1. Introduction
Alongside subcutaneous tissue and skeletal muscles, the skin is an important
structural layer of acupuncture points (acupoints), which are special sites on
the body surface that receive a variety of physical stimuli to modulate
functional disorders of inner organs. Our researches and some other related
studies have shown that subcutaneous mast cells (MCs) are present at greater
densities in acupoints, and their activation plays an initiating role in the
mechanism of acupuncture (AP) [1], an ancient oriental therapeutic approach
gradually becoming more accepted worldwide. During AP, fine needles are inserted
into certain acupoints and subsequent manipulations generate mechanical
stimulation on acupoint, 240–280 mN in force, and 10–15 mN mm
in torque [2]. These signals are transmitted to the wider and deeper space by
subcutaneous collagen fibers twisting around the needles [3]. Our studies
in vitro reveal the mechanosensitivity of MCs and tests performed
in vivo have uncovered such characteristic contributes to AP analgesia
[4, 5]. Additionally, MCs can also be activated by biological agents. Substance P
(SP) and calcitonin gene related peptide (CGRP) released from
the nerve terminals due to needling stimulation can induce adject MCs to
degranulate [6].
Our previous work in vivo had shown that MCs-released histamine was
involved in the trigger mechanism of AP analgesic effect via activating H1
receptors [4]. In the current work, we wondered whether serotonin
(5-hydroxytryptamine, 5-HT) was also involved in this process, which is another
endogenous substance exported from subcutaneous MCs [7]. In rat acupoints,
5-HT-immunopositive MCs are present in subcutis and dermis, and their
degranulation occurs in response to AP [8, 9]. 5-HT has a controversial effect in
pain mechanism, in which pain or analgesia relies on which subtype of 5-HT
receptors (5-HTRs) is activated [10]. Considering the tissue components of
acupoints, 5-HTR and 5-HTR might be expressed [11], and they mediate
the sustained and transient pain processing on rat neurons, respectively [12].
Therefore, the current work tried to address whether MCs-released 5-HT also
contributes to AP-analgesia via binding to some 5-HTRs or 5-HTRs
subtypes.
5-HT-related signaling interacts with purinergic signals that are widely
involved in pain mechanism [13]. Adenosine is a vital mediator for
anti-nociceptive effect in AP analgesia via activating adenosine A1 receptors (A1Rs) [14]. Adenosine
triphosphate (ATP), as the precursor of adenosine, had been found to
mechano-sensitively release from MCs in vitro [5, 15]. Our recent
publication confirmed in vivo the needling-induced interstitial ATP
(inters. ATP) accumulation in the interstitial space of acupoint [16]. In type II
cells of rat’s carotid body, exogenous 5-HT (exo-5-HT) increases [Ca]i
partially via activating pannexin-1 (Panx-1) channels, implying 5-HT might
facilitate ATP release [17]. Hence, we also wondered whether 5-HT is involved in
AP-analgesia by mediating ATP release.
Our results verified that in acute adjuvant arthritis (AA) rat model,
MCs-associated 5-HT accumulated in treated acupoint and contributed to
AP-analgesia. The underlying mechanism might be that mechanosensitive 5-HT
release promote ATP secretion and initiate the downstream signalings.
Although these considerable basic studies have been executed to explore the
underlying mechanisms, it is still not fully understood. A better understanding
of how a fine needle to trigger the anti-nociceptive signals at the acupoint
would shed light on the initiation mechanism of AP and provide guidance for
proper clinical operation.
2. Materials and Methods
2.1 Animals
SPF-grade male Sprague-Dawley (SD) rats weighing 200 20 g were used. The
rats were purchased from Zhejiang Viton Lihua Experimental Animal Technology Co.,
Ltd., and maintained at the Animal Experimental Center of Shanghai University of
Traditional Chinese Medicine. We performed the animal experiments in accordance
with the procedures approved by the Animal Experimental Center (Animal Ethics
No.: PZSHUTCM200911014) and implemented the relevant provisions from the Guidance
Suggestions for the Care and Use of Laboratory Animals formulated by the Ministry
of Science and Technology of the People’s Republic of China.
2.2 Rat Model of AA
We used the rat model of AA established as previously described [16]. Briefly,
50 L complete Freund’s adjuvant (CFA) (Sigma-Aldrich, St. Louis, MO, USA)
was injected into the left ankle joint cavity of rats anesthetized with 1.5%
isoflurane. Local swelling and behavioral disability appeared within 24 h.
2.3 AP Treatments
A single course of AP treatment for 20 min was performed at Zusanli (ST 36)
acupoint of the affected side of conscious rats. According to our researches and
related studies [4], the ST 36 has a desirable anti-nociceptive effect on AA
rats. ST 36 is located at the posterolateral side of knee joint, about 5 mm below
the fibula capitulum. A stainless-steel needle (0.18 mm 13 mm, Cloud
Dragon Medical Equipment Company, Wujiang, China) was gently inserted to an
approximate depth of 7 mm. AP procedure was dominated by twirling-rotating motion
(~100 times/min), supplemented with lifting-thrusting motion
(~80 times/min).
2.4 Behavioral Tests
Pain thresholds of the injured hind plantar were determined to reflect the pain
levels. Mechanical allodynia (paw withdrawal threshold, PWT) and thermal
hyperalgesia (paw withdrawal latency, PWL) were evaluated to determine the pain
level. The timeline and methods for behavioral tests was referred to our
published work [16, 18]. Briefly, CFA was administrated on day 0 and AP was
performed 2 days later. Pain thresholds were determined for three times, just
before CFA injection, just before AP intervention, and after 30 min-AP treatment.
Thermal hyperalgesia was measured 2 h after the mechanical allodynia assessment.
