1 Ajinomoto Co., Inc., 1-1, Suzuki-Cho, Kawasaki-Ku, Kawasaki-Shi, 210-8681 Kanagawa, Japan
2 Ajinomoto Bio-Pharma Services, San Diego, CA 92121, USA
§Present addresses: Exelixis Inc., Alameda, CA 94502, USA
Academic Editor: Graham Pawelec
Abstract
Background: Trastuzumab-emtansine (T-DM1, commercial name: Kadcyla) is well-known antibody-drug conjugate (ADC) and was first approved for human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer. This molecular format consisting of trastuzumab and maytansinoid payload (emtansine) is very simple, however, T-DM1 has wide heterogeneity due to non-specific conjugation, lowering its therapeutic index (TI). Methods: To overcome this issue during the chemical modification of the random conjugation approach to generate T-DM1, we developed a novel chemical conjugation technology termed “AJICAP®” for modification of antibodies in site-specific manner by IgG Fc-affinity peptide based reagents. Results: In this study, we compared site-specific maytansinoid-based ADCs synthesized by AJICAP and T-DM1 in rat safety studies. The results indicated an increase in the maximum tolerated dose, demonstrating an expansion of the AJICAP-ADC therapeutic index compared with that of commercially available T-DM1. Gram scale preparation of this AJICAP-ADC and the initial stability study are also described. Conclusions: Trastuzumab-AJICAP-maytansinoid produced by this unique chemical conjugation methodology showed higher stability and tolerability than commercially available T-DM1.
Keywords
- antibody drug conjugate
- maytansinoid
- toxicology study
- site-specific conjugation
- AJICAP
Trastuzumab-emtansine (T-DM1, commercial name: Kadcyla) is a maytansinoid-based antibody-drug conjugate (ADC), which was first approved for patients with human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer [1]. Since its success in 2013, more than 10 ADCs been introduced into the market [2, 3].
