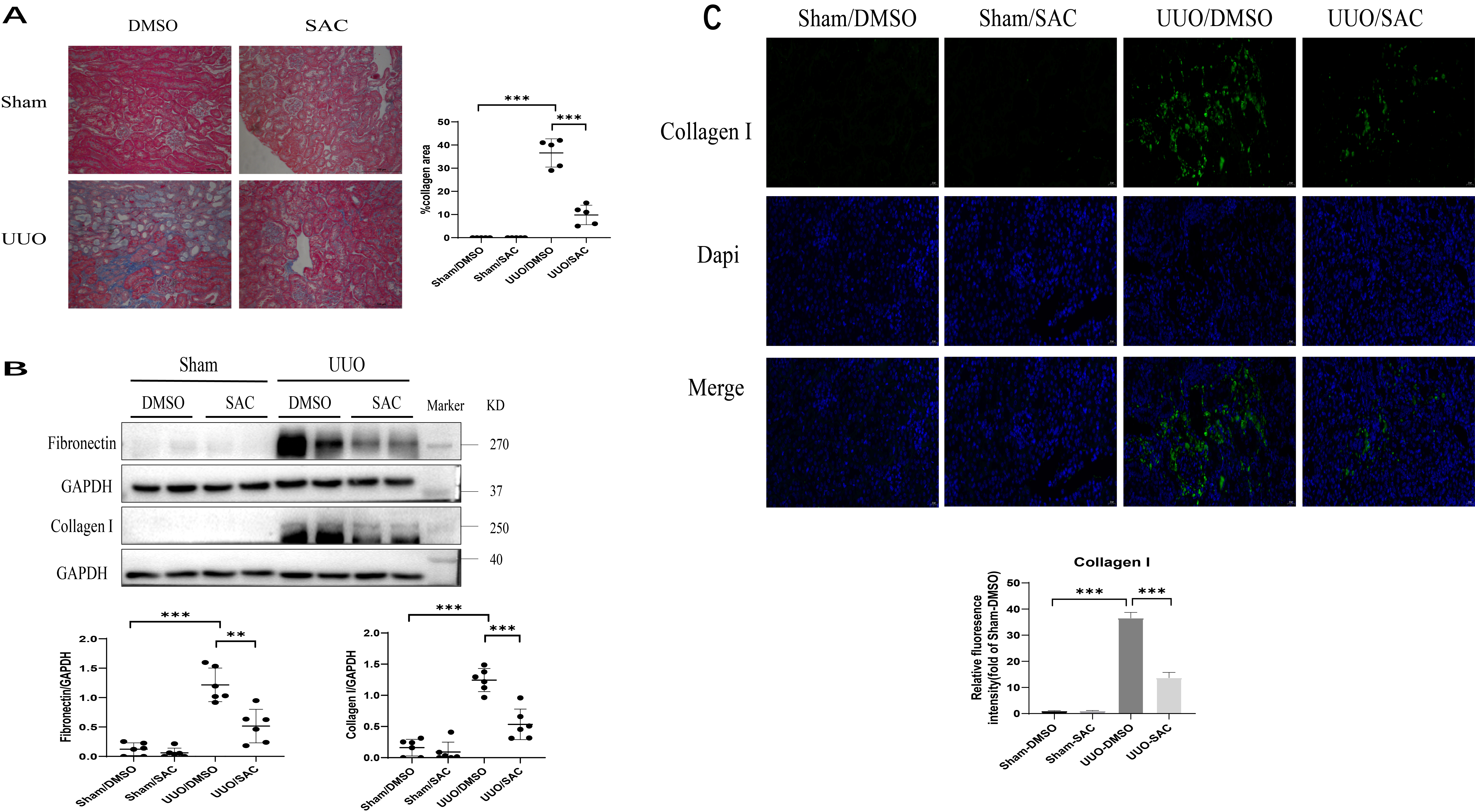 Fig. 1.