2.5 Reagents and Solutions
All stock solutions were stored at –20 °C and diluted into working solutions to
final concentrations when used. Sodium cromolyn (CRO, final
concentration: 0.02 g/mL, 20 L/paw, #C0399, Sigma-Aldrich, St. Louis, MO,
USA), a MC stabilizer was pre-injected into the left ST 36 in layers 10 min prior
to AP treatment, with half receiving 5 mm subcutaneous injections
into the muscle and the other half receiving 2 mm subcutaneous
injections under the dermis. Granisetron hydrochloride injection (Gran., 1
mg/mL, 20 L/paw, National medicine permission number:
H20043610) was procured from Guorui Pharmaceutical Co. Ltd.
(China National Pharmaceutical Group, Beijing, CN). Methiothepin mesylate (Meth., #T2190,
TopScience Co., Ltd., Shanghai, CN) were dissolved in dimethyl sulfoxide
(DMSO), and the final concentration was 5 mg/mL, 20 L/paw.
WAY-100635 (WAY., 2 mg/mL, 20 L/paw, #W108, Sigma-Aldrich,
St. Louis, MO, USA), ARL67156 (ARL, 100 M, 50 L/paw, #A265,
Sigma-Aldrich, St. Louis, MO, USA), 2-Chloro-N6-cyclopentyladenosine (CCPA, 0.04
mg/mL, 20 L/paw, #C7938, Sigma-Aldrich, St. Louis, MO, USA),
Suramin (100 M, 50 L/paw, #574625,
Sigma-Aldrich, St. Louis, MO, USA) and Pyridoxal phosphate-6-azo tetrasodium salt
hydrate (PPADS, 100 M, 50 L/paw, #P178, Sigma-Aldrich, St. Louis,
MO, USA) were prepared with distilled water. All the agonists and antagonists
were pre-injected into the left ST 36 in layers prior to AP treatment. CRO (25
M), Compand 48/80 (16 M, #C2313, Sigma-Aldrich, St. Louis, MO,
USA), Meth. (100 nM), Gran. (100 nM) and WAY. (100 nM) were introduced to cells
20 minutes prior to the application of mechanical stimulation or exo-5-HT and
were present during the entire process. Preparation of bath solution and 50%
hypotonic solution was described in our previous work [5].
2.6 Acupoint Injection
In order to intervene certain corresponding signals at the acupoints, 20–50
L related reagents were pre-injected into the left ST 36 in layers 20 min
prior to AP treatment.
2.7 Microdialysis
The performance of microdialysis technology referred to our published work [19].
Briefly, after rat was anesthetized with 1.5% isoflurane, a linear microdialysis
probe (CMA 31, CMA Microdialysis AB, Kista, Sweden) was implanted at a distance
of 4–6 mm from ST 36. The dialysis solution, Hank’s balanced salt solution
(HBSS, Corning Cellgro, Manassas, VA, USA), was controlled by syringe pumps
(NE-1000, New Era Pump Systems, Farmingdale, NY, USA) and continuously perfused
at a rate of 1 L/min. Subsequently, rat was restrained in the rat fixator
and was gradually awakened from anesthesia. After a 2 h recovery period,
microdialysis outflow was collected for 30 minutes per sample.
2.8 Determination of 5-HT, ATP and Adenosine in the Microdialysates
5-HT and ATP in the microdialysate were determined by ELISA kit (HZ-5-HT-RA,
Zhen Shanghai and Shanghai Industrial Co., Ltd., CN) and luciferin-luciferase
assay (L-L, Sigma-Aldrich, St. Louis, MO, USA), respectively. The procedures were
performed according to the manufacturer’s instructions. The light emission was
measured by a microplate reader (Synergy Mx, BioTek, Winooski, VT, USA).
Calibrations were executed before and after each dertermination.
Adenosine levels was performed by high-performance liquid chromatography (HPLC)
as previously described [4]. The adenosine standard was checked both before and
after the measurement, and the concentrations were 3000 nM, 1000 nM, 300 nM, and
100 nM. The detection results of the animal samples were compared to the standard
according the peak areas, and the concentration was calculated.
2.9 Tissue Sectioning and MC Staining
Tissue sectioning and MC Staining referenced to our previous article [4].
Briefly, after the final behavioral test, a 5 5 5 mm
specimen of ST 36 was taken and 5 m paraffin sections were prepared. After
staining with toluidine blue, the MCs were dark purple in color. MCs with a
blurred boundary, or dispersed granules were counted as degranulated MCs.
2.10 Western Blotting
The performance of western blotting referred to related research [20]. We used
rabbit monoclonal antibodies: GAPDH (#ab8245, Abcam, Waltham, MA, USA), 5-HTR
(#ab85615, Abcam, MA, USA), 5-HTR (#ab140486, Abcam, MA, USA),
5-HTR (#PA1-41069, Thermo Fisher Scientific, Waltham, MA, USA), 5-HTR
(#PA5-106542, Thermo Fisher Scientific, MA, USA), Connexin 43 (#71-0700, Thermo
Fisher Scientific, MA, USA), Pannexin 1 (#488000, Thermo Fisher Scientific, MA,
USA) and P2Y (#P6487, Sigma-Aldrich, St. Louis, MO, USA). The proteins
were then incubated with peroxidase-conjugated goat anti-rabbit IgG (#7074, Cell
Signaling Technology, MA, USA). A visualizer was then used to observe the stripe,
and the grayscale value was calculated using Image J v1.8.0 (National Institutes of
Health, Bethesda, MD, USA).