ADCs have becoming an innovative cancer medicines that consist of monoclonal antibodies (mAbs) and cytotoxic payloads via chemical linkage. This molecular concept is simple; however, most ADCs currently in the market have wide heterogeneity owing to non-specific conjugation. These nonspecific conjugations are classified into two main categories: using reduced interchain native cysteine and using native lysine. The “interchain-break” cysteine-based methodology is the major manufacturing approach to produce “semi-random” ADC including mainly eight drug-to-antibody ratio (DAR) species (DAR = 0, 2, 4, 6, 8) [4]. This conjugation provides six ADCs (Adcetris, Polivy, Padcev, Blenrep, Zynlonta and Tivdak) approved by U.S. Food and Drug Administration (FDA). Native lysine conjugation is a traditional conjugation technology using activated ester groups (such as NHS) [5]. These high reactive compounds enable to react with exposed lysine residue to form stable covalent bond between antibodies and drug-linkers. This methodology produced three FDA-approved ADCs (Mylotarg, Kadcyla and Besponsa). T-DM1 (commercially name: Kadcyla) is produced by natural conjugation with available lysine residues, thereby resulting in a heterogeneous distribution of high potent payload-linker over multiple ADC sites; this process could lead to a clinically insufficient and narrow therapeutic index (TI) [6].
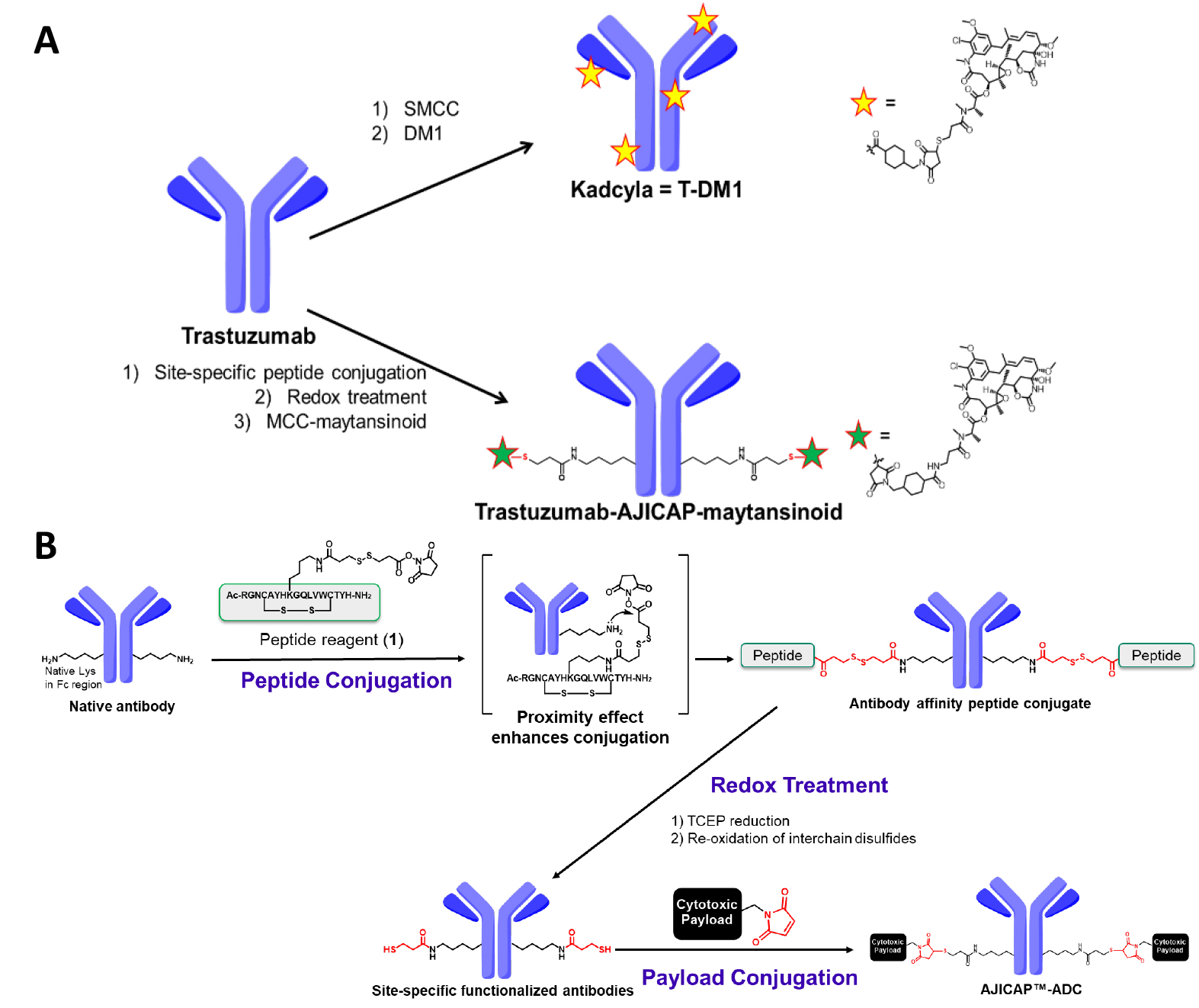
In addition to limited in vivo profile, the heterogeneity of T-DM1 could be problematic from a chemical manufacturing and control point-of-view. Some HPLC analyses, which are the most common approaches for determining the DAR, fail to provide clear chromatographic results [7, 8]. To overcome ADC heterogeneity, several site-specific conjugation methods to produce more homogeneous ADCs have been developed [9]. Thus, our research group conceived a proprietary technology utilizing an Fc-affinity peptide, which can install a bi-orthogonal group onto antibodies in a site-selective manner, thereby enabling the site-specific ADCs syntheses (Fig. 1) [10, 11].
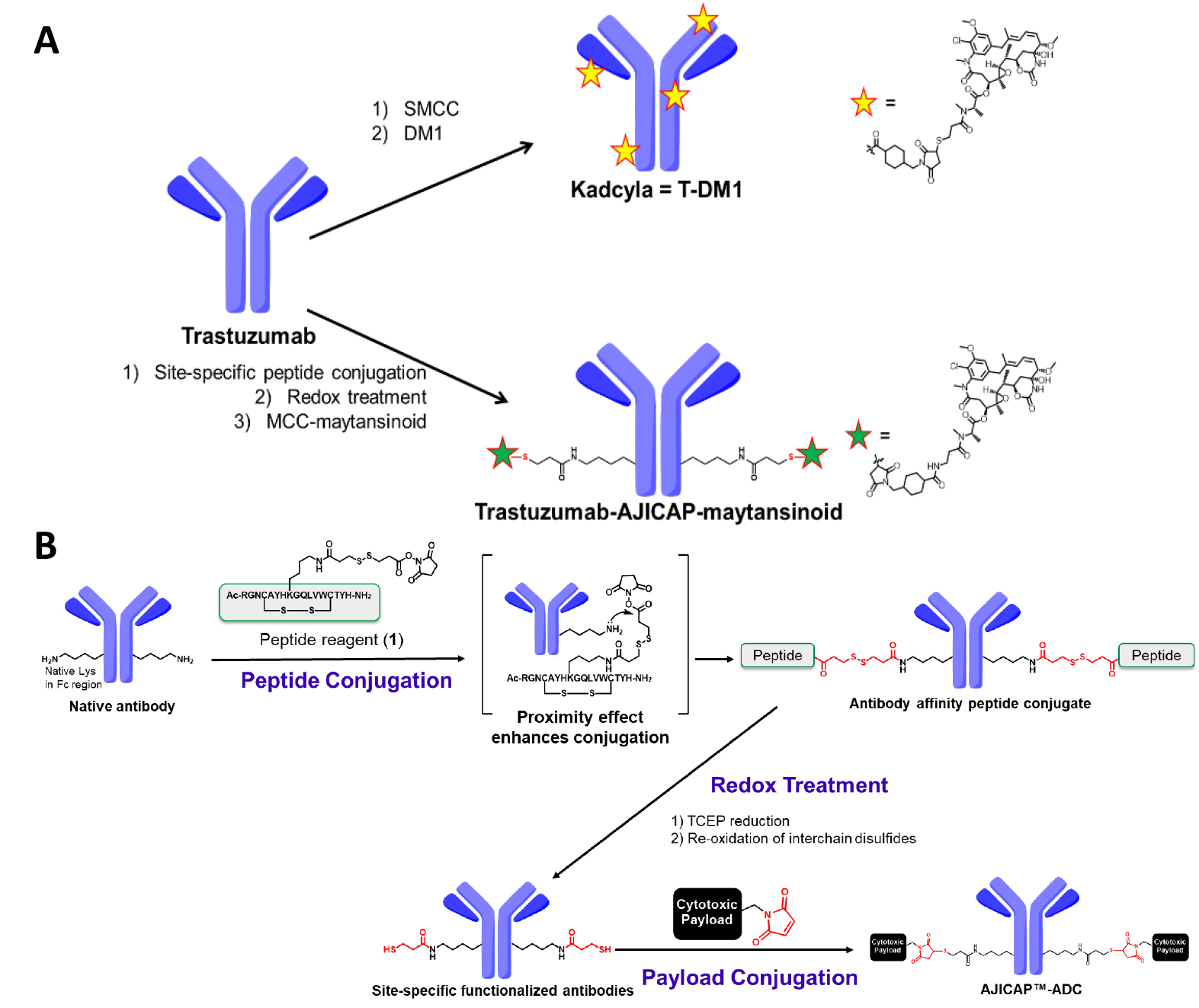 Fig. 1.