Fig. 1.SAC inhibits renal tubulointerstitial fibrosis in obstructed
mouse kidneys. Sham or unilateral ureter obstruction (UUO) operation was
performed on mice (n = 6), followed by two weeks of treatment with DMSO or
salvianolic acid C (SAC). (A) Renal fibrosis was assessed by Masson’s trichrome
staining and then quantified. Bars = 100 µm. (B) The expression of
fibronectin and collagen I were analyzed by Western blotting and then quantified.
(C) Representative immunofluorescent images of collagen I staining in sham or UUO
mouse kidneys. These were also quantified. DAPI (nuclei) = blue, collagen I =
green. Bar = 20 µm.
N = 6 in each experimental group. One representative of
at least three independent experiments is shown. Data represents the
mean
The effects of SAC on anti-fibrosis were further studied in mice with chronic aristolochic acid nephropathy (AAN). Masson’s trichrome revealed mild staining in the interstitial areas of AAN kidneys (Fig. 2A). Treatment with SAC for two weeks significantly reduced the positive areas shown by Masson’s trichrome staining in AAN kidneys (Fig. 2A). Fibronectin and collagen I expression were increased in AAN kidneys relative to the control kidneys. SAC treatment was observed to reduce the expression of these ECM proteins (Fig. 2B). Up-regulation of collagen I in the interstitial areas was further confirmed by immunofluorescent staining observed in AAN kidneys relative to control kidneys (Fig. 2C). SAC treatment significantly reduced positive areas of collagen I staining in AAN kidneys (Fig. 2C).
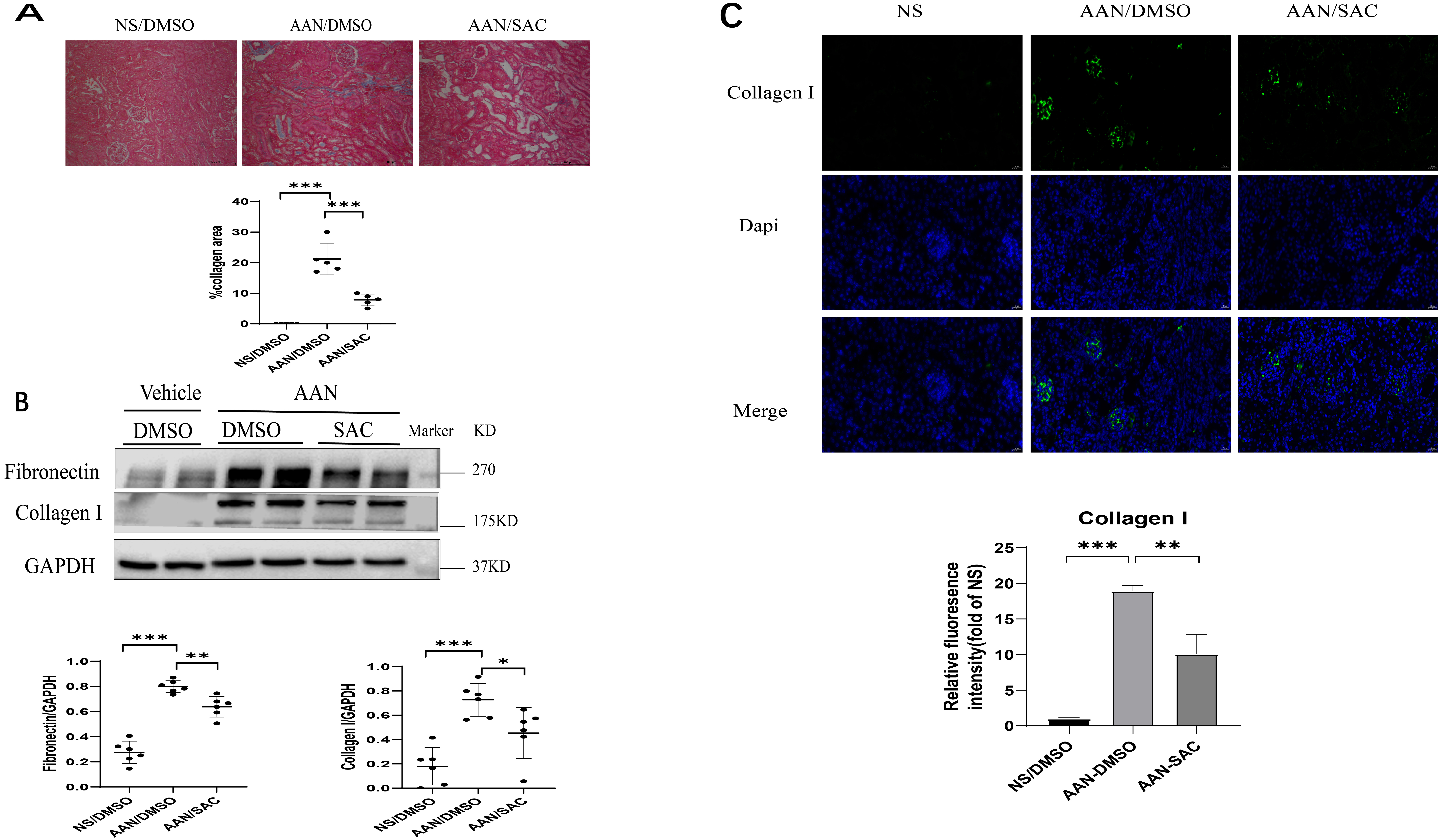 Fig. 2.
Fig. 2.SAC attenuates the development of renal tubulointerstitial
fibrosis in mice with aristolochic acid nephropathy (AAN). Mice (n = 6) were injected
intraperitoneally with aristolochic acid I (AAI) or normal saline (NS) once a
week for two weeks. One week after the second injection of AAI or NS, mice were
treated with DMSO or SAC (10 mg/kg) for two weeks. (A) Renal fibrosis was
assessed by Masson’s trichrome staining and then quantified.
Bar =100 µm. (B) The expression of fibronectin and collagen