2.11 Cell Origin and Culture
The human MC (HMC-1) cell line was kindly provided by Dr J. H. Butterfield (Mayo
Clinic) and cultured in Roswell Park Memorial Institute (RPMI) 1640 medium. Rat basophilic leukemia (RBL-2H3) cell line
was purchased from the Cell Bank of Chinese Academy of Sciences (Shanghai, CN)
and cultured in Minimum Essential Medium (MEM) medium. The culture medium was supplemented with 10% Fetal Bovine Serum (FBS), 100
units/mL penicillin and 100 units/mL streptomycin in a 5% CO humidified
atmosphere at 37 °C.
2.12 Mechanical Stimulation
Hypotonic shock was introduced to HMC-1 cells to induce degranulation, and
tryptase or ATP release. The entire procedure referenced to our article [15].
Briefly, 1–2 mL of HMC-1 cell suspension (0.5–1.5 10 cells/mL)
was gently introduced into the polycarbonate filter chamber, and superfused with
bath solution at 1.3 mL/min. After an equilibration period, hypotonic solution
(200 mOsm/Kg HO) was introduced to the cells and the perfusate was
collected at 1 min. Hypotonic environment is a common method to stimulate cells
with force [21]. The cell becomes swollen and its cellular membrane is extended,
then the corresponding downstream signals are initiated. In our previous work, we
used hypotonic shock to activate mast cells in vitro to mimic manual AP
[5, 15, 22]. Tryptase or ATP were assessed by ELISA (#IMM001, Sigma-Aldrich, St.
Louis, MO, USA) and luciferase-luciferin assay. For 5-HT release measurements, we
stimulated the cells by medium displacement. HMC-1 cells grew in the 96-well
plate containing 100 L culture medium. Half of the medium was gently
pipetted up and down ten times with a pipette gun. Subsequently, 5-HT in the cell
suspension was qualified by ELISA assay.
2.13 Exo-5-HT Stimulation
For each experiment, RPMI 1640 and MEM medium was used to further dilute 10 mM
5-HT stock solutions to acquire various required concentrations of 5-HT.
5-HT-sensitive ATP released from HMC-1 cells in response to exogenous 5-HT
(exo-5-HT, #H9523, Sigma-Aldrich, St. Louis, MO, USA) in the range of 1 nM–10
M for 15 min. Released ATP levels in the fractions were quantified by L-L
assay, as described in 2.8. Based on our preliminary results, when exo-5-HT
concentration 50 M, cell mortality tended to be above 80%, and
the large increase of ATP at this time might be caused by cell death.
2.14 Statistical Analysis
The measurement data are expressed as the mean standard error (SE)
values. The data were analyzed using SPSS 25.0 (IBM Co., Armonk, NY, USA). The
figures were prepared by GraphPad Prism 8.0 software (GraphPad Software, San
Diego, CA, USA). Differences among multiple groups were tested with one-way or
two-way analysis of variance (ANOVA), followed by Fisher’s least significant
difference (LSD.) test. Where only two groups were compared, a two-sample
t-test was used. When data were non-normally distributed, a Mann-Whitney
U test was performed. We considered p 0.05 to be statistically
significant.
3. Results
3.1 5-HT Released from MCs in Response to AP and Contributed to
Analgesic Effect
We revealed that 30 min-needling caused 5-HT accumulation in the interstitial
space of treated acupoint. Inters. 5-HT concentration remarkably increased from
5.5 ng/mL 0.2 ng/mL (time = 0) to 11.4 ng/mL 0.4
ng/mL (t = 30 min) (n = 4, p 10) gradually restored
to the baseline level (Fig. 1A). Inters. 5-HT amount was significantly
potentiated from baseline of 156.8 pg 5.8 pg (-30–0 min) to 252.5 pg
7.2 pg (0–30 min) (p = 1.2 10, n = 4) (Fig. 1B). Such potentiation effect lasted for at least 120 min. AP-induced 5-HT
mobilization was canceled by the presence of CRO (0.02 g/mL, 20 L), a
stabilizer of MCs, which was pre-injected in ST 36 before AP intervention (Fig. 1A,B). These results suggest that needling activate MCs in acupoint to release
5-HT.
 Fig. 1.
Fig. 1.
AP mobilized 5-HT release from MCs in acupoint, which
contributed to AP-analgesic effect (n = 4–10, mean SE). (A) Time-course
of inters. 5-HT concentration in response to needling on AA model rats in the
absence and presence of CRO (0.02 g/mL, 20 L) pre-injected in ST 36 (n =
4). Inters. 5-HT was collected by microdialysis technique and determined by
ELISA. ### p 0.001 vs. baseline (time = 0 min); ** and ***,
p 0.01 and 0.001 vs. +CRO, respectively. Two-way ANOVA and
LSD. test were used. (B) Changes of inters. 5-HT amount in response to
AP in the absence and presence of CRO (n = 4). They were calculated based on the
area under the curve in (A). ## and ###, p 0.01 and 0.001 vs.
baseline (time = -30–0 min); *, ** and ***, p 0.05, 0.01 and
0.001 vs. +CRO, respectively. (C,D) Effects of AP treatment on CFA-induced
tactile allodynia (PWT) and thermal hyperalgesia (PWL) of injured-side hindpaws,
respectively. ### p 0.001 vs. baseline (Day 0).
p 0.001 vs. Day 2, before AP.