Fig. 1.Summary of the conjugation chemistry for ADC production. (A) Upper: Synthesis of T-DM1 by traditional native lysine conjugation; Lower: AJICAP site-specific conjugation to synthesize trastuzumab-AJICAP-maytansinoid. T-DMI, Trastuzumab-emtansine; ADC, antibody-drug conjugate. (B) Reaction scheme of AJICAP technology.
This platform, termed “AJICAP”, has been advantageous in several studies. First, its proof of concept was reported in 2019, showing highly efficient and versatile conjugation applied to several mAb subtypes such as IgG1, IgG2, and IgG4 [10]. Peptide mapping analysis of the resulting homogeneous ADC confirmed its conjugation site-occupancy at Lys 248 in the antibody Fc region [12]. The larger scale ADC preparation using a glass reactor vial based on the good manufacturing practice strategy indicated the high productivity and robustness of the AJICAP technology [13]. In addition, site-specific AJICAP-ADCs have been used as a demonstration tool to develop novel analytical approaches [14].
In contrast to the numerous synthetic/analytical reports on AJICAP-ADCs, there have been limited studies on their biological profile. Furthermore, no studies have performed a direct comparison between AJICAP-ADC and commercially available ADCs.
In order to provide a TI comparison between AJICAP-ADCs and traditional ADCs in the market, we decided to demonstrate the rat toxicology study of trastuzumab-AJICAP-maytansinoid (DAR = 2) as compared to commercially available T-DM1 (DAR = 3.4). The combination of this safety study and a previously reported efficacy study enables comparison of the TI, thereby resulting in a wide enhancement of TI by AJICAP technology [15]. To supply a sufficient quantity of trastuzumab-AJICAP-maytansinoid, gram-scale ADC preparation was also performed, thus, supporting the robustness of the previously established conjugation process [13]. To adapt the AJICAP method of conjugation, a cysteine-reactive maytansinoid (MCC-maytansinoid, Fig. 1) was synthesized as previously reported [16]. The stability of trastuzumab-AJICAP-maytansinoid was assessed to determine its appropriate storage conditions [17, 18]. Overall, the results described herein support the previously reported advantages of AJICAP conjugation platform enabling site-specific ADC production.
Human IgG1 trastuzumab (Herceptin®) was purchased from Roche Pharmaceutical Company (Basel Switzerland). MCC-maytansinoid (catalog no: TCRS-1262) was purchased from Abzena (San Diego, CA, USA). PNGase F and GlycoBuffer 2 were purchased from New England Biolabs (Ipswich, Massachusetts, UK). All other chemical reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA).
The SoloVPE spectroscopy system (C Technologies, Inc. Bridgewater, NJ, USA) provided ADC concentration.
Site-specific thiol intermediate was produced by a previously established procedure [13].
To a solution of site-specific thiol intermediate (6.8 mg/mL, 1.63 g) in a PBSE buffer (50 mM phosphate buffered saline (PBS), 10 mM ethylenediaminetetraacetic acid (EDTA), pH 7.4), dimethylformamide (DMF) (15 mL) and a 10-mM DMF solution of MCC-maytansinoid (10 eq., 11.7 mL) were added to form a mixture, which was then incubated at 20 °C. After 2 h, a small amount of the reaction mixture (0.5 mL) was sampled for the in-process control (IPC) analysis. Subsequently, the reaction mixture was quenched with an excess amount of a 50-mM aqueous solution of N-acetyl cysteine (NAC) and then incubated at 25 °C for 15 min. This reaction mixture was purified with a tangential flow filtration (TFF) system using a Sartocon Slice 200 Eco Hydrosart membrane (30 kDa; Sartorius) and diafiltration (DF) buffer as the conjugation buffer at an antibody concentration of 20 mg/mL. Next, buffer exchange of this solution was performed using a TFF system with a Sartocon Slice 200 Eco Hydrosart membrane (30 kDa; Sartorius) and DF buffer as the formulation buffer (20 mM histidine containing 5% trehalose, pH 5.2) at an antibody concentration of 5.7 mg/mL. Trastuzumab-AJICAP®-maytansinoid 4 (1.67 g, 98% yield) was then afforded in the formulation buffer.