I were analyzed by Western blotting and then quantified. (C) Representative
immunofluorescent images of collagen I staining in control or AAN mouse kidneys.
These were also quantified. DAPI (nuclei) = blue, collagen I = green.
Bar =20 µm. N = 6 in each
experimental group. One representative of at least three independent experiments
is shown. Data represents the mean
Direct effects of SAC on kidney cells were further investigated in
vitro by using the rat fibroblast cell line NRK-49F, as well as the human kidney
proximal tubular epithelial cell line HK2. The mRNA levels of fibronectin,
collagen I and collagen III in NRK-49F cells were reduced by SAC in
dose-dependent fashion from 10 to 100 µM (Fig. 3A). Western blot analysis
showed that SAC also dose-dependently reduced fibronectin and collagen I protein
expression in these cells from 30 µM to 100 µM (Fig. 3B). The
dose-dependent (10 µM to 100 µM) inhibition of fibronectin expression
by SAC was further shown in HK2 cells stimulated with TGF-
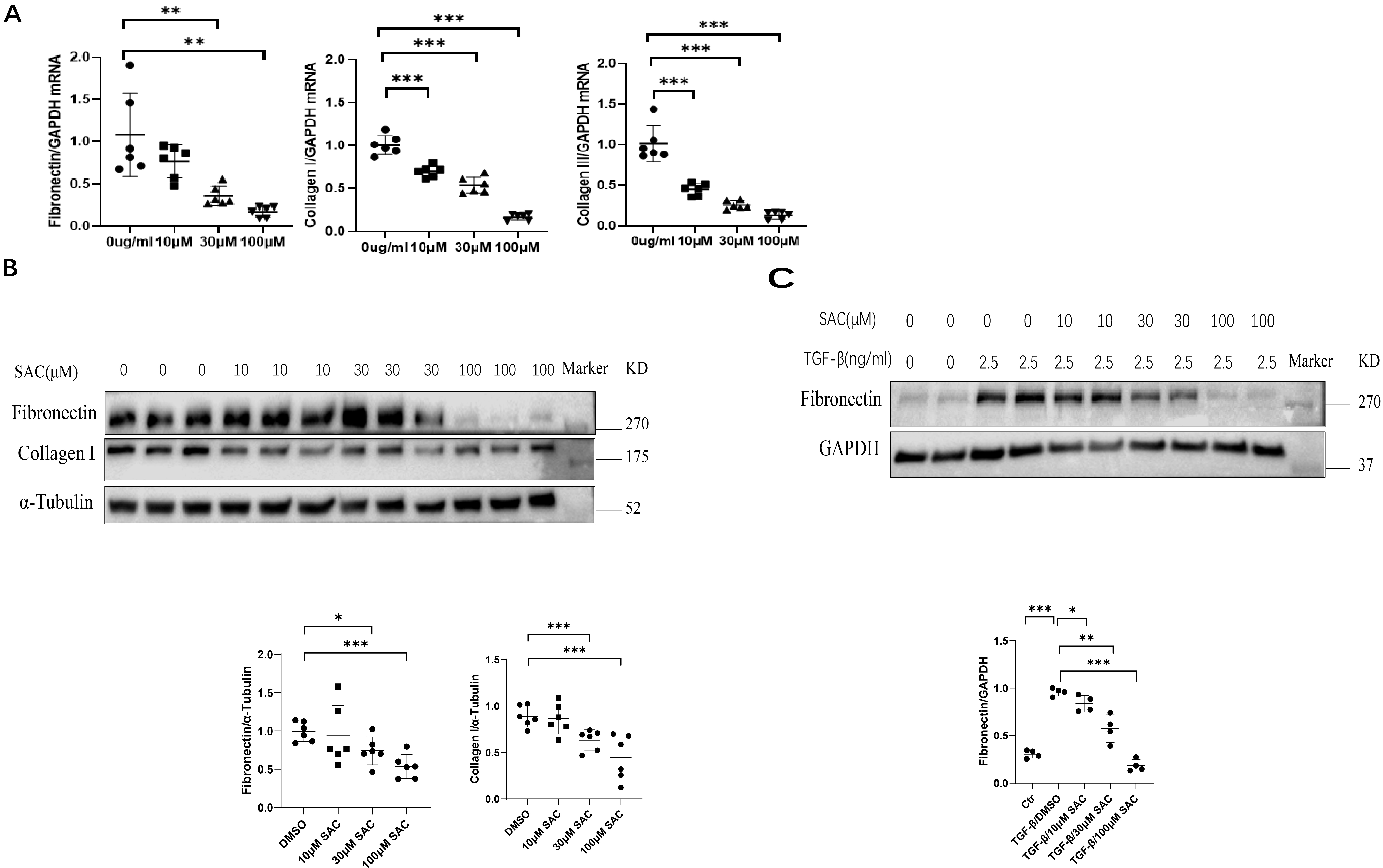 Fig. 3.