Two-way ANOVA and LSD. test were used to perform within-group
comparisons. (E,F) Effects of pre-co-injection of Methiothepin (Meth.) and
Granisetron (Gran.) in ST 36 on AP-relieved PWT and PWL, respectively. Data
illustrate the final behavioral tests. ** and ***, p 0.01 and 0.001,
respectively. One-way ANOVA and LSD. test were used to perform
between-group comparisons. (G) AP/Mechanical stimulation induced MCs to
degranulate. Allows point to degranulated MCs. (i) Paraffin sections prepared
from ST36 of AA model group (left) and AP group (right). MCs were stained with
toluidine blue and were marked in purple. (ii) Connective tissue slices isolated
from ST 36 of normal rat before (left) and after (right) hypotonic shock. (iii)
HMC-1 cells before (left) and after (right) hypotonic shock. In (ii) and (iii),
the tissue slice and HMC-1 cells were incubated in 200 mOSm/kg H2O-hypotonic
solution for 25 min and 15 min, respectively. The plasma membrane of degranulated
MCs became rough and the granules scattered around the cells. Scale bar: 50
m. Mech. stim.: Mechanical stimulation. (H) Release of tryptase, ATP and
5-HT from HMC-1 cells in response to 50% hypotonic shock (n = 7–10).
Unpaired t-test was performed for two groups’ comparison. *** p 0.001. In (E–G), numbers above each column denote sample size (n).
Behavioral test revealed that CFA-treated rats suffered from pain
hypersensitivity, exhibiting tactile allodynia (PWT) (reduced from 54.9 g
1.1 g to 11.1 g 1.4 g, n = 6, p = 2.4 10 vs.
baseline) and thermal hyperalgesia (PWL) (reduced from 19.6 s 0.8 s to
6.6 s 0.1 s, n = 6, p = 3.6 10 vs. baseline),
but clearly benefited from 30 min-AP at ST 36 (PWT: 43.2 g 2.8 g, n = 6,
p = 2.4 10 vs. before AP; PWL: 15.7 s 1.4 s,
n = 6, p = 1.7 10 vs. before AP) (Fig. 1C,D).
Based on our previous research, the microdialysis recovery percentage was in the
range of 25–40% [19]. Considering 20 dilution by diffusion and/or
convection into the bulk extracellular volume [19, 23], the
real concentration of 5-HT at the cell surface near the AP site was more than
500 ng/mL (2.8 M), indicating some 5-HT receptors might be activated [17, 24, 25]. Considering the expressions and effects in pain processing in acupoints
[11, 12], we tried to verify the role of 5-HTRs and R. Co-injection
of Methiothepin (5 mg/mL, 20 L), a nonselective
antagonist of 5-HTRs, and Granisetron (1 mg/mL, 20
L), a selective antagonist of 5-HTR, in ST 36 prior to AP suppressed
the anti-nociceptive effect of AP on PWT (13.2 g 1.0 g, n = 6, p
= 1.3 10 vs. AP) and PWL (6.3 s 0.7 s, n = 6,
p = 0.0014 vs. AP) (Fig. 1E,F). Additionally, compared
with normal rats, CFA induced more 5-HT mobilization in rats
(Supplementary Fig. 1), suggesting MCs in acupoint changed dynamically
with the ankle arthritis.
AP-induced subcutaneous MCs degranulation is the basis for triggering the
analgesic mechanism [3, 4], which was confirmed in the current work (Fig. 1G,i).
In addition, MCs in the connective tissue slice ex vivo prepared from
rat ST 36 (Fig. 1G,ii) and cultured HMC-1 cells in vitro (Fig. 1G,iii)
also degranulated in response to mechanical stimulation of hypotonic shock (200
mOsm/Kg HO) that was used to mimic needling stimulation. Determination of
the mediator in cellular suspension showed that when HMC-1 cells were perturbed
with 50% hypotonic shock, tryptase release, a classic index
for MCs degranulation [26], was potentiated from 23.5 ng/mL 2.4 ng/mL of
control to 41.5 ng/mL 2.9 ng/mL (n = 10, p = 0.0004, vs.
Control) (Fig. 1H). In addition, secretion of stress-sensitive molecular ATP and
5-HT were also strengthened (ATP: from 16.1 1.2 ng/mL of control to 32.1
3.2 ng/mL, n = 8–10, p = 0.0001 vs. Control; 5-HT: from 4.1
ng/mL 0.2 ng/mL of control to 7.4 ng/mL 0.3 ng/mL, n = 7–8,
p = 1.1 10 vs. Control) (Fig. 1H). 5-HT is well known
to be released in the manner of degranulation [27]. While, unexpectedly,
mechanosensitive ATP release was neither suppressed by CRO nor enhanced by
Compand 48/80 (Supplementary Fig. 2A), but tryptase release was
remarkably affected (Supplementary Fig. 2B), indicating that ATP
secreted from MCs in a degranulation-independent manner.
3.2 5-HT Induced ATP Release from MCs via Acivation of 5-HTR
5-HT is reported to induced ATP release from type II cells of the rat carotid
body [17]. Mechanosensitive ATP release is discovered in various mammalian cells
[21], including MCs [5]. Needling-induced ATP transient accumulation in treated
acupoint is supposed as one of the primary steps toward AP-analgesic mechanism by
providing the precursor for adenosine [16, 19]. Besides primary mechanosensitive
ATP release, secondary ATP secretion had been found in MCs in our previous work
[15]. Subsequently, we tried to verified whether 5-HT could mediate MCs to
release ATP. Fig. 2A shows the dependency of ATP generation on the concentration
of exogenous 5-HT (exo-5-HT) (1 nM–10 M). When the concentration of
exo-5-HT reached 1 M and 10 M, extracellular ATP level was
significantly potentiated from baseline of 15.7 nM 2.5 nM to 43.6 nM
5.3 nM and 107.3 nM 3.4 nM, respectively (Fig. 2A). 5-HT
concentration greater than 10 M was not determined because cells viability
was affected (Supplementary Fig. 3).