Trastuzumab-AJICAP-maytansinoid (50
Q-TOF MS analysis was performed using a PLRPS 1000 Å 8
The EIC analysis was performed using Q-TOF MS system with m/z ratio of 1103.4 (M+).
The reductive pre-treatment was performed as previously reported [19].
RP-HPLC analysis was performed according to a previously reported study [19].
HIC HPLC analysis was performed according to a previously reported study [20].
Three sets of standard solutions of trastuzumab-AJICAP-maytansinoid (4) were prepared in a formulation buffer. All the samples were stored at various temperatures (–80, –20, 4, 25, and 37 °C) for 4 weeks. Aggregation was then analyzed using size-exclusion chromatography (SEC)-HPLC.
Aggregation analysis using SEC was performed as previously reported [20].
Sixty 8-week-old female Sprague-Dawley rats (Charles River Japan, Tokyo, Japan) were provided ad libitum access to a standard diet (Oriental Yeast, Tokyo, Japan) and water. Following the acclimatization period (1 week), the animals were stratified by body weight and randomly assigned to the following groups: (1) control group, treated with the vehicle (histidine buffer); (2) two T-DM1 groups, treated with 20 mg/kg and 60 mg/kg; and (3) three trastuzumab-AJICAP-maytansinoid (4) groups, treated with 20 mg/kg, 60 mg/kg, and 120 mg/kg. Each group consisted of five animals for blood chemistry test as well as five animals for clinical signs and body weight measurements. All rats were administered once through intravenous injection via the caudal vein using a butterfly needle, a 30-mL polypropylene syringe, and a syringe pump (Pump 11 Elite, Harvard Apparatus). The experimental procedures were approved by the institutional ethics committee.
The animals were observed once daily for any clinical signs. Individual body weights were measured on Days 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 19, 20, and 21; the first day of administration was defined as Day 0.