Fig. 3.SAC reduces ECM protein expression in renal cells. Rat renal
fibroblasts (NRK-49F) were starved for 12 h, then treated for 24 h with different
concentrations (10 µM, 30 µM, 100 µM) of SAC. (A) The mRNA
expression levels for fibronectin, collagen I and collagen III were analyzed by
qPCR. (B) The expression of fibronectin and collagen I were analyzed by Western
blotting and then quantified. (C) HK2 human renal epithelial cells were starved
overnight, stimulated with TGF-
The effect of SAC on EMT was evaluated in vivo as well as in
vitro. Snail expression in UUO and AAN kidneys was increased compared to their
respective controls (Fig. 4A,B). However, treatment with SAC reduced renal
expression of Snail in these mouse models. Increasing doses of SAC (10 µM
to 100 µM) also progressively inhibited N-cadherin and vimentin expression
in TGF-
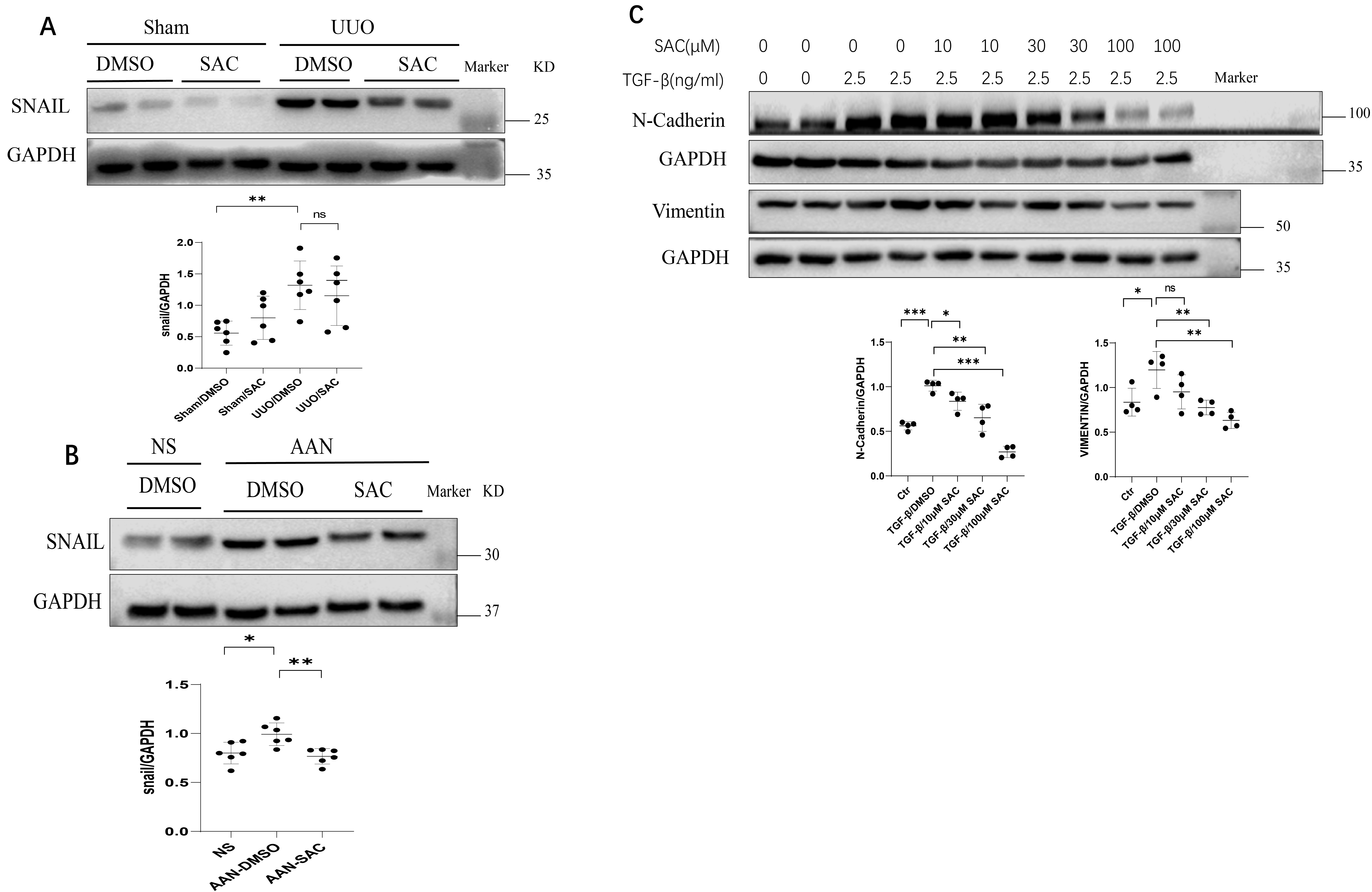 Fig. 4.