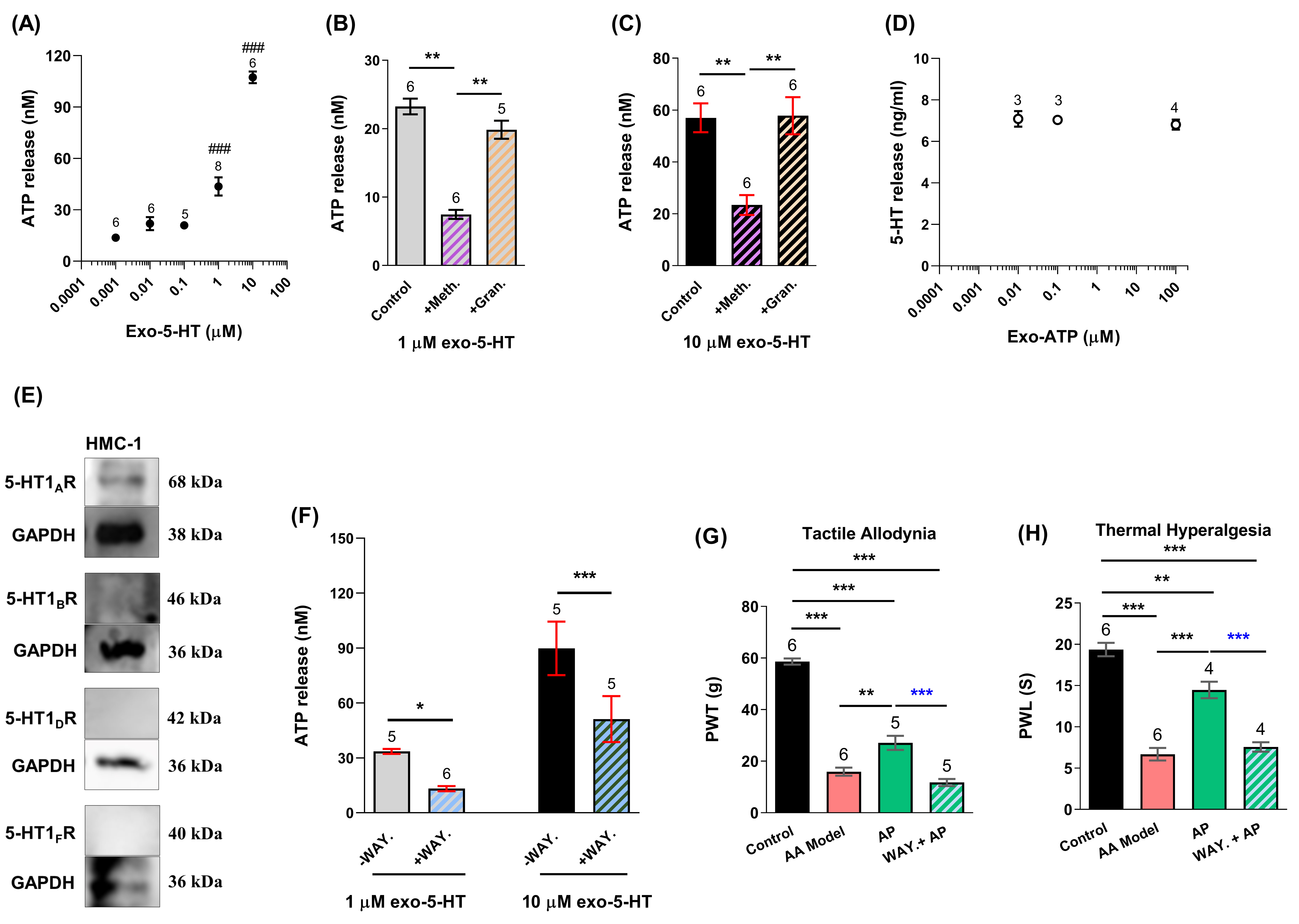 Fig. 2.
Fig. 2.
5-HT receptors mediated ATP release and contributed to AP
analgesia (n = 3–8, mean SE). (A) Concentration-dependent mediation of
ATP release from HMC-1 cells by 1 nM–10 M exo-5-HT). ### p
0.001 vs. baseline (absent of exo-5-HT). (B,C) Effects of 5-HT receptors
inhibition on exo-5-HT induced ATP release from HMC-1 cells. Meth. (100 nM) or
Gran. (100 nM) was introduced to cells 10 min before 5-HT intervention and was
present during the entire process. ** p 0.01. (D) Response of 5-HT
release from HMC-1 cells to 10 nM–100 M exogenous ATP intervention. (E)
WB determination of 5-HT subtypes identification on HMC-1 cells.
These are representative blots from n = 4–6 recordings. (F) Effects of
5-HTR inhibition on exo-5-HT induced ATP release from HMC-1 cells.
WAY-100635 (WAY., 100 nM) was introduced to cells 10 min before exo-5-HT
intervention and was present during the entire process. * p 0.05 and
*** p 0.001. (G,H) Effect of 5-HT receptor-inhibition on
AP-relieving tactile allodynia and thermal hyperalgesia of the injured plantar,
respectively. WAY. (2 mg/mL, 20 L) was pre-injected at ST 36. Data
illustrate the final behavioral tests. ** and ***, p 0.01 and 0.001,
respectively. Numbers above each column/circle denote sample size (n). In (F),
Unpaired t-test was performed. In the rest panels, one-way
ANOVA and LSD. test were used.
Pretreatment HMC-1 with Methiothepin (100 nM) significantly impaired ATP release
induced by 1 M- and 10 M-exo-5-HT as well. In contract, Granisetron
(100 nM) had null effect (Fig. 2B,C). Similar results were also obtained from rat
MCs, RBL2H3 cell line (Supplementary Fig. 4). However, exogenous ATP had
null effect on 5-HT release (Fig. 2D). These results suggest that 5-HT mediates
ATP release from MCs via activating 5-HTRs, especially 5-HTRs.