Blood chemistry parameters were evaluated 2 d after administration. The animals were fasted overnight and anesthetized with isoflurane prior to collecting blood from the caudal vena cava. Heparin-anticoagulated plasma samples were evaluated using an automated clinical chemistry analyzer (TBA-120FR; Toshiba Medical Systems, Inc., Tokyo, Japan) to determine aspartate (AST) and alanine aminotransferase (ALT) levels. EDTA-anticoagulated blood samples were evaluated using an automated hematology analyzer (ADVIA2120, Siemens Healthineers AG., Munich, Germany) to determine platelet (PLT) counts.
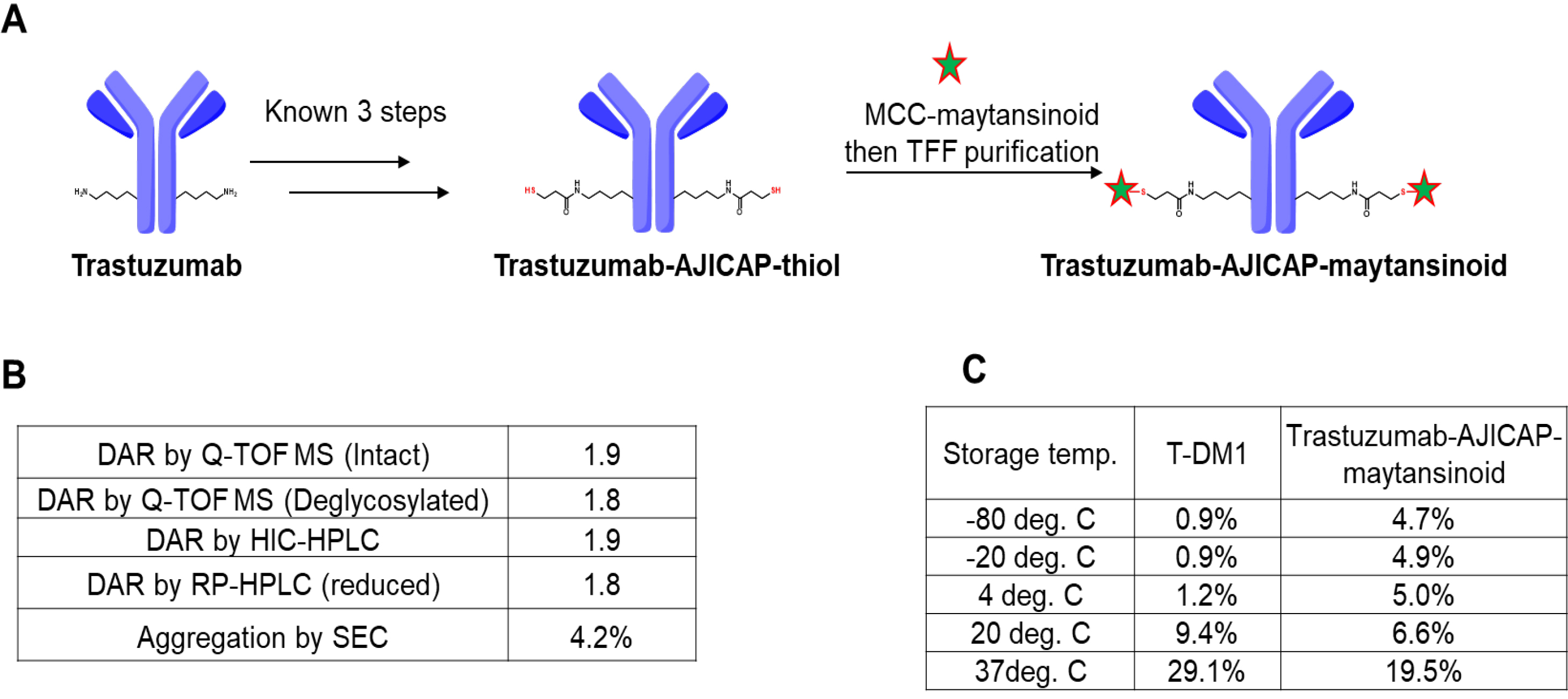
Using a well-established previously described approach [13], a site-specific thiol-modified mAb, the intermediate ADC precursor, was prepared from trastuzumab, which is the same antibody as that in T-DM1 upon completion of a gram-scale synthesis (Fig. 2). The final step in ADC preparation is the conjugation of this thiol intermediate with MCC-maytansinoid (Fig. 2A). This reaction completion was detected by IPC analysis using HIC HPLC, as per a previous good laboratory practice (GLP) material preparation [13]. Purification to remove residual drug linkers was performed in a straightforward manner using tangential flow filtration (TFF), which is a commonly used technique that can be applied to kilogram-scale ADC manufacturing [21, 22]. Compared to MC-VC-MMAE, which was previously used for preparing AJICAP-ADC, TFF could clear MCC-maytansinoid from the ADC composition. This can be attributed to the hydrophobicity difference of each drug linker. MC-VC-MMAE is relatively more hydrophobic than MCC-maytansinoid [23], thus, requiring careful monitoring of its clearance level. The removal rate of highly potent MCC-maytansinoid drug-linker was calculated by the extracted ion chromatogram (EIC) analysis, which showed that the rate was supposed to be near the detection limit in this analysis (data not shown).
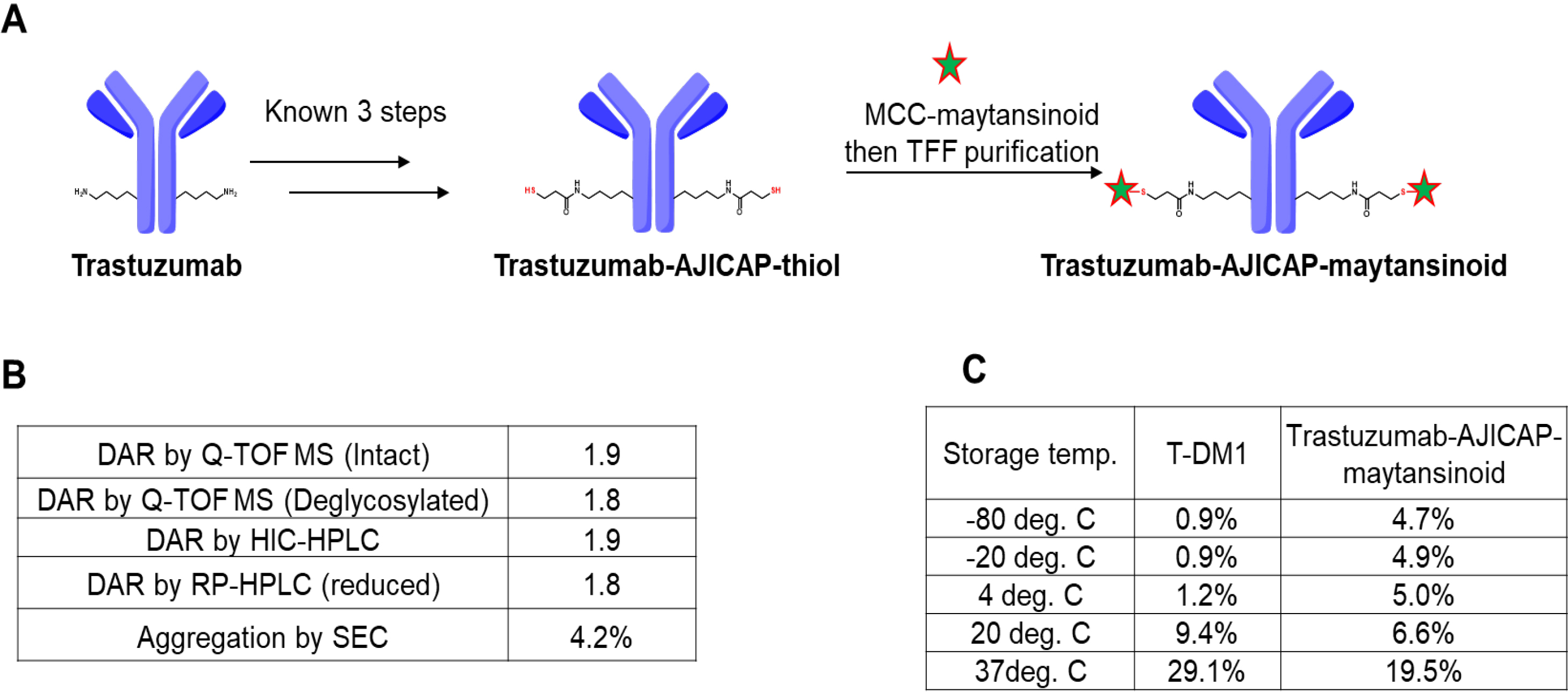 Fig. 2.