Fig. 4.SAC inhibits epithelial-mesenchymal transition in fibrotic
kidneys and renal fibrotic cells. (A) The expression of Snail in sham or UUO
kidneys (n = 6) was analyzed by Western blotting and then quantified. (B) The expression
of Snail in NS or AAN kidneys (n = 6) was analyzed by Western blotting and then
quantified. Data represents the mean
We next evaluated the effects of SAC on Smad3 signaling in vivo and
in vitro. Fig. 5A,B show that Smad3 phosphorylation was enhanced in
fibrotic kidneys of UUO or AAN mice compared to their controls. SAC treatment
decreased Smad3 phosphorylation (pSmad3) levels in UUO and AAN kidneys (Fig. 5A,B). Moreover, SAC inhibited pSmad3 in TGF-
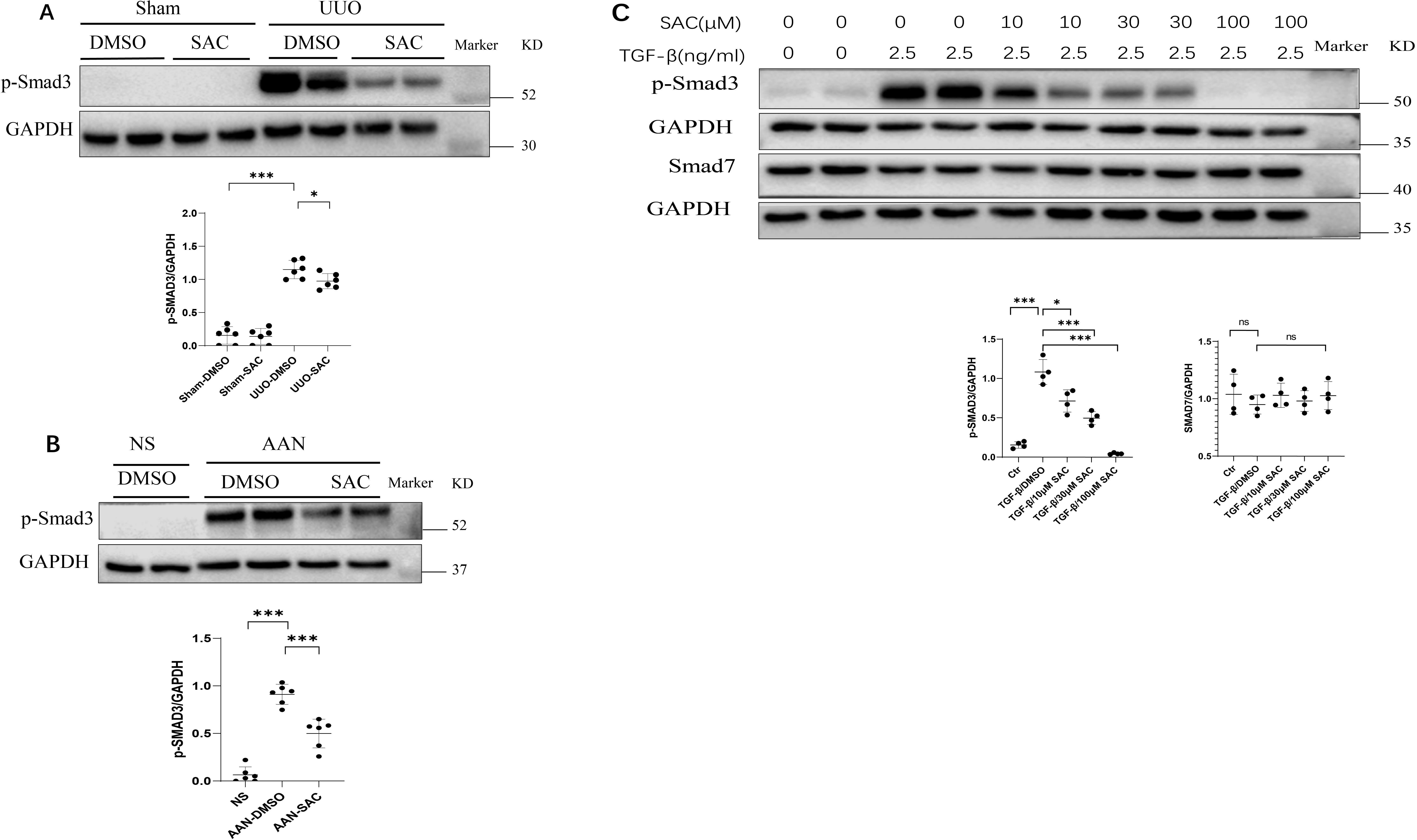 Fig. 5.