5-HTRs family comprises A, B, D, E and F subtypes. Currently, all subtypes
except for E have been reported to be involved in transducing of peripheral pain
[28]. Among them, subtype A has been repeatedly confirmed to be expressed in
human and rat MCs. Our WB determination revealed that
5-HTR markedly expressed in HMC-1 cells (Fig. 2E). Subsequently, we
examined the effect of WAY-100635 (WAY.), a specific antagonist of 5-HTR.
The result uncovered that pretreatment HMC-1 with WAY. (100 nM) significantly
impaired ATP release induced by 1 M (n = 5–6, p = 2.8
10 vs. -WAY.) and 10 M (n = 5, p = 0.0020 vs. -WAY.)
exo-5-HT (Fig. 2F). Taken together, these results indicate that 5-HT mediates ATP
release from MCs via activating 5-HTR subtype.
Based on these findings, we returned to the test in vivo. Behavioral
tests found that pre-injection of WAY. (2 mg/mL, 20 L)
in ST 36 canceled AP analgesic effect on PWT (n = 5–6, p = 8.1
10 vs. AP) and PWL (n = 4–6, p = 0.0003 vs. AP) (Fig. 2G,H). Taken these results together, we infer that contribution of MC-associated
5-HT release to AP analgesia mechanism might be due to mediate ATP release from
MCs or other adjacent cells by activating 5-HTR.
3.3 Needling-Induced Inters. ATP Mobilization in Acupoint was
Involved in AP Analgesia Possibly via Producing Adenosine or Propagating AP
Signals
AP-induced adenosine accumulation and activation of local adenosine ARs
contribute to initiate analgesia [14]. Such adenosine mobilization might
partially rely on the hydrolyzation of ATP. Our microdialysate determination
revealed that both adenosine and ATP in the interstitial space of treated
acupoint were significantly potentiated by needling (adenosine, from
baseline of 58.4 nM 6.9 nM to 76.8 nM 9.1 nM, n
= 9, p = 0.0250; ATP, from baseline of 3.4 nM 1.4 nM to 56.0 nM
12.5 nM, n = 4, p = 0.0269). More importantly, the presence of
ARL (100 M, 50 L), a non-specific inhibitor of ecto-ATPase, in
acupoint further potentiated AP-induced ATP accumulation (136.3 nM 22.7
nM, n = 4, p = 0.0258 vs. AP group) (Fig. 3A), indicating the activities
of ecto-ATPase and hydrolyzation of inters. ATP existed in the treated acupoint.
Further behavioral tests demonstrated that AP analgesic effect was impaired by
ARL (100 M, 50 L) (n = 4, p = 0.0233), and was duplicated
by CCPA (0.04 mg/mL, 20 L), an agonist of adenosine A1Rs (n = 5,
p = 0.9773) (Fig. 3B). Taken together, these findings suggest that
mobilization of inters. ATP via primary or secondary release contributes to
anti-nociceptive mechanism of AP by promoting adenosine production.
 Fig. 3.
Fig. 3.
AP-induced the mobilization of adenosine (Ado) and inters. ATP in the
treated acupoint contributed to the anti-nociceptive effect of AP (n = 4–9, mean
SE). (A) Changes of Adenosine and ATP levels in the interstitial space in
ST 36 of AA model rats before (-AP) and after (+AP) needling. Adenosine was
determined with HPLC. ATP was assayed with L-L method. ARL (100 M, 50
L) was pre-injected at ST 36 20 min prior to AP. * p 0.05 by
paired t-test. # p 0.05 by unpaired t-test. (B)
Involvement of inters. ATP hydrolyzation and Adenosine A1 receptors in
AP-analgesic effect. ARL (100 M, 50 L) or CCPA (0.04 mg/mL, 20
L) was pre-injected at ST 36. ** and ***, p 0.01 and 0.001,
respectively. (C,D) Effects of P2 receptor-inhibition on AP-analgesia in tactile
allodynia and thermal hyperalgesia, respectively. *, ** and ***, p
0.05, 0.01 and 0.001, respectively. In (B–D), data illustrate the final
behavioral tests and one-way ANOVA and LSD. test were used.
Numbers above each column denote sample size (n).
Calcium wave propagation (CWP) is supposed as the basis of ATP
regeneration, which facilitates the localized propagation of biological signals
[29]. CWP has been uncovered in mouse treated acupoint [30]. Our previous work
had found a similar CWP and cascaded ATP release in HMC-1 cells when perturbed by
hypotonic shock, in which primary mechanosensitive ATP released induced secondary
ATP release via activating P2Y or P2X receptors [15].
The present work found an additional 5-HT-induced secondary ATP release. We
hypothesized that besides as the precursor of adenosine, such cascaded ATP
release could amply and propagate the localized needling signals. Our WB
determination revealed that some components for forming CWP/ATP regeneration,
including Panx-1, connexin 43 and P2Y were expressed in HMC-1 cells
(Supplementary Fig. 5). Subsequently, further behavioral tests observed
that inhibition of local purinergic receptors (P2 receptors) by Suramin (100
M, 50 L) (Relative PWT: reduced from 0.9
0.0 to 0.7 0.1, n = 4, p = 0.0057 vs. AP and Relative PWL:
reduced from 0.9 0.0 to 0.6 0.0, n = 4, p = 0.0269 vs.
AP) or PPADS (100 M, 50 L) (Relative PWT: reduced from 0.9
0.0 to 0.7 0.1, n = 4, p = 0.0009 vs. AP and Relative PWL:
reduced from 0.9 0.0 to 0.8 0.1, n = 4, p = 0.0749 vs.