Fig. 2.Synthesis and analysis of trastuzumab-AJICAP-maytansinoid. (A) Synthetic scheme of the gram-scale ADC preparation. (B) Analytical summary of resulting ADC. (C) Aggregation comparison of T-DM1 (literature data) and trastuzumab-AJICAP-maytansinoid (tested data) at different temperatures after 4 weeks of storage. T-DMI, Trastuzumab-emtansine.
Three different analytical methods were used to determine the DAR of trastuzumab-AJICAP-maytansinoid (Fig. 2B). Q-TOF MS (Intact ADC: Supplementary Fig. 1, deglycosylated ADC: Supplementary Fig. 2), RP-HPLC (Supplementary Fig. 3) and HIC-HPLC (Supplementary Fig. 4) analyses revealed that this ADC has a high DAR value (1.8–1.9). The DAR results were slightly different among the three methods; however, this observation was also reported in several previous studies [24, 25, 26]. Aggregation percentage was within acceptance level (Supplementary Fig. 5).
Next, a thermal stability study of trastuzumab-AJICAP-maytansinoid was examined to estimate the suitable formulation and storage conditions towards forthcoming manufacturing processes (Fig. 2C). Several previous studies, including ours, had reported stability assessments results of T-DM1 in peer-reviewed literature; and we followed the same procedures for one of the processes applied to trastuzumab-AJICAP-maytansinoid [13, 16, 17, 18].
T-DM1 was found to be relatively stable at lower temperatures (
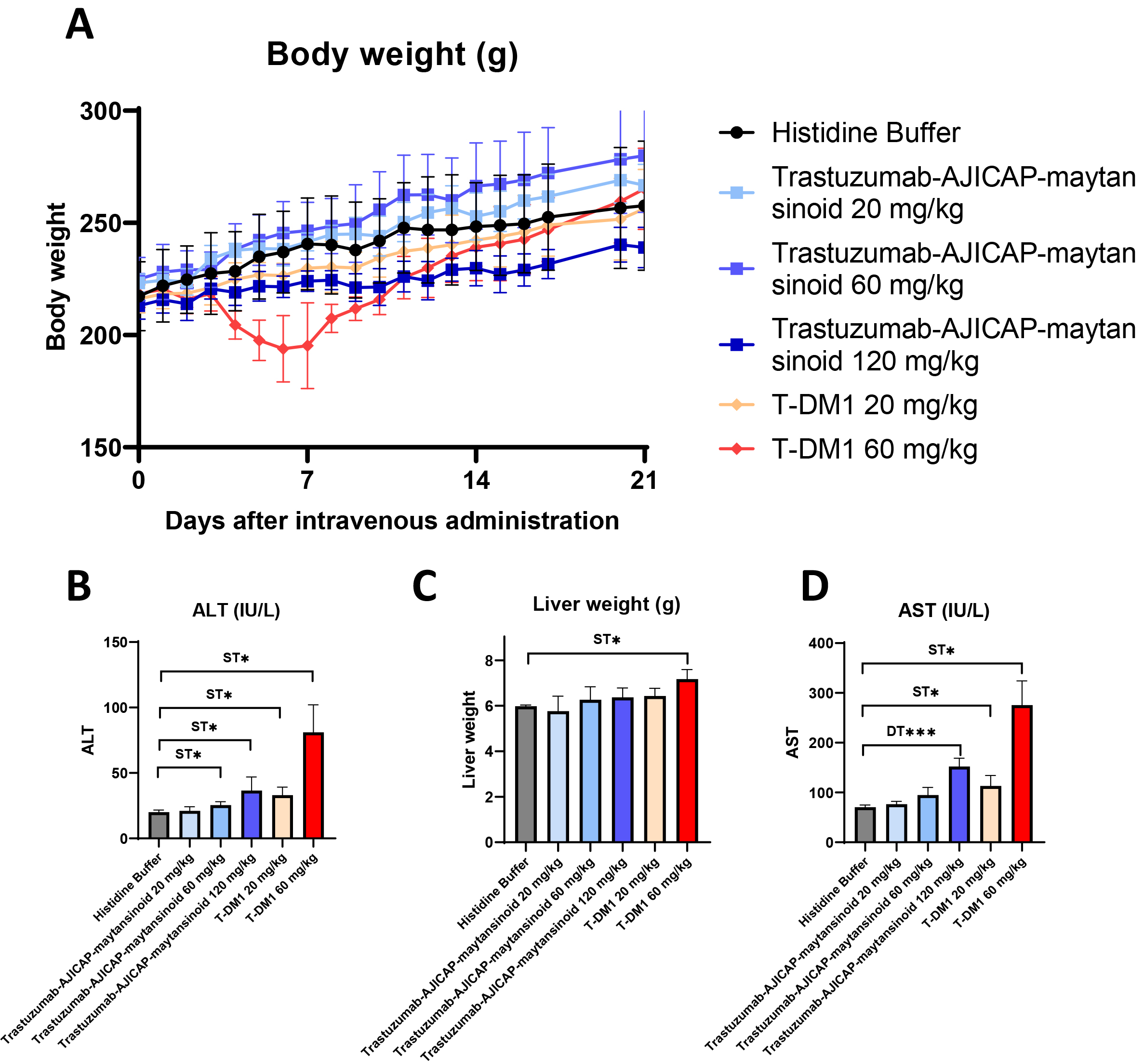
A rat acute toxicity study was performed for the MTD determination of the ADCs (Fig. 3). Mortality or severe toxicity was observed with T-DM1 at a dose of 40 mg/kg; however, no toxicity was observed with AJICAP-ADC even at a dose of 120 mg/kg (Fig. 3A). Decreases in body weight or PLT levels and increased AST levels were not observed at doses of up to 120 mg/kg trastuzumab-AJICAP-maytansinoid and 20 mg/kg T-DM1. Additionally, Liver weight did not change significantly at a 120 mg/kg. Thus, the MTD of trastuzumab-AJICAP-maytansinoid and T-DM1 was estimated to be at least 120 and 20 mg/kg, respectively. Neutrophil, reticulocyte, lymphocyte and platelet count data supported these estimations, considering the mode of action of maytansinoids (Supplementary Fig. 6) [28, 29]. An exploratory rat safety toxicology study showed that the rats showed better tolerance