Fig. 5.SAC inactivates the Smad signaling pathway in fibrotic kidneys
and renal fibrotic cells. (A) The expression of phosphorylated Smad3 (pSmad3) in
sham or UUO kidneys (n = 6) was analyzed by Western blotting and then quantified. (B) The
expression of pSmad3 in NS or AAN kidneys (n = 6) was analyzed by Western blotting and
then quantified. (C) HK2 human renal epithelial cells were starved overnight,
then stimulated with TGF-
The beneficial effects of SAC were recently demonstrated in cisplatin-induced
acute kidney injury [10]. The effects of SAC on kidney fibrosis, however, are
still unknown. To examine the role played by SAC in kidney tubulointerstitial
fibrosis, we established the UUO and AAN mouse models. We showed that two weeks
of treatment with SAC reduced collagen deposition in UUO or AAN kidneys, as
observed with Masson’s staining. Western blot and immunofluorescence methods
revealed down-regulation of two ECM proteins (fibronectin and collagen I)
following SAC treatment in these two mouse models. The anti-fibrotic effects of
SAC on kidney cells were further studied using renal fibroblasts and
TGF-
TGF-
A characteristic of kidney fibrosis is the upregulation of EMT markers [9]. The
transcriptional factor Snail is an important molecular switch for the EMT program
and has been involved in renal tubulointerstitial fibrosis [9]. Two studies in
gastric cancer and renal fibrosis found that salvianolic acid B inhibits EMT
[12, 29]. In the present study, SAC was observed to reduce the expression of
N-cadherin and vimentin in a dose-dependent fashion in TGF-
We conclude that SAC inhibits EMT and attenuates kidney tubulointerstitial
fibrosis through the signaling pathway for TGF-
The data used to support the findings of this research are available upon request.
MW, DC and CY conceived and coordinated the study. MW wrote the paper. JL conducted the in vitro experiments. MW, DH and JL performed the animal experiments. JL performed and analyzed the Western blotting. All authors reviewed the results and approved the final version of the manuscript.
The animal ethics committee of the Shanghai University of Traditional Chinese Medicine approved this study (PZSHUTCM18111601).
Not applicable.
This work was supported by Key Disciplines Group Construction Project of Pudong Health Bureau of Shanghai (PWZxq2017-07), The Three Year Action Plan Project of Shanghai Accelerating Development of Traditional Chinese Medicine (ZY(2018-2020)-CCCX-2003-08) and National Natural Science Foundation of China (81873617 and 82170747) to CY, Scientific Research Foundation of Shanghai Municipal Commission of Health and Family Planning (201740193) to MW, 2021 Research Project of Blood Purification Center Branch of Chinese Hospital Association (CHABP2021-13) to DC.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.