AP) partially suppressed the AP-relieved tactile allodynia and thermal
hyperalgesia (Fig. 3C,D). This finding implies that besides adenosine and A1Rs,
ATP/ADP and related P2 receptors in acupoint also play a role to mediate AP
analgesia and its underlying mechanism might be the ATP-related CWP.
4. Discussion
4.1 Local MC-Associated 5-HT Reflected the State of Acupoints
5-HT-immunopositive MCs are present in acupoints and become more when the
acupoints are sensitized [8, 9]. Acupoint sensitization means acupoints
transformed dynamically from a “silent” state to an “activated” one when the
body is suffering from some corresponding diseases [31]. Neurogenic inflammation
is one of the biological bases for acupoint sensitization due to
dorsal root reflex or axon reflex [32, 33]. In the present work, we noticed that
compared to normal rats, ST 36 in model group had higher inters. 5-HT level at
baseline (Supplementary Fig. 1). Here, the onset site and treated
acupoint are innovated by tibial nerve and peroneal nerve, respectively, branches
of sciatic nerve, suggesting that they are innovated by the same or adjacent
peripheral ganglia and spinal segments. Similarly, in knee osteoarthritis rats,
5-HT together with tryptase and histamine are upregulated in the nearby
acupoints, Yanglingquan acupoint and Heding acupoint rather than Weizhong
acupoint [34]. The greatest benefit of acupoint sensitization is to facilitate AP
to exert better therapeutic effect. Our previous work had revealed that AP
modulated higher ATP accumulated in AA rats than in normal ones [19]. Systematic
review demonstrates the comparative superiority of needle stimulation of
sensitized points over non-sensitized points, especially for some pain syndromes
[31].
Degranulation of local subcutaneous MCs induced by needling has been often
reported [1]. While the certain associated mediators are not well quantitatively
studied. Beside adenosine assessed in our previous work [4], the current study
clearly recorded MC-associated 5-HT mobilization (Fig. 1A,B). Based on the
microdialysis recovery percentage [19] and considering dilution by diffusion
and/or convection into the bulk extracellular volume [23], the real concentration
of inters. 5-HT at the cell surface was more than 500 ng/mL (2.8 M). This
concentration is within the range we used in our test in vitro (1 and 10
M), which is sufficient to activate 5-HTR (Fig. 2F). Additionally,
this concentration range can activate 5-HTR [24], 5-HTR [17] and
5-HTR [25], implying various functions are involved, for example, influence
the differentiation, proliferation, migration, adherence and life-span of skin
cells, regulate the tonus of blood vessels and influence nerve transmission [35].
The potentiated inters. 5-HT could last for more than 2.5 h (Fig. 1A,B). It might
contribute to the maintenance of AP effect.
4.2. 5-HT Induced ATP Release by Activating 5-HTR
The activation of MCs can easily establish a positive feedback
loop. Matrix metalloproteinase-9 [36] or ATP [15] secreted from MCs, in turn,
activate MCs. In the present work, unidirectional regulation of 5-HT on ATP
release via activation of 5-HTR was another example of this
self-activation mechanism (Fig. 2A). Other cells can also be activated, as
long as they express 5-HTR, for example, melanocytes [37]. Similar finding
has been reported in type II cells of rat’s carotid body, in which exo-5-HT
increases [Ca] partially via activating Panx-1 channels, implying
5-HT might facilitate ATP release [17]. Although we demonstrated that MCs express
Panx-1 channels (Supplementary Fig. 5), whether they assisted ATP
release still need further determination.
Additionally, interaction between 5-HT mediated signaling and ATP-related P2X
receptors have been characterized. While it is mainly inhibitory rather than
facilitative. In submucosal neurons of guinea pigs, whole-cell recordings reveal
that 5-HTRs and P2X channels negatively modulate each other when they are
simultaneously activated [38]. 5-HTR and P2X2 channels were found to
co-immunoprecipitate constitutively and be associated in clusters in cultured
myenteric neurons of guinea pig as well as expression cells [39]. 5-HTRs in
pelvic afferent neurons is up-regulated in mice lacking P2X2 or P2X3 receptor
genes [25].
4.3. Inters. ATP Contributed to AP-Analgesia as
the Precursor of Adenosine
We demonstrated an upregulation of inters. ATP in the treated acupoint by
needling (Fig. 3A), and prevention its hydrolysis by ARL impaired AP-analgesic
effect (Fig. 3B). These findings imply that ATP mobilization and subsequent
hydrolysis are vital steps towards antinociception during needling. The
aggregated inters. ATP originates from cell lysis and non-lytic release as well.
Regarding the former, needle piercing and subsequent manipulation can cause
tissue damage. As reported, damage-associated molecules, high mobility group box
1 protein (HMGB1) and toll-like receptor 4 (TLR4), increases in the treated
acupoint of normal rats by 2-min needling [40]. The non-lytic
release of ATP results from the primary ATP secretion via
activating mechanosensitive channels [1] and secondary ATP release induced by ATP
or 5-HT, as we discussed above. But unlike 5-HT, mechanosensitive ATP release via
non-degranulation approach (Supplementary Fig. 2A).
Adenosine, as the downstream product of ATP, aggregates in acupoints too [14, 41]. The current work and other similar study [14] observed that pharmacologic
activation of adenosine A1R in acupoints had antinociceptive effect (Fig. 3B), in
which A1R are supposed to be situated on local nerve terminals [14]. Like ATP,
adenosine might also release out via lytic and non-lytic approaches [42].