to trastuzumab-AJICAP-maytansinoid than to T-DM1. Given the limited dosing in our study, the MTD of trastuzumab-AJICAP-maytansinoid was estimated to be at least 120 mg/kg, whereas the MTD of T-DM1 was estimated to be at least 20 mg/kg. This initial safety analysis indicated that the conjugation approach generated a stable ADC, although further biological/analytical studies are currently ongoing, such as PK. In previous report [15], a rat PK study showed that the trastuzumab-AJICAP-MMAE ADC is stable in blood circulation compared to native cysteine-based stochastic ADC. The measured levels of total antibody from trastuzumab-AJICAP-MMAE indicated a half-life similar to that expected for parent trastuzumab. Furthermore, in vitro binding assay using biolayer interferometry method concluded that trastuzumab-AJICAP-maytansinoid does not affect FcRn interactions [30]. These results suggest that site-specific conjugated ADCs at Lys248 result in a superior profile with regard to both safety and in vivo stability compared to the approved ADC, T-DM1.
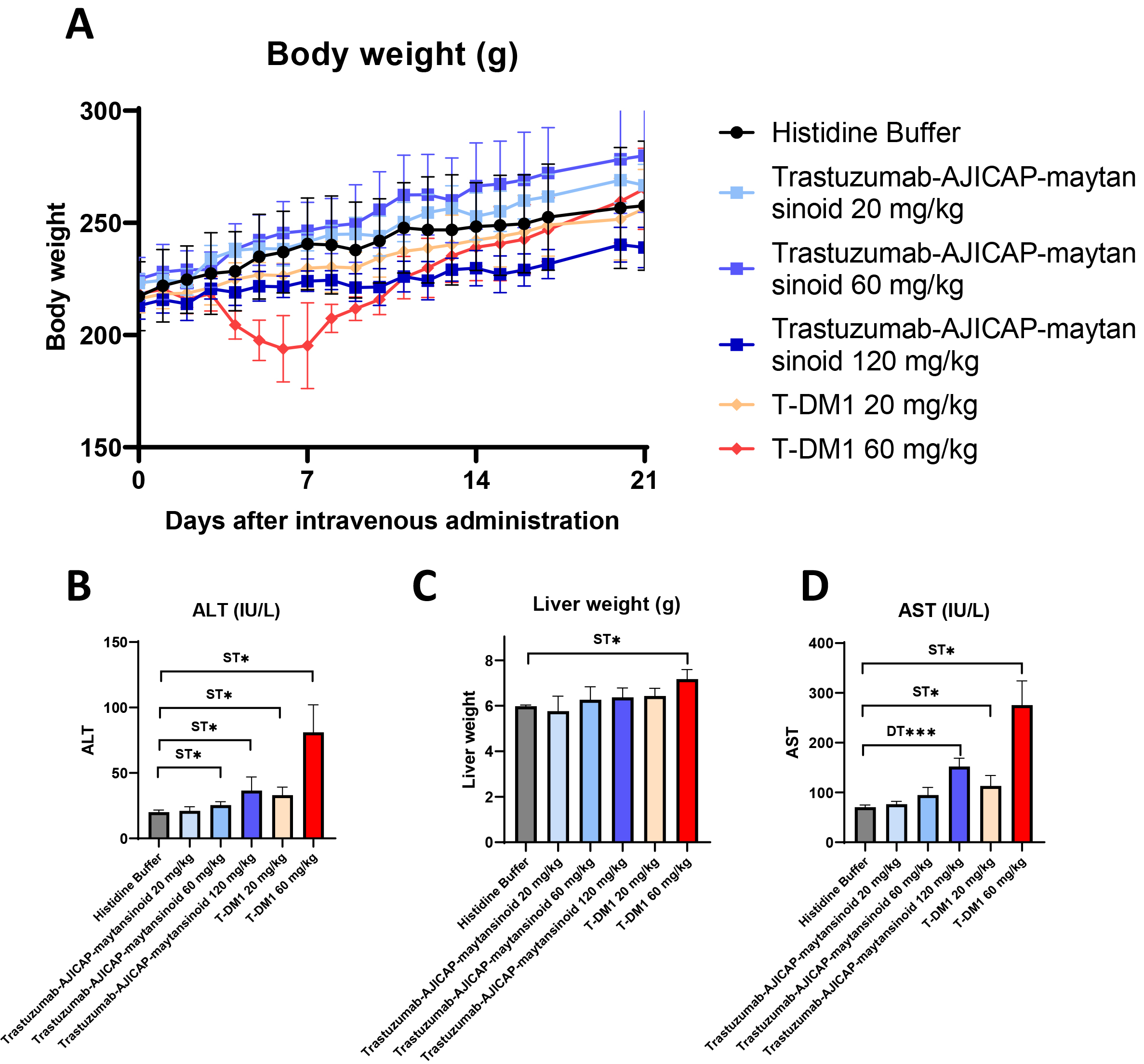 Fig. 3.
Fig. 3.Rat acute toxicity study to determine the MTD of ADCs.
(A) Body weight change (N = 5 female SD rats). (B) Liver weight in rat serum at
2 days after administration (N = 5 female SD rats. (C) AST in rat serum at 2 d
after administration (N = 5 female SD rats). (D) ALT levels in rat serum at 2 d
after administration (N = 5 female SD rats). MTD, maximum tolerated dose; ADCs,
antibody-drug conjugates; AST, aspartate aminotransferase; ALT, alanine
aminotransferase. Significantly different from Histidine Buffer:
* p
Furthermore, in a previous study, we reported that the minimum effective dose (MED) of both ADCs was 5 mg/kg based on the NCI-N87 xenograft study. Thus, the TI of trastuzumab-AJICAP-maytansinoid was estimated to be qualitatively greater than that of T-DM1, considering their respective MTDs and MEDs (Table 1).
| ADC | Minimum effective dose (MED) | Maximum tolerated dose (MTD) |
| T-DM1 (Kadcyla) | ||
| Trastuzumab-AJICAP-maytansinoid |
In conclusion, we applied a full chemical conjugation technology, AJICAP, to produce a maytansinoid-based site-specific ADC. The scale-down manufacturing process using TFF purification effectively removed the residual drug linkers from the final ADC composition, thus, producing DAR of nearly 2.0 ADCs without including a significant level of aggregates. The resulting site-specific ADCs showed sufficient long-term stability under different storage conditions.
Furthermore, the comparative toxicology study between trastuzumab-AJICAP-maytansinoid and commercially available T-DM1 indicated that ADCs produced by this unique chemical conjugation methodology enables therapeutic index expansion. Therefore, this platform conjugation technology has a strong potential to produce next-generation ADCs with high homogeneity and significant in vivo stability.
T-DMI, Trastuzumab-emtansine; ADCs, antibody-drug conjugates; HER2, human epidermal growth factor receptor 2; TI, therapeutic index; DAR, drug-to-antibody ratio; DMF, dimethylformamide; MPA, mobile phase A; MPB, mobile phase B; FA, formic acid; AST, aspartate aminotransferase; ALT, alanine aminotransferase; PLT, platelet; TFF, tangential flow filtration; EIC, extracted ion chromatogram; Q-TOF MS, Quadrupole time-of-flight mass spectrometry; MED, minimum effective dose; MTD, maximum tolerated dose.
TS—Animal study, Validation, Formal analysis, Data curation and Writing—original draft preparation. KY—Conceptualization and ADC preparation. YO—Animal study, Formal analysis, Data curation. TF—Resource, Stability assessment and ADC preparation. TN, AN and YK—Methodology and Validation. BAM—Supervision, Writing—review and editing. YM—Conceptualization, Resources, ADC preparation, Writing—original draft preparation, review and editing. TO—Conceptualization, Funding acquisition, Supervision, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Not applicable.
The authors wish to express the gratitude to colleagues from Ajinomoto Co., Inc., as follows: Natsuki Shkida, Reiko Yuji, Kazutoshi Takahashi, Kunio Nakata and Kazutaka Shimbo for useful opinions on ADC analysis; Akira Chiba for support and encouragement during manuscript preparation.
This research received no external funding.
The authors declare no conflict of interest. YM and BAM are serving as the Guest editors of this journal. We declare that YM and BAM had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to GP.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/j.fbl2708234.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.