We inferred the presence of ecto-ATPases in the acupoint based on the
reinforcing effect of ARL on inters. ATP accumulation (Fig. 3A). NTPDases family
is the major nucleotide-hydrolyzing enzymes, which degrade ATP to AMP with
intermediate formation of ADP [43]. Our previous work revelated that rats ST 36
acupoint expressed mRNA of NTPDase-1, -2,-3 [16]. Combined with ecto-AMPase, for
example, ecto-5’-nucleotidase (Nt5e), can fulfill the production of adenosine. In
the skin, Langerhans cells express NTPDase-1 [44], keratinocytes express
NTPDase-3 [45] and Nt5e [46]. Behavioral evidences in mice show that
nucleotidases prostatic acid phosphatase (PAP) [47] and Nt5e [48, 49] have
anti-nociceptive effects at spinal cord level via promoting adenosine production.
Injection of PAP into Weizhong acupoint (BL 40) has analgesic effect similar to
AP treatment [50].
4.4 Inters. ATP may Facilite Localized Propagation of Needling
Signals
Needling manipulations generate mechanical stimulation that is transmitted to
the wider and deeper space by subcutaneous collagen fibers twisting around the
needles [51, 52]. This is the “physical propagation” of needling signals [3].
In addition, “biochemical propagation” also occurs. As reported, AP-generated
acoustic shear waves can cause [Ca] shock and CWP in vitro
and in vivo [30]. CWP permits regenerative signal amplification. It has
been proposed that non-neural cells respond to mechanical stimulation by ATP
release and send signals to nerve terminals via CWP [30]. CWP formation is
commonly based on ATP regeneration [29]. Compelling evidence indicates that
ATP-mediated ATP release involves P2YRs [53], among which P2Y1R is the most
documented [54, 55]. Our previous work had observed a similar CWP phenomenon in
cultured MCs in response to mechanical stimulation, which relied on the
activation of P2Y and P2X receptors via “ATP-induced ATP release”
mechanism [15]. 5-HT-mediated ATP release uncovered in this work
is a good complement to ATP regeneration, then facilitate CWP formation. The
necessary components for CWP, including P2Y1 receptors, Connexin 43 and Panx-1
channels are expressed in mice acupoint (ST 36) [20]. In the present work, WB
determined their presence in MCs (Supplementary Fig. 5). Furthermore,
Panx-1 [56] and connexin 43 [57] are been reported to express
in skeletal muscle and fibroblasts that are also main tissues contained in
acupoints. Hence, besides as the precursor of adenosine, inters. ATP in acupoint
might exert function of transmitting the needling signals. This hypothesis was
partially confirmed by the inhibitory effect of Suramin and PPADS on ATP
regeneration in MCs in our previous work [15] and AP-analgesic effect in the
present study (Fig. 3C,D).
4.5 Contribution of 5-HT to AP Analgesia is Might via Direct
Activating 5-HTR
We found that 5-HTRs, especially 5-HTR, in acupoint played role in the
initiation mechanism of AP-analgesia (Fig. 1E,F and Fig. 2G,H). Beside
facilitating needling signals as we discussed above, 5-HT might contribute to AP
analgesic effect by activating 5-HTR on the adjacent
peripheral nerve endings. As we investigated previously, AP-activated MCs were
eventually involved in the analgesic effect via transmitting the biological
signals to the nearby nerve endings, then ascending to central nerve system [58].
5-HTRs class represents the most complex subtype families of 5-HTRs, which
comprise of five receptor subtypes, 5-HT, 5-HT, 5-HT,
5-HT and 5-HT. Although 5-HTR activation is reported to
elicit hyperalgesia [59, 60], later researches reveal its anti-nociceptive
effect. Peripheral sensory nerve endings are the main location of 5-HT
subtype [61]. Administered intrathecally with
()-8-hydroxydipropylaminotetralin hydrobromide (8-OH-DPAT), a 5-HTR
agonist, was to suppressed the second phase of formalin-induced aversive
responses in the 5,7-dihydroxytryptamine treated rats [62]. Another research
using 8-OH-DPAT also suggested an analgesic action is exerted by 5-HTR in
the formalin model of tonic nociceptive pain in rats [63]. It still needs further
work to explore whether 5-HT mediates AP analgesia by activating 5-HTR
situated on nearby nerve endings.
Abbreviations
5-HT, 5-hydroxytryptamine, serotonin; 5-HTRs, 5-HT receptors; 5-HTRs,
5-HT1 receptors; 5-HTRs, 5-HT1A receptors; 5-HTRs, 5-HT3 receptors;
AA, adjuvant arthritis; AP, acupuncture; ARL67156,
6-N,N-Diethyl---dibromomethylene-D-adenosine-5’-triphosphate;
ATP, adenosine triphosphate; [Ca]i, intracellular calcium concentration;
CCPA, 2-Chloro-N6-cyclopentyladenosine; CFA, complete Freund’s adjuvant; CRO,
sodium cromolyn; CWP, calcium wave propagation; DMSO, dimethyl sulfoxide; Inters.
5-HT, interstitial 5-HT; Inters. ATP, interstitial ATP; ELISA, enzyme linked
immunosorbent assay; Exo-5-HT, exogenous 5-HT; HBSS, Hank’s balanced salt
solution; HPLC, High-performance liquid chromatography; MCs, mast cells; Panx-1,
pannexin 1; PPADS, pyridoxal phosphate-6-azo tetrasodium salt hydrate; P2
receptors, purinergic P2 receptors; PWL, paw withdraw latency; PWT, paw withdraw
threshold; WAY-100635,
N-[2-[4-(2-Methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinylcyclohexanecarboxamide
maleate salt.
 Fig. 1.
Fig. 1. Fig. 2.
Fig. 2. Fig. 3.
Fig. 3. Fig. 4.
Fig. 